Abstract
Sepsis is the leading cause of death amongst critically ill patients in intensive care units, and treatment options are limited. Therapies developed against the pro-inflammatory stage have failed clinically; therefore new approaches that target the host immune response in sepsis are necessary. Increasing evidence suggests that a major pathophysiological event in sepsis is immune suppression, often resulting in secondary fungal, bacterial, or viral infections. Recent studies from animal sepsis models and patient samples suggest that cytokines such as IL-7, IL-15, GM-CSF as well as co-inhibitory molecule blockade, such as anti-PD-1 and anti-BTLA, may have utility in alleviating the clinical morbidity associated with sustained sepsis. This review discusses some of these novel immunomodulatory agents and evaluates their potential use as therapeutics.
Keywords: sepsis, immunosuppression, co-inhibitory molecules, cytokines, immuno-modulatory agents
What is sepsis?
Sepsis is the 10th leading cause of death in the USA and the leading cause of death amongst critically ill patients. Approximately 250,000 cases of sepsis lead to fatalities in the USA annually [1–3]. In 1991, a North American consensus conference defined sepsis as the host’s immune response (systemic inflammatory response syndrome; SIRS) to injury and/or infectious stimuli in the presence of a known (or strongly suspected) infection. This same conference also defined criteria to qualify SIRS (see Glossary) [4]. Thus, sepsis is caused by the host’s immune response to a bacterial, viral, and/or fungal infection, which derives from the abdomen, skin, lungs, and/or urinary tract. Sepsis has historically thought to be hallmarked by two distinct stages: pro-inflammatory and anti-inflammatory. Typically, the pro-inflammatory stage predominates first. This is sometimes referred to as a cytokine storm, where the innate immune system releases cytokines systemically to mobilize the host’s ability to combat infection, while also recruiting members of the adaptive system to mount an intense immune response. The anti-inflammatory stage, referred to as CARS (compensatory anti-inflammatory response syndrome), was proposed initially to follow SIRS [5], and is usually defined as the body’s inability to respond to a defined antigenic and/or infectious challenge.
While these stages have been considered as distinct entities in time (the pathological process), most experimental therapies were initially directed against the pro-inflammatory stage. These initial therapeutic approaches were solely based on the premise that high concentrations of pro-inflammatory mediators produced in response to septic and/or infectious stimuli could cause marked organ injury in an experimental and as such the clinical setting, thereby, culminating in the demise of critically ill patients.
Currently, therapies directed against sepsis consist of antibiotics to the known and often presumed pathogen(s) along with primarily supportive care, which, while often sustaining patients through acute episodes of distress, frequently gives way to a state of chronic morbidity lasting days to weeks, consuming substantial health care resources. Unfortunately, contemporary pharmacological and molecular biological approaches to alleviate the healthcare costs associated with these chronic sepsis-related hospital stays are nonexistent. This timely review considers whether the immune suppressive stage, often associated with such chronic sepsis related deaths, could be targeted in order to improve overall patient outcomes. Immunomodulatory agents or novel therapeutics that enhance immune cell function are potentially promising and should be investigated in a clinical setting. Traditionally, most of the unsuccessful therapies directed at sepsis have focused on the pro-inflammatory stage, and have not specifically addressed sepsis-induced immune suppression. Here, we propose a “new normal”, in contrast to pro-inflammatory therapies, which encourages the application of cytokines and co-inhibitory molecule antagonists to improve immune responses in critically ill patients.
Sepsis, pro-inflammation, and unsuccessful targets
Multiple anti-inflammatory immunomodulatory therapies have been developed to combat the pro-inflammatory stage of sepsis. However, these immunomodulatory agents, such as anti-endotoxin (LPS, lipopolysaccharide), anti-tumor necrosis factor (TNF)-α, interleukin (IL)-1β and Toll-like receptor (TLR)-4 inhibitors [6–8], have proven unsuccessful in clinical trials. The notion that septic morbidity is simply the sequelae of an overzealous pro-inflammatory response to infectious challenge implies that the pathophysiological process of sepsis is a more complex condition in which multiple mediators are released, inducing a highly multifaceted immunological and host tissue response.
Further, contrary to what was previously thought, recent data suggests that both the pro-inflammatory and anti-inflammatory stages of the host immune response to severe injury and/or sepsis often occur simultaneously [9]. Irrespective, the amplitude of the initial pro-inflammatory phase appears to be dependent on a variety of factors including pathogen virulence and load, as well as the patient’s own baseline co-morbidities [10]. Although the accumulation of pro-inflammatory cytokines in human patients correlates with early mortality, late (chronic) sepsis related deaths appeared to be often associated with overt evidence of immune suppression and dysfunction (reviewed in [9]). In fact, more than 70% of the sepsis related deaths occur after the first three days, with many deaths following weeks after sepsis onset [11]. Hypothetically, the septic patients that survive the early pro-inflammatory phase and then enter a prolonged counter-regulatory or anti-inflammatory phase were indices of immune suppression, or immune paralysis [12].
Septic immunosuppression defined
Immunosuppression is especially common in elderly patients that develop sepsis, since the immune system of the aged is often impaired, resulting in reduced efficacy in fighting invading pathogens [2, 13]. Lymphoid cell loss, often resulting in the diminished capacity to fight and eliminate pathogens, is a primary feature of immune suppression in critically ill patients. The diminished capacity of septic patients to fight and eliminate infections often leads to the development of secondary infections, mediated by otherwise weakly virulent or opportunistic organisms, for example Stenotrophomonas, Acinetobacter, Enterococus, Pseudomonas, and Candida. [11, 14]. Additional evidence for immunosuppression can be seen in the high incidence of reactivation of latent viruses such as cytomegalovirus and herpes simplex virus [15, 16].
It has been shown in both animal models and human patient samples that there is a significant loss of lymphocytes, dendritic cells, gastrointestinal epithelial cells as well as thymocytes during sepsis. The majority of immune cell loss has been associated with programmed cell death, mediated by both death-receptor (extrinsic) and mitochondrial-mediated (intrinsic) apoptotic pathways [17–21]. When apoptotic signaling pathway mediators and byproducts, such as Fas, FasL, B cell lymphoma-2 (Bcl-2), Bid, and caspase 8 are inhibited, overall survival in septic animal models improves [22–26]. In addition, septic patients have decreased T cell receptor (TCR) diversity compared to healthy controls [27]. While most studies have suggested that lymphoid cell loss during sepsis was a partial explanation for immune suppression, recent studies have also begun to focus on non-lymphoid cell loss, such as endothelial cells [28, 29]. However, as we learn more about what regulates lymphoid cell loss during sepsis, it also substantiates the idea that targeting a sole cytokine and/or a single mediator by itself may not be sufficient to improve morbidity and mortality in septic patients. One study [30] suggested that it might be preferable to stabilize vascular integrity or consider other pathways instead of a single aspect from the “cytokine storm”, which often results in local tissue edema, organ failure and shock [31].
Sepsis, the anti-inflammatory stage and the need for novel immuno-adjuvant therapy
Even though it was proposed that critically ill patients died from immune suppression resulting from an inadequate immune response in the presence of secondary opportunistic viral, fungal, and/or bacterial infections, novel therapeutic options have largely not been pursued as it has been assumed this might potentiate the deleterious aspects of pro-inflammation [32]. Therefore, novel biomarkers to identify immune suppression and/or the pathological mechanisms that underpin the immune suppressive state would seem to be of significant value.
A recent post-mortem study that examined spleen and lung samples from forty septic patients found that there was increased expression of co-inhibitory receptors and their ligands on lymphocytes and non-immune cell populations in lung and splenic tissue samples. This suggests that immunomodulatory agents may not only help to combat sepsis, but also aid in understanding why the pro-inflammatory stage of sepsis is followed by immune suppression [33, 34]. Other research groups have demonstrated that the immunosuppressive state is due to a variety of pathological mechanisms including apoptosis of immune effector cells [35], the development of T cell anergy and/or unresponsiveness of immune cells. These latter effects potentially being due to the up-regulation of cell-surface markers that inhibit cell function, cellular exhaustion due to increased expression of negative co-stimulatory (co-inhibitory) receptors/proteins, for example programmed cell death receptor-1 (PD-1) and cytotoxic T-lymphocyte antigen-4 (CTLA-4), as well as a shift toward a more immune suppressive cytokine profile [36], increased T regulatory cell activity [37], and decreased human leukocyte antigen D-related (HLA-DR) expression [38].
Therefore, novel therapeutic approaches in sepsis directed at decreasing mortality should expand the focus beyond agents that only treat the initial infectious agent, to those that augment the host immune response to it.
Cytokines as immunomodulatory therapeutic agents in sepsis
One family of the new immunotherapeutic agents with great potential in the treatment of septic patients is IL-7 (Table 1). IL-7 is a pleuripotent cytokine, which is mainly produced by stromal cells and is absolutely required for T cell development, homeostasis, and maintenance. This is illustrated by the absence of T cells in humans with severe combined immunodeficiency (SCID) due to mutations in the IL-7 signaling pathway [39, 40].
Table 1.
The clinical relevance of immunomodulatory cytokines in sepsis*.
| IL-7 | IL-15 | GM-CSF | |
|---|---|---|---|
| Protein |
|
|
|
| Cellular Expression |
|
|
|
| Function |
|
||
| Clinical relevance in sepsis |
|
|
IL-7, interleukin 7; TCR, T cell receptor; DTH, delayed type hypersensitivity; IFN-y, interferon-gamma; IL-5, interleukin-15; NK, natural killer; IL-12, interleukin-12; GM-CSF, granulocyte colony stimulating factor; HLA-DR, human leukocyte antigen-D-related; APC, antigen presenting cell; IL-1, interleukin-1; TNF, tumor necrosis factor-α; IL-6 interleukin-6.
Postmortem studies of sepsis patients showed significant apoptosis-induced loss in cells of the innate and adaptive immune system [33] [35]. IL-7 not only induces homeostatic proliferation of T cells during times of lymphopenia [41], but also possesses anti-apoptotic properties. IL-7 prevents sepsis-induced apoptotic depletion of CD4+ and CD8+ T cells by modulating the expression of pro- and anti-apoptotic members of the B cell lymphoma 2 (BCL-2) family [42, 43]. In a murine model of sepsis, IL-7 therapy led to up-regulation of the anti-apoptotic protein Bcl-2 and down regulation of pro-apoptotic proteins, including p53 upregulated modulator of apoptosis (Puma) and Bcl-2 interacting mediator of cell death (Bim) [44, 45], preventing apoptosis-induced depletion of T cells. Other beneficial properties of IL-7 include its ability to reverse sepsis-induced depression of T cell cytokines, such as interferon (IFN)-γ, an important activator of monocyte/macrophages, and increased expression of cell adhesion molecules, such as lymphocyte function associated antigen (LFA)-1 and very late antigen (VLA)-4, thereby facilitating lymphocyte trafficking to infection sites. The overall immune stimulating properties of IL-7 have been demonstrated by its ability to restore the delayed type hypersensitivity (DTH) response toward a known antigen, often a response strongly decreased in septic individuals (Figure 1) [44].
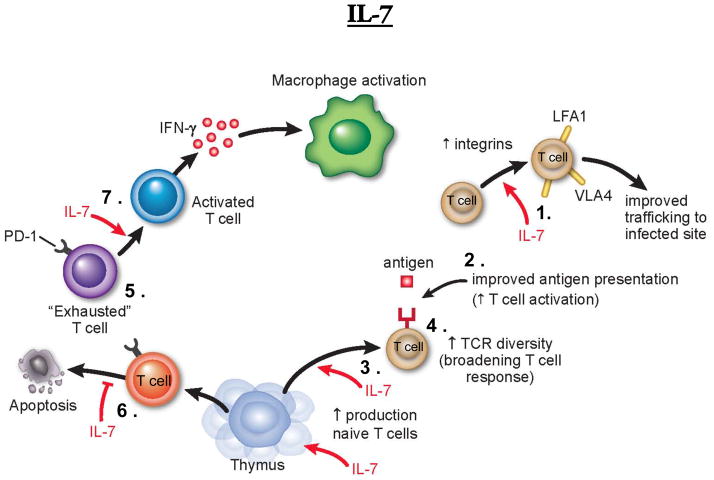
Figure 1. The potential immune-stimulatory effects of IL-7 in sepsis.
Interleukin 7 (IL-7) is mainly produced by stromal and parenchymal cells and is absolutely required for T cell maintenance. IL-7 has multiple beneficial effects that act to enhance immune function. These include: (1) increased expression of integrins, resulting in enhanced cell trafficking to the infection site and (2) increased T cell activation and CD4+ and CD8+ T cell proliferation (3). IL-7’s affects on T cell expansion leads to a significant increase in TCR repertoire diversity (4), thereby improving T cell activation to subdominant antigens. IL-7 can also aid in reversing the exhausted phenotype (5), decreasing apoptosis of immune effector cells (6) and aiding in efficient macrophage activation (7). IL-7’s immune stimulatory effects can also improve crosstalk between the innate and adaptive branch of the immune system, and therefore restore immune function under septic conditions. Abbreviations: TCR, T cell receptor; LFA-1, lymphocyte function associated antigen 1; VLA-4, very late antigen 4; PD-1, programmed cell death 1; IFN-y; interferon-gamma.
Preclinical experiments testing the potential of IL-7 as an immunostimulatory therapy in sepsis demonstrated that the IL-7 pathway remains intact in septic patients, and that ex vivo IL-7 treatment of patient cells significantly improved lymphocyte functions including proliferation, IFN-γ production, and Bcl-2 induction [46]. Additionally, IL-7 treatment increases T cell receptor diversity that should result in a broadened and more robust immune response against pathogens [47, 48]. IL-7 has been implicated in multiple clinical trials, mainly for viral infections, for example HIV and hepatitis C, and for cancer [49]. Results indicated a doubling of circulating CD4+ and CD8+ T cells, but not regulatory T cells, as well as an increase in spleen and peripheral lymph node size [50–53]. These findings are highly desirable in patients with sepsis in which T cells are constantly depleted by apoptosis. Due to the broad stimulating effects of IL-7 on the immune system, clinical trials with IL-7 in critically ill patients are highly anticipated.
Another immuno-adjuvant molecule that is showing promise in the early stages of testing in clinically relevant models of sepsis is IL-15. IL-15 is a pleuripotent cytokine closely related to IL-7, and is essential for natural killer (NK) cell development, survival and cell function. Additionally, IL-15 stimulation increases NK cell cytotoxicity [54]. IL-15 also supports homeostasis of memory CD8+ T cells, survival of epithelial γδ T cells, activation and proliferation of naïve CD8+ T cells upon TCR stimulation as well as B cell proliferation and antibody production. IL-15 is one of the most promising tools for cancer immunotherapy [55]. In contrast to IL-7, IL-15 has potent immuno-stimulatory and proliferative effects on NK cells, NKT cells, CD8+ T cells, and dendritic cells (Figure 2).
Figure 2. The potential immune-stimulatory effects of IL-15 in sepsis.
Interleukin 15 (IL-15) is a potential candidate to improve immune function under septic conditions. IL-15 binds to the high-affinity IL-15Rα chain and trans-presented to an opposing IL-2Rβ/yc receptor complex on NK cell, NKT cell or CD8 T cell through cell-cell contact. IL-15 signaling improves activation, proliferation, as well as cytotoxicity of stimulated cells leading to increased expression of interferon gamma (IFN-γ) and IFN-α. Furthermore, IL-15 prevents apoptosis of immune effector cells by up-regulating expression of anti-apoptotic proteins. Abbreviations: IL-15Rα, Interleukin-15 receptor alpha chain; IL-2Rβ, Interleukin-2 receptor beta chain; γc, common gamma chain; NK, natural killer cell; IFNγ, interferon gamma; IFNα, interferon alpha.
The role of IL-15 in the development and activation of effector and memory T cells, NK cells and NKT cells as well as its pro-survival effects on neutrophils [56], makes IL-15 a promising potential candidate for immunotherapy in sepsis (Table 2). IL-15 has shown anti-tumor activity in several preclinical mouse tumor models [57]. In fact, combination therapy with anti-programmed cell death receptor ligand-1 (αPD-L1) or anti-CTLA-4 antibodies further increased the anti-tumor activity of IL-15 [58]. IL-15 also demonstrated efficacy when administered as an adjuvant in vaccine based murine tumor models [59]. IL-15 has also been studied in relevant pre-clinical models of sepsis. IL-15 treatment of septic mice decreased apoptosis of immune competent cells by increasing Bcl-2 expression and improved overall survival [60]. It has also been reported that IL-15 had a positive impact in reducing sepsis-induced muscle-wasting in mice [61].
Table 2.
Co-inhibitory molecules that have been implicated in human ICU patient samples and septic animal models*.
| Co-inhibitory Molecule | PD-1 | PD-L1 | BTLA | CTLA-4 |
|---|---|---|---|---|
| Expression levels from septic animals and patient samples | Increased levels on monocytes and lymphocytes from human ICU patients [33]. PD-1 expression levels increased on peritoneal macrophages, T and B cells in septic animal models [36]. |
Increased expression levels on monocytes and lymphocytes from human patients [83]. PD-L1 is upregulated on monocytes and NKTcells from peripheral blood of septic animals [80]. PD-L1 expression is increased on neutrophils and macrophages from the infectious site in septic animals [80]. PD-L1 on splenic macrophages increased in animal septic models [80]. |
Increased on macrophages, neutrophils, and monocytes in septic animals [85]. Increased % on circulating CD4+ T cells from septic patients vs. ICU patients [87]. |
Increased on CD8+T, CD4+ T, and regulatory T cell populations from septic animals [83]. Septic patients had significantly increased expression levels CTLA-4 positive expressing CD4+ T cells [33]. |
| Effects on survival | PD-1/- animals are protected from septic morbidity and mortality [36]. Mice treated with anti-PD-1 (antibody blockade) have improved survival from sepsis [78]. |
Anti-PD-L1 (antibody blockade) treated animals are protected from sepsis-induced mortality [79]. PD-L1−/− mice are protected from septic morbidity and mortality [80]. |
BTLA−/− mice exposed to septic challenge are protected from septic induced mortality [85]. Mice treated with anti-BTLA have improved survival in LPS models [86]. |
CTLA-4’s effect on survival is dose-dependent in septic animals [84]. |
| Proposed mode of action | PD-1 impacts the phagocytic ability of macrophages in septic animals [36]. | Increased PD-L1 expression on neutrophils may serve as a predicted outcome indicator of septic-induced mortality and morbidity [80]. | Suppresses dendritic cell and macrophage pro-inflammatory cytokine secretion in LPS endotoxin models [86]. Contributes to lymphoid cell loss seen during sepsis, since BTLA−/− septic animals have decreased apoptosis [87]. |
Acts like PD-1 to suppress T cell effector functions, but improves survival in secondary fungal infections [81, 84]. |
PD-1, programmed cell death receptor-1; ICU, intensive care unit; PD-L1, programmed cell death ligand-1; NKT, natural killer T; BTLA, B and T Lymphocyte attenuator, LPS, lipopolysaccharide; CTLA-4, cytotoxic T lymphocyte antigen-4
Granulocyte-Macrophage colony stimulating factor (GM-CSF) is a third promising cytokine that shows potential in increasing immune function in septic patients. Neutrophils are one of the first lines of defense in controlling invading pathogens. During sepsis, multiple defects in neutrophil function have been reported, including impaired clearance of bacteria, reduced reactive oxygen species [62], and diminished chemotactic activity [63, 64]. Several studies have reported that impaired neutrophil function precedes the patient’s susceptibility to nosocomial infections [65]. Sepsis is hallmarked by the reduced ability of monocytes to secrete pro-inflammatory cytokines, such as TNF-α and IL-6 in response to bacterial challenges [66] as well as a persistent low expression of HLA-DR resulting in impaired antigen presentation (Figure 3) [67]. Since 1998, GM-CSF studies have been performed in an attempt to reverse monocyte/macrophage dysfunction as well as enhance polymorphonuclear leukocyte numbers and boost pathogen clearance. Two recent phase 2 trials of GM-CSF that targeted the treatment of septic patients with low monocyte HLA-DR, showed beneficial effects by reducing the severity of illness score, shortening time on mechanical ventilation, enhancing blood monocyte HLA-DR expression and by improving pro-inflammatory cytokine production [68]. GM-CSF has also shown potential in a pediatric sepsis study. Sepsis-induced immunosuppression was determined ex vivo by assessing lipopolysaccharide-stimulated whole blood TNF-α productive capacity. Patients with a TNF-α response below 200 pg/ml were treated with GM-CSF. GM-CSF treatment restored TNF-α production and decreased the occurrence of nosocomial infections [69]. More clinical trials of GM-CSF are being planned and the results of these new trials are awaited with much interest.
Figure 3. GM-CSF improves immune function in sepsis.
Granulocyte-macrophage colony stimulating factor (GM-CSF), released from activated lymphoid cells (T cells, B cells or NK cells) as well as macrophages/monocytes or mast cells, acts in two major ways to increase immune function. First, GM-CSF leads to mobilization of myeloid cells such as dendritic cells, monocytes and granulocytes from the bone marrow, thereby significantly increasing myeloid cell counts. Second, GM-CSF also directly acts on these cells by increasing survival, maturation, activation and migration to the site of infection. Furthermore, GM-CSF treatment reverses critically low human leukocyte antigen D-related (HLA-DR) expression on antigen presenting cells (APCs) in septic patients, leading to improved immune function. Abbreviations: GM-CSF, granulocyte-macrophage colony stimulating factor; HLA-DR, human leukocyte antigen D-related; NK cell, natural killer cell.
Co-inhibitory cell surface molecules and sepsis
In the past few years awareness of a novel set of immunological regulatory molecules, known as the co-inhibitory cell surface molecule family, has grown, as has interest in their potential therapeutic roles against septic inflammation. These co-inhibitory molecules were first thought of as negative regulators of TCR-mediated activation and cytokine release involved in the adaptive immune response. One of the most well studied co-inhibitory receptor molecules is programmed cell death receptor-1 (PD-1) and its ligand, programmed cell death receptor ligand-1 (PD-L1) [70]. PD-1 is expressed on T-/B-lymphocytes, myeloid and dendritic cells, while PD-L1 is expressed by epithelial, endothelial, and antigen presenting cells (APCs) such as monocytes, macrophages, and dendritic cells [71]. PD-1/PD-L1 binding usually results in the tyrosine phosphorylation of the PD-1 internal immunoreceptor tyrosine based inhibitory (ITIM) or switch motif (ITSM) domain, leading to the recruitment of phosphatases such as Src homology region 2 domain-containing phosphatase (SHP)-1/SHP-2, resulting in an inhibitory signal for T cells and negatively regulating their activation. T cell differentiation is also blocked by down-regulation of Bcl-XL expression [72]. Since PD-1 is up-regulated on CD4+ and CD8+ T cells in viral infections and cancer, it has frequently been associated with the phenomenon of “T cell exhaustion”, thought to be a result of sustained viral antigen exposure within these states [73, 74]. Both anti-PD-1 and anti-PD-L1 therapy have shown significant success in clinical trials against cancer and, thus, it has been proposed they could have similar beneficial effects in septic patients, since both disease states exhibit significant aspects of immune suppression [34, 75, 76].
During sepsis, immune suppression takes the form of immune cell deactivation as there is extensive leukocyte apoptosis. PD-1, its ligand and other co-inhibitory cell surface family members have been suggested to be markers of “T cell anergy”, or immune cell deactivation in injured or critically ill patients. Expression analysis from enriched human T lymphocyte populations showed that there was increased co-inhibitory receptor expression on various members of the lymphoid and myeloid cell lineages [77], and this has encouraged further investigation of the role of PD-1 and other co-inhibitory cell surface family members in sepsis. In an animal model of sepsis, PD-1 deficient animals were protected from sepsis-induced lethality [36]. These animals also exhibited decreased bacterial load and improved anti-microbial and anti-inflammatory responsiveness when exposed to septic challenge. This study supported the novel concept that PD-1 expression on monocytes/macrophages, as well as lymphocytes, could also contribute to immune dysfunction encountered in the experimentally septic mouse, and potentially a critically ill patient. Similar results were found when septic animals were post-treated with an anti-PD-1 neutralizing antibody [78]. Another study using PD-L1 blocking antibody showed improved survival in septic animals and decreased lymphocyte apoptosis [79]. This was further confirmed in PD-L1 deficient animals exposed to experimental septic challenge, which had improved survival rates compared to wild-type animals [80]. Anti-PD-1 and anti-PD-L1 therapy in animals initially exposed to septic challenge also improved survival rates after secondary fungal infections [81]. Importantly, septic shock and trauma patients have been reported to have increased levels of PD-1 and PD-L1 on their monocyte and T lymphocyte populations [82, 83], which again suggests that PD-1 and other co-inhibitory cell surface molecules can be not only potential indicators of immune suppression, but also potentially successful therapeutic agents.
In addition to PD-1, other co-inhibitory family members, such as B- and T-lymphocyte attenuator (BTLA) and CTLA-4, have also been studied in septic animal models. Expression levels of CTLA-4 increased on CD8+, CD4+, and regulatory T lymphocyte populations in animals that underwent cecal ligation and puncture (CLP; a sepsis animal model), while anti-CTLA-4 post-treatment therapy improved overall sepsis-induced lymphocyte apoptosis and survival of secondary fungal infections in mice that initially were exposed to septic challenge [81, 84]. BTLA-deficient animals that underwent CLP were also protected from sepsis-induced mortality [85]. Septic animals exhibited increased numbers of inflammatory monocytes, macrophages, and neutrophils that express BTLA [85]. Recently, a study using an anti-BTLA antibody further confirmed that animals with LPS-induced endotoxin shock have improved survival [86]. This same study also identified that one of BTLA’s modes of action could be by serving to suppress pro-inflammatory cytokine production from dendritic cells and macrophages [86]. Further, increased BTLA expression on CD4+ T cells from septic patients correlated with development of subsequent nosocomial infections and with a longer length of hospital stays [87].
Cell surface co-inhibitory molecules, immunomodulation, and the critically ill patient
Although more studies are being performed to investigate the roles of cell surface co-inhibitory molecules in critically ill patient and animal models, few investigators have considered whether these molecules function alone or cooperatively in vivo with other cell surface molecules and/or if there is functional redundancy between each molecule [88]. Most of what we know about these molecules as suggested therapies, particularly CTLA-4, and PD-1, has come from clinical trials in cancer and studies of their roles in viral infections, suggesting that these two molecules block regulatory T cell effector functions, encourage robust CD8+ T cell responses against tumors and paralyze anti-viral T cell motility [89, 90]. However, recent reports have suggested that PD-1 and PD-L1 function quite differently, and possibly separately in septic animal models. For example, macrophages from septic animals still had increased PD-1 expression in the absence of PD-L1 [80]. In addition, while PD-1 plays an important role in the ability of macrophages to phagocytose and clear bacteria, PD-L1 has a minor role in this capacity [80]. These co-inhibitory molecules may also have different modes of action based on their expression sites during infection. For example, it has also been proposed that PD-L1 expression in the liver plays a role in attenuating acute liver injury in sepsis animal models [91, 92]. Based on experimental animal and human data with PD-1, we propose that an ideal anti-PD-1 therapy in sepsis would enhance monocyte/macrophage phagocytic function as well as T cell function, because the expression of PD-1 on macrophages/monocytes potentially also leads to sepsis-induced immune dysfunction (Figure 4) [36, 88].
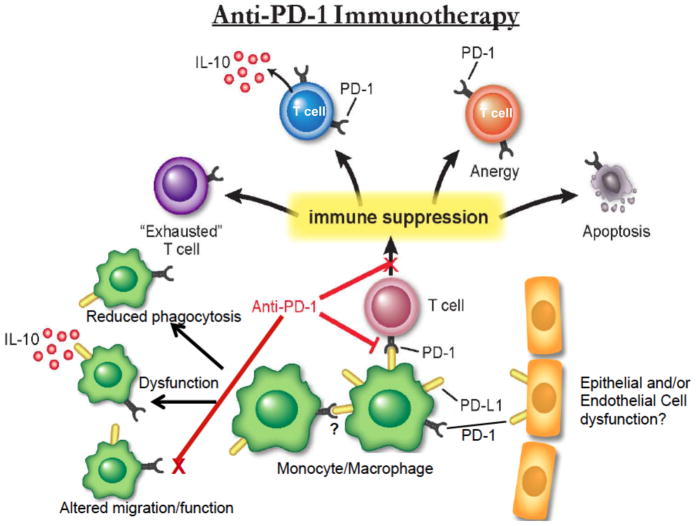
Figure 4. Anti-PD-1 immunotherapy proposed for septic patients.
The co-inhibitory molecule, programmed death receptor-1 (PD-1), and its ligand (PD-L1) are up-regulated on monocytes, macrophages, and T lymphocytes during sepsis. PD-L1 has also been shown to be increased on epithelial and endothelial cell populations in septic animal models, and may play a role in barrier function. Future potential anti-PD-1 therapy, indirectly impacting the effects of PD-L1, would improve immune cell function, particularly monocyte/macrophage function, phagocytosis, and their ability to secrete anti-inflammatory cytokine, interleukin-10 (IL-10), directly addressing the immune suppressed state in the critically ill patient.
Structurally, PD-1, BTLA, and CTLA-4 are classified within the same B7/CD28 gene family, and contain an ITIM as well as an ITSM domain [93]. The downstream signaling cascades of these co-inhibitory cell surface molecules also have some overlap, including their targeting phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) pathway, which is a result of association with SHP-1 and SHP-2 upon tyrosine phosphorylation of their ITIM/ITSM domains [94]. However, if these molecules have similar beneficial effects in human clinical trials against sepsis to those seen in inducing the regression of tumors in late stage cancer patients, and how they might function in sepsis is still open for discussion and investigation (Box 1). A study with CTLA-4 in septic animal models, however, suggests that the affects of CTLA-4 on survival rates were dose-dependent [84]. Finally, we must also consider what type of patient in intensive care units should receive this therapy, when would it be administered and do these co-inhibitory molecules potentially have synergistic effects, requiring their combined blockade to truly provide effective treatment? The overarching question that still remains, circles when would be the appropriate time to administer such therapy. Administration of anti-co-inhibitory molecules may be based on individual patient sample profiles of their co-inhibitory expression levels.
Box 1. Outstanding questions.
How will future novel therapies to treat sepsis be designed to potentiate the host immune response, and which cell populations will be targeted?
Cytokines and co-inhibitory molecules are potential immuno-modulatory agents that address sepsis-induced lymphocyte apoptosis, but when should they be administered?
Co-inhibitory cell surface molecules, such as CTLA-4 and PD-1, have shown promise in clinical trials against cancer, but will these agents have the same effects in critically ill patients and what is their primary mode of action in a clinically setting of sepsis?
Concluding remarks and future perspectives
As scientists and clinicians alike continue to delve deeper into studying the pathophysiology of sepsis and come to a better understanding of how sepsis modulates the host immune response, novel therapeutic agents will be developed. We propose here that therapeutic agents could potentiate the immune response. More specifically, agents such as IL-7, IL-15, GM-CSF, anti-PD-1 and anti-BTLA will target the immunosuppressed state in critically ill patients. However, as these cytokines and co-inhibitory molecules function quite differently to target aspects of immune suppression, if and how they will be used for critically ill patients, remains to be determined.
Highlights.
Sepsis is the leading cause of death in critically ill patients and novel therapies are necessary.
Immune suppression is frequently associated with sepsis related deaths.
Therapies or immunomodulatory agents that enhance the immune response should be considered.
Cytokines and co-inhibitory receptors represent potential immunomodulatory agents.
Acknowledgments
Supported in part by National Institutes of Health (NIH) grants T32 GM065085 (N.A.H.), R01 GM046354 (A.A.), R01 GM107149 (A.A.), R01 GM055194 (R.S.H.) and R01 GM044118 (R.S.H.).
Glossary
- B and T lymphocyte attenuator (BTLA)
BTLA is expressed by T and B lymphocytes; it is an immunoglobin (Ig) domain superfamily member that contains a cytoplasmic tyrosine based inhibitory motif. The ligand for BTLA is herpesvirus entry mediator (HVEM). The interactions between BTLA and HVEM involve bidirectional signaling and potentiate negative immune responses. Generally, T cell activation is negatively regulated upon HVEM engagement to BTLA
- Cecal ligation and puncture (CLP)
The CLP method is a clinical comparable rodent model of polymicrobial peritonitis used in order to investigate the pathological changes associated with sepsis/septic shock seen in patients
- Co-inhibitory cell surface molecules
These are cell surface molecules, such as PD-1, BTLA, and CTLA-4 that belong to the CD28/B7 gene family and have been shown to be up-regulated on monocytes, macrophages, and lymphocyte populations in patient samples and animals exposed to experimental septic challenge. Traditionally, these molecules are thought to negatively regulate T cell receptor (TCR) mediated activation as well as cytokine release from immune cells of the adaptive immune system and have been extensively investigated in the processes of viral infection and cancer
- Compensatory anti-inflammatory response syndrome (CARS)
CARS is usually succeeded by the pro-inflammatory phase during sepsis, and is hallmarked by increased anti-inflammatory cytokines, such as IL-10, increased lymphocyte apoptosis, diminished human leukocyte antigen (HLA) receptors on monocytes and decreased cytokine responses by monocytes as well as lymphocytes
- Cytokine storm
The excessive or uncontrolled release of pro-inflammatory cytokines in the context of an acute tissue damage and/or infection, such as traumatic injury/shock/ischemia-reperfusion and/or sepsis
- Cytotoxic-T-lymphocyte-antigen 4 (CTLA-4)
CTLA-4, also known as CD152, is expressed on the surface of T lymphocytes, and a member of the Ig superfamily that competes with CD28 for binding to co-stimulatory molecules, CD80 and CD86, on antigen presenting cells. CTLA-4 binding prevents T cell proliferation, expansion, and activation
- Cytokines
Interleukins-7 (IL-7) and -15 (IL-15) are closely related, and have been shown to improve immune cell function (particularly, CD8+ T, NK, and dendritic cells) and decrease lymphocyte apoptosis in sepsis animal models. Granulocyte-macrophage colony stimulating factor (GM-CSF), however, is a cytokine that improves neutrophil and macrophage functions, and increases HLA-DR expression on monocytes. These cytokines, if directed to the appropriate patient, may address the immune suppressed state in critically ill patients
- Immunomodulatory therapy
Ideal immunomodulatory therapy directed against sepsis would boost overall patient immunity, drive lymphocyte effector functions, decrease lymphoid apoptosis and ultimately mitigate the development of immune suppression, which has been often associated with onset of secondary infections and death amongst critically ill patients
- Immune suppression
the host’s inability to mount an appropriate immune response to a defined antigenic stimuli and/or infectious challenge
- Programmed cell death receptor-1 (PD-1)
The co-inhibitory receptor molecule, PD-1 is expressed on T and B cells (lymphocytes), myeloid cells, natural killer (NK) cells, and NKT cells. Upon ligation by its ligand, programmed cell death ligand-1 (PD-L1), transduces an inhibitory signal to the leukocyte during the course of activation
- Systemic inflammatory response syndrome (SIRS)
The American College of Chest Physicians and the Society for Critical Care Medicine established criteria for SIRS. There are four categories, including body temperature, respiratory rate, white blood cell count, and heart rate. These criteria are not necessarily related to infection
- T cell anergy
T lymphocytes become functionally inactive or “anergic” following an antigen encounter, typically in the absence of adequate co-stimulatory signaling, yet still remain viable in circulation and can regain responsiveness to select T cell stimuli under the appropriate conditions
- T cell exhaustion
This is a state of T cell dysfunction, which is typically defined by decreased lymphocyte effector functions and sustained expression of inhibitory receptors, the results of which the lymphocyte is non-responsive to exogenous stimuli. T cell exhaustion prevents the clearance of bacterial and chronic viral infections
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angus DC, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, et al. The epidemiology of sepsis in the United States from 1979 through 2000. New Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Gaieski DF, et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 4.Vincent JL, et al. Sepsis definitions: time for change. Lancet. 2013;381:774–775. doi: 10.1016/S0140-6736(12)61815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward NS, et al. The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Clin Chest Med. 2008;29:617–625. viii. doi: 10.1016/j.ccm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraham E, et al. p55 Tumor necrosis factor receptor fusion protein in the treatment of patients with severe sepsis and septic shock. A randomized controlled multicenter trial. Ro 45-2081 Study Group. JAMA. 1997;277:1531–1538. [PubMed] [Google Scholar]
- 7.Opal SM, et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA. 2013;309:1154–1162. doi: 10.1001/jama.2013.2194. [DOI] [PubMed] [Google Scholar]
- 8.Osuchowski MF, et al. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177:1967–1974. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 9.Hotchkiss RS, et al. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. New Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 11.Otto GP, et al. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care. 2011;15:R183. doi: 10.1186/cc10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volk HD, et al. Clinical aspects: from systemic inflammation to ‘immunoparalysis’. Chem Immunol. 2000;74:162–177. doi: 10.1159/000058753. [DOI] [PubMed] [Google Scholar]
- 13.Reber AJ, et al. Immunosenescence and Challenges of Vaccination against Influenza in the Aging Population. Aging and Disease. 2012;3:68–90. [PMC free article] [PubMed] [Google Scholar]
- 14.Kollef KE, et al. Predictors of 30-day mortality and hospital costs in patients with ventilator-associated pneumonia attributed to potentially antibiotic-resistant gram-negative bacteria. Chest. 2008;134:281–287. doi: 10.1378/chest.08-1116. [DOI] [PubMed] [Google Scholar]
- 15.Luyt CE, Kaiser L. Virus detection in patients with severe pneumonia: still more questions than answers? Am J Respir Crit Care Med. 2012;186:301–302. doi: 10.1164/rccm.201206-1119ED. [DOI] [PubMed] [Google Scholar]
- 16.Limaye AP, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotchkiss RS, et al. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci USA. 1999;96:14541–14546. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotchkiss RS, et al. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T-and B-cell-deficient mice. Crit Care Med. 1997;25:1298–1307. doi: 10.1097/00003246-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Hotchkiss RS, et al. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. 2005;174:5110–5118. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 20.Hotchkiss RS, et al. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 21.Coopersmith CM, et al. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA. 2002;287:1716–1721. doi: 10.1001/jama.287.13.1716. [DOI] [PubMed] [Google Scholar]
- 22.Chung CS, et al. Deficiency of Bid protein reduces sepsis-induced apoptosis and inflammation, while improving septic survival. Shock. 2010;34:150–161. doi: 10.1097/SHK.0b013e3181cf70fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wesche-Soldato DE, et al. In vivo delivery of caspase-8 or Fas siRNA improves the survival of septic mice. Blood. 2005;106:2295–2301. doi: 10.1182/blood-2004-10-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung CS, et al. Inhibition of Fas/Fas ligand signaling improves septic survival: differential effects on macrophage apoptotic and functional capacity. J Leukoc Biol. 2003;74:344–351. doi: 10.1189/jlb.0102006. [DOI] [PubMed] [Google Scholar]
- 25.Hotchkiss RS, et al. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 26.Coopersmith CM, et al. Overexpression of Bcl-2 in the intestinal epithelium improves survival in septic mice. Crit Care Med. 2002;30:195–201. doi: 10.1097/00003246-200201000-00028. [DOI] [PubMed] [Google Scholar]
- 27.Venet F, et al. Decreased T-cell repertoire diversity in sepsis: a preliminary study. Crit Care Med. 2013;41:111–119. doi: 10.1097/CCM.0b013e3182657948. [DOI] [PubMed] [Google Scholar]
- 28.Hutchins NA, et al. Kupffer Cells Protect Liver Sinusoidal Endothelial Cells from Fas-Dependent Apoptosis in Sepsis by Down-Regulating gp130. Am J Pathol. 2013;182:742–754. doi: 10.1016/j.ajpath.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNeal SI, et al. The dual functions of receptor interacting protein 1 in fas-induced hepatocyte death during sepsis. Shock. 2011;35:499–505. doi: 10.1097/SHK.0b013e31820b2db1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.London NR, et al. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Trans Med. 2010;2:23ra19. doi: 10.1126/scitranslmed.3000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison C. Sepsis: calming the cytokine storm. Nat Rev Drug Discovery. 2010;9:360–361. doi: 10.1038/nrd3162. [DOI] [PubMed] [Google Scholar]
- 32.Hotchkiss RS, et al. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boomer JS, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hotchkiss RS, et al. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hotchkiss RS, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Huang X, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci USA. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venet F, et al. Regulatory T cell populations in sepsis and trauma. J Leukoc Biol. 2008;83:523–535. doi: 10.1189/jlb.0607371. [DOI] [PubMed] [Google Scholar]
- 38.Landelle C, et al. Low monocyte human leukocyte antigen-DR is independently associated with nosocomial infections after septic shock. Intensive Care Med. 2010;36:1859–1866. doi: 10.1007/s00134-010-1962-x. [DOI] [PubMed] [Google Scholar]
- 39.Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–655. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]
- 40.Puel A, et al. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat Genetics. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 41.Hennion-Tscheltzoff O, et al. TCR triggering modulates the responsiveness and homeostatic proliferation of CD4+ thymic emigrants to IL-7 therapy. Blood. 2013;121:4684–4693. doi: 10.1182/blood-2012-09-458174. [DOI] [PubMed] [Google Scholar]
- 42.Vassena L, et al. Treatment with IL-7 prevents the decline of circulating CD4+ T cells during the acute phase of SIV infection in rhesus macaques. PLoS pathogens. 2012;8:e1002636. doi: 10.1371/journal.ppat.1002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chetoui N, et al. Interleukin-7 promotes the survival of human CD4+ effector/memory T cells by up-regulating Bcl-2 proteins and activating the JAK/STAT signalling pathway. Immunology. 2010;130:418–426. doi: 10.1111/j.1365-2567.2009.03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unsinger J, et al. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol. 2010;184:3768–3779. doi: 10.4049/jimmunol.0903151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li WQ, et al. Interleukin-7 regulates Bim proapoptotic activity in peripheral T-cell survival. Mol Cell Biol. 2010;30:590–600. doi: 10.1128/MCB.01006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venet F, et al. IL-7 restores lymphocyte functions in septic patients. J Immunol. 2012;189:5073–5081. doi: 10.4049/jimmunol.1202062. [DOI] [PubMed] [Google Scholar]
- 47.Sportes C, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morre M, Beq S. Interleukin-7 and immune reconstitution in cancer patients: a new paradigm for dramatically increasing overall survival. Target Oncol. 2012;7:55–68. doi: 10.1007/s11523-012-0210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mackall CL, et al. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. 2011;11:330–342. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenberg SA, et al. Altered CD8(+) T-cell responses when immunizing with multiepitope peptide vaccines. J Immunother. 2006;29:224–231. doi: 10.1097/01.cji.0000190399.98802.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sportes C, et al. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin Cancer Res. 2010;16:727–735. doi: 10.1158/1078-0432.CCR-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lundstrom W, et al. IL-7 in human health and disease. Semin Immunol. 2012;24:218–224. doi: 10.1016/j.smim.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levy Y, et al. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin Infect Dis. 2012;55:291–300. doi: 10.1093/cid/cis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu J, et al. Interleukin 15 promotes antigen-independent in vitro expansion and long-term survival of antitumor cytotoxic T lymphocytes. Clin Cancer Res. 2002;8:3877–3884. [PubMed] [Google Scholar]
- 55.Ochoa MC, et al. Interleukin-15 in gene therapy of cancer. Curr Gene Ther. 2013;13:15–30. doi: 10.2174/156652313804806561. [DOI] [PubMed] [Google Scholar]
- 56.Pelletier M, et al. Mechanisms involved in interleukin-15-induced suppression of human neutrophil apoptosis: role of the anti-apoptotic Mcl-1 protein and several kinases including Janus kinase-2, p38 mitogen-activated protein kinase and extracellular signal-regulated kinases-1/2. FEBS letters. 2002;532:164–170. doi: 10.1016/s0014-5793(02)03668-2. [DOI] [PubMed] [Google Scholar]
- 57.Waldmann TA, et al. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 2011;117:4787–4795. doi: 10.1182/blood-2010-10-311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu P, et al. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res. 2010;16:6019–6028. doi: 10.1158/1078-0432.CCR-10-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steel JC, et al. Interleukin-15 and its receptor augment dendritic cell vaccination against the neu oncogene through the induction of antibodies partially independent of CD4 help. Cancer Res. 2010;70:1072–1081. doi: 10.1158/0008-5472.CAN-09-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inoue S, et al. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J Immunol. 2010;184:1401–1409. doi: 10.4049/jimmunol.0902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim HC, et al. Role of IL-15 in Sepsis-Induced Skeletal Muscle Atrophy and Proteolysis. Tuberc Respir Dis. 2012;73:312–319. doi: 10.4046/trd.2012.73.6.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alves-Filho JC, et al. Neutrophil paralysis in sepsis. Shock. 2010;34(Suppl 1):15–21. doi: 10.1097/SHK.0b013e3181e7e61b. [DOI] [PubMed] [Google Scholar]
- 63.Kovach MA, Standiford TJ. The function of neutrophils in sepsis. Curr Opin Infect Dis. 2012;25:321–327. doi: 10.1097/QCO.0b013e3283528c9b. [DOI] [PubMed] [Google Scholar]
- 64.Cummings CJ, et al. Expression and function of the chemokine receptors CXCR1 and CXCR2 in sepsis. J Immunol. 1999;162:2341–2346. [PubMed] [Google Scholar]
- 65.Stephan F, et al. Impairment of polymorphonuclear neutrophil functions precedes nosocomial infections in critically ill patients. Crit Care Med. 2002;30:315–322. doi: 10.1097/00003246-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 66.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 67.Manjuck J, et al. Decreased response to recall antigens is associated with depressed costimulatory receptor expression in septic critically ill patients. J Lab Clin Med. 2000;135:153–160. doi: 10.1067/mlc.2000.104306. [DOI] [PubMed] [Google Scholar]
- 68.Meisel C, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180:640–648. doi: 10.1164/rccm.200903-0363OC. [DOI] [PubMed] [Google Scholar]
- 69.Hall MW, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37:525–532. doi: 10.1007/s00134-010-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ceeraz S, et al. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013;34:556–563. doi: 10.1016/j.it.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 74.Hirano F, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 75.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. New Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laudanski K, et al. Cell-specific expression and pathway analyses reveal alterations in trauma-related human T cell and monocyte pathways. Proc Natl Acad Sci USA. 2006;103:15564–15569. doi: 10.1073/pnas.0607028103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brahmamdam P, et al. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol. 2010;88:233–240. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Y, et al. PD-L1 blockade improves survival in experimental sepsis by inhibiting lymphocyte apoptosis and reversing monocyte dysfunction. Crit Care. 2010;14:R220. doi: 10.1186/cc9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang X, et al. Identification of B7-H1 as a novel mediator of the innate immune/pro-inflammatory as well as a possible myeloid prognostic marker in sepsis. J Immunol. 2014 doi: 10.4049/jimmunol.1302252. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang KC, et al. Blockade of the negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit Care. 2013;17:R85. doi: 10.1186/cc12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y, et al. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit Care. 2011;15:R70. doi: 10.1186/cc10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guignant C, et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care. 2011;15:R99. doi: 10.1186/cc10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Inoue S, et al. Dose-dependent effect of anti-CTLA-4 on survival in sepsis. Shock. 2011;36:38–44. doi: 10.1097/SHK.0b013e3182168cce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shubin NJ, et al. BTLA expression contributes to septic morbidity and mortality by inducing innate inflammatory cell dysfunction. J Leukoc Biol. 2012;92:593–603. doi: 10.1189/jlb.1211641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kobayashi Y, et al. B and T lymphocyte attenuator inhibits LPS-induced endotoxic shock by suppressing Toll-like receptor 4 signaling in innate immune cells. Proc Natl Acad Sci USA. 2013;110:5121–5126. doi: 10.1073/pnas.1222093110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shubin NJ, et al. B and T lymphocyte attenuator expression on CD4+ T-cells associates with sepsis and subsequent infections in ICU patients. Crit Care. 2013;17:R276. doi: 10.1186/cc13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ayala A, et al. Sepsis-Induced Potentiation of Peritoneal Macrophage Migration Is Mitigated by Programmed Cell Death Receptor-1 Gene Deficiency. J Innate Immun. 2013 Nov 16; doi: 10.1159/000355888. ePub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duraiswamy J, et al. Dual Blockade of PD-1 and CTLA-4 Combined with Tumor Vaccine Effectively Restores T-Cell Rejection Function in Tumors. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zinselmeyer BH, et al. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J Exp Med. 2013;210:757–774. doi: 10.1084/jem.20121416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hutchins NA, et al. Kupffer cells potentiate liver sinusoidal endothelial cell injury in sepsis by ligating programmed cell death ligand-1. J Leukoc Biol. 2013;94:963–970. doi: 10.1189/jlb.0113051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu W, et al. PD-L1 Blockade Attenuated Sepsis-Induced Liver Injury in a Mouse Cecal Ligation and Puncture Model. Mediat Inflamm 2013. 2013:361501. doi: 10.1155/2013/361501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Watanabe N, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 94.Okazaki T, et al. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci USA. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]