Abstract
Taiwan has very high incidence and prevalence of chronic kidney disease (CKD), which easily progresses to end-stage renal disease (ESRD). The association between inflammation and CKD has been explored in several studies. ORAI1 functions as a pore-forming subunit of the store-operated calcium channels which are involved in the regulation of immune system. Hence, we conducted a case-control study to determine whether the genetic polymorphisms of ORAI1 gene is a susceptibility factor to CKD and its clinical features in a Taiwanese population. Five hundred seventy-nine CKD patients from a hospital-based CKD care program were included in the study. Five tagging single nucleotide polymorphisms (tSNPs) of ORAI1 were selected from the genotyping data of the Han Chinese population from the HapMap project. Among these polymorphisms, rs12313273 was found to be significantly associated with elevated serum calcium levels, which has been linked to increased risk of death in CKD patients. To have a better management of serum calcium, we suggest that ORAI1 polymorphisms might be used as a potential biomarker for initiating non-calcium-based phosphate binder in CKD patients in the future.
1. Introduction
Chronic kidney disease (CKD) is an important global public health concern because of its high incidence, prevalence, morbidity, and mortality [1]. According to the US Renal Data System (USRDS) report, Taiwan has the highest incidence and prevalence of end-stage renal disease (ESRD) [2]. The prevalence of CKD in Taiwan was 9.8–11.9% and owing to the differences in the data sources, study subjects, and definition of CKD, the reasons behind this high incidence and prevalence are multifactorial [3].
CKD has been well known to be associated with low-grade inflammation, endothelial dysfunction, and platelet activation, even among those in the early stage of CKD [4]. Serum levels of the proinflammatory cytokines, such as IL-1, IL-6, CRP, and TNF-α were significantly high in CKD patients [5–8], and these inflammation markers may replace albumin, which is currently used as the predictive marker for mortality, to predict patient outcomes [9].
Calcium signaling controls diverse cellular functions such as enzyme metabolism, muscle contraction, immune response, and cell cycle regulation [10, 11]. In nonexcitable cells such as T cells and B cells, immunological reactions are regulated via Ca2+ entry mainly through store-operated calcium channels [12]. ORAI1 consists of four transmembrane domains and functions as a pore-forming subunit of the store-operated calcium channels [13]. Functional analysis of ORAI1- (also called CRACM1-) deficient mice revealed dysfunction of mast cells and attenuation of cytokine (TNF-α and IL-6) release [14].
Recent studies on the genetic susceptibility and the progression of CKD have yielded promising results [15–17]. The results of a genome-wide association study showed that several loci were associated with CKD and estimated glomerular filtration rate (eGFR) [16]. The evolution of ApoL1 variants as survival factors may have contributed to the high prevalence of renal disease among African Americans [17]. To the best of our knowledge, there is no previous research established regarding the association between genetic polymorphism of ORAl1 and the severity of CKD in Taiwanese population. Therefore, in this case-control study, we examined the association of the ORAI1 genetic polymorphisms with CKD susceptibility, eGFR, and serum phosphorus and calcium levels.
2. Materials and Methods
2.1. Study Subjects and Data Collection
Five hundred seventy-nine unrelated CKD patients (323 (55.8%) men; age range, 18–90 years old; mean age, 61 ± 14 years old) were included in the study at the time of their enrolment for the CKD Care Program at the Kaohsiung Medical University Hospital, Kaohsiung, Taiwan; written informed consent was obtained from all patients. All included patients were >18 years of age, and their detailed clinical history was recorded as part of the CKD Care Program. The study protocol conformed to the Declaration of Helsinki and was approved by the Institutional Review Board of the Kaohsiung Medical University Hospital. Serum creatinine levels were calculated using a modified kinetic Jaffe reaction. eGFR was estimated using the abbreviated equation developed in the Modification of Diet in Renal Disease Study [18], and the cases were categorized according to the staging system described in the Kidney/Dialysis Outcome Quality Initiative Clinical Practice Guidelines for CKD: Evaluation, Classification, and Stratification [19]. The patients were divided into two groups according to their eGFR: patients with eGFR above 45 mL/min/1.73 m2 were classified as having early-stage CKD [3, 20, 21], whereas those with lower eGFR were classified as having late-stage CKD. In Taiwan, the “nationwide CKD preventive project with multidisciplinary care program” implemented by Health Promotion Administration divided CKD patients into “early” and “pre-ESRD” stages, according to the eGFR ≥45 mL/min/1.73 m2 or <45 mL/min/1.73 m2 [22]. Different treatment strategy and management plans are applied in those two groups. In our study, we divided patients into two groups as above to investigate the differences of genetic polymorphism. Their clinical history and biochemical data were recorded.
2.2. DNA Extraction
Venous blood was collected from the patients during medical visit, stored at 4°C, and processed on the same day. The blood was centrifuged to separate serum and cells. DNA extraction from the blood cells involved an initial treatment with 0.5% SDS lysis buffer followed by treatment with protease K (1 mg/mL, for the digestion of nuclear protein) for 4 h at 60°C. Total DNA was harvested using the Gentra extraction kit and was precipitated using 70% alcohol.
2.3. SNP Selection
From the HapMap database (http://www.hapmap.org, HapMap Data Rel 27 PhaseII+III, Freb09, on NCBI B36 assembly, dbSNP b126), five tagging single nucleotide polymorphisms (tSNPs) of ORAI1 (rs12313273, rs6486795, rs7135617, rs12320939, and rs712853) with minor allele frequency (MAF) >10% and r 2 > 0.8 were selected from chromosomal region 120,545,838–120,561,329 of the Han Chinese population in Beijing (CHB). A graphical overview of the physical and chromosomal location of the five tSNPs is shown in Figure 1. Two ORAI1 polymorphisms (rs12313273 and rs1232093) were located in the promoter region, two (rs6486795 and rs7135617) in the intron region, and one (rs712853) in the 3′-untranslated region (UTR).
Figure 1.
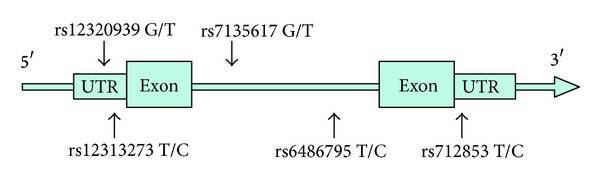
A graphical overview of the genotyped polymorphisms identified in relation to the exon/intron structure of the human ORAI1 gene.
2.4. Genotyping
Genotyping was performed using TaqMan PCR. In brief, TaqMan probes were first labeled with different fluorescent markers. PCR primers and TaqMan probes were designed to target the 5 tSNPs. Reactions were performed in 96-well microplates in the ABI 9700 Thermal Cycler (Applied Biosystems, Foster City, USA) and fluorescence was detected and analyzed using the System SDS software version 1.2.3.
2.5. Statistical Analysis
The genotype distribution of the five tSNPs was tested for Hardy-Weinberg equilibrium (HWE). The Chi-square test was used for comparing the genotype distribution or allele frequencies of the early-stage and late-stage CKD patients. One-way ANOVA was used to assess the difference in mean values of the eGFR and the serum levels of calcium and phosphate in the groups created based on genotyping results. All statistical analyses above were performed using the JMP 8.0 statistical software. Linear regression and logistic regression were used to adjust the influence of age in eGFR and CKD staging, which were performed using the SNPassoc 1.9-1 statistical software. A P value < 0.05 was considered significant.
3. Results
3.1. Association between ORAI1 tSNPs and eGFR in the CKD Patients
Patient characteristics are shown in Table 1. We tested whether genetic polymorphisms in ORAI1 are associated with eGFR in CKD patients. None of the tSNPs were found to be significantly associated with CKD susceptibility. We further adjusted our result by age which showed no significant associations (Table 2).
Table 1.
Basal characteristics of patients with chronic kidney disease.
| Characteristics | Patients with CKD |
|---|---|
| Number of subjects | 579 |
| Gender: male, number (%) | 323 (55.8%) |
| Age (years)a | 61.1 ± 13.7 |
| Range (years) | 18–90 |
aMeans ± SD.
Table 2.
Difference in the value of eGFR among CKD patients stratified by different ORAI1 genotypes.
| SNP | Genotype | Sample number (%) | eGFRa |
|---|---|---|---|
| rs12320939 | TT | 122 (21.2) | 31.75 ± 24.28 |
| TG | 295 (51.2) | 32.26 ± 24.04 | |
| GG | 159 (27.6) | 31.34 ± 24.28 | |
| P value | 0.9229 | ||
| Adjusted P valueb | 0.8764 | ||
|
| |||
| CC | 50 (8.7) | 34.64 ± 23.93 | |
| rs12313273 | CT | 245 (42.5) | 31.08 ± 22.45 |
| TT | 281 (48.8) | 31.90 ± 24.70 | |
| P value | 0.6229 | ||
| Adjusted P valueb | 0.7209 | ||
|
| |||
| TT | 98 (17.0) | 29.06 ± 20.71 | |
| rs7135617 | TG | 285 (49.6) | 32.71 ± 24.31 |
| GG | 192 (33.4) | 32.29 ± 25.44 | |
| P value | 0.4138 | ||
| Adjusted P valueb | 0.3691 | ||
|
| |||
| CC | 71 (12.3) | 33.49 ± 25.23 | |
| rs6486795 | CT | 271 (47.0) | 31.79 ± 22.61 |
| TT | 235 (40.7) | 31.93 ± 24.90 | |
| P value | 0.8619 | ||
| Adjusted P valueb | 0.8608 | ||
|
| |||
| CC | 56 (9.7) | 34.36 ± 26.12 | |
| rs712853 | CT | 238 (41.4) | 31.95 ± 24.39 |
| TT | 281 (48.9) | 31.53 ± 23.09 | |
| P value | 0.7220 | ||
| Adjusted P valueb | 0.7038 | ||
aMeans ± SD. bAdjusted age by linear regression.
3.2. Association of ORAI1 tSNPs in Early- and Late-Stage CKD Patients
Next, we evaluated whether the genotype and allele frequency of ORAI1 were associated with the stage of CKD. After being adjusted by age using logistic regression, no association was observed between tSNPs and the stage of CKD (Table 3).
Table 3.
Genotyping and allele frequency of ORAI1 gene in chronic kidney disease patients.
| Genotype | Late stage (%) (n = 453) |
Early stage (%) (n = 126) |
Allele | Late stage (%) (n = 453) |
Early stage (%) (n = 126) |
Genotype P value |
Dominant P value |
Recessive P value |
Allelic P value |
|
|---|---|---|---|---|---|---|---|---|---|---|
| rs12320939 | TT | 94 (20.8) | 28 (22.4) | T | 424 (47.0) | 115 (46.0) | 0.6482 | 0.4482 | 0.7955 | 0.7363 |
| TG | 236 (52.3) | 59 (47.2) | G | 478 (53.0) | 135 (54.0) | |||||
| GG | 121 (26.8) | 38 (30.4) | ||||||||
|
| ||||||||||
| rs12313273 | CC | 36 (8.0) | 14 (11.3) | C | 271 (30.0) | 74 (29.8) | 0.3780 | 0.5522 | 0.3148 | 0.9939 |
| CT | 199 (44.0) | 46 (37.1) | T | 633 (70.0) | 174 (70.2) | |||||
| TT | 217 (48.0) | 64 (51.6) | ||||||||
|
| ||||||||||
| rs7135617 | TT | 80 (17.7) | 18 (14.5) | T | 378 (41.9) | 103 (41.5) | 0.4551 | 0.6285 | 0.3425 | 0.8567 |
| TG | 218 (48.3) | 67 (54.0) | G | 524 (58.1) | 145 (58.5) | |||||
| GG | 153 (33.9) | 39 (31.5) | ||||||||
|
| ||||||||||
| rs6486795 | CC | 53 (11.8) | 18 (14.2) | C | 323 (35.8) | 90 (35.7) | 0.5856 | 0.6638 | 0.4536 | 0.9574 |
| CT | 217 (48.1) | 54 (42.9) | T | 579 (64.2) | 162 (64.3) | |||||
| TT | 181 (40.1) | 54 (42.9) | ||||||||
|
| ||||||||||
| rs712853 | CC | 46 (10.2) | 10 (8.0) | C | 274 (30.4) | 76 (30.4) | 0.6072 | 0.6717 | 0.4667 | 0.9998 |
| CT | 182 (40.4) | 56 (44.8) | T | 626 (69.6) | 174 (69.6) | |||||
| TT | 222 (49.3) | 59 (47.2) | ||||||||
Late stage: eGFR <45, early stage: eGFR ≥45.
All P values had been adjusted by age using logistic regression.
3.3. Association between the ORAI1 Polymorphisms and Serum Calcium Levels in CKD Patients
Abnormalities in the levels of calcium, phosphorus, and intact parathyroid hormone (PTH) are evident early in CKD patients who are not on dialysis [19]. Since abnormalities in calcium and phosphate levels are associated with increased mortality and CKD progression in non-dialysis-dependent CKD patients [23, 24], we also investigated the associations between ORAI1 genetic polymorphisms and serum calcium concentration. We found that rs12313273 was significantly associated with serum calcium levels in CKD patients (Table 4). We also observed that patients with the CC genotype of rs12313273 showed significantly higher calcium levels than those with other genotypes did. However, we found no correlation between the genetic polymorphisms and the serum phosphorus levels.
Table 4.
Difference in the value of Ca2+ and phosphorous among CKD patients stratified by different ORAI1 genotype.
| SNP | Genotype | Sample number (%) | Calcium (mg/dL)a | P value | Phosphorous (mg/dL)a | P value |
|---|---|---|---|---|---|---|
| rs12320939 | TT | 122 (21.2) | 9.32 ± 0.53 | 0.0528 | 4.26 ± 1.02 | 0.5243 |
| TG | 295 (51.2) | 9.10 ± 0.94 | 4.25 ± 1.02 | |||
| GG | 159 (27.6) | 9.17 ± 0.80 | 4.37 ± 1.02 | |||
|
| ||||||
| rs12313273 | CC | 50 (8.7) | 9.32 ± 0.61 | 0.0389* | 4.33 ± 0.89 | 0.0831 |
| CT | 245 (42.5) | 9.23 ± 0.57 | 4.18 ± 1.01 | |||
| TT | 281 (48.8) | 9.08 ± 1.03 | 4.38 ± 1.03 | |||
|
| ||||||
| rs7135617 | TT | 98 (17.0) | 9.21 ± 0.87 | 0.1017 | 4.42 ± 1.05 | 0.3290 |
| TG | 285 (49.6) | 9.09 ± 0.81 | 4.24 ± 0.97 | |||
| GG | 192 (33.4) | 9.25 ± 0.85 | 4.29 ± 1.07 | |||
|
| ||||||
| rs6486795 | CC | 71 (12.3) | 9.33 ± 0.60 | 0.1586 | 4.36 ± 1.08 | 0.2622 |
| CT | 271 (47.0) | 9.17 ± 0.77 | 4.21 ± 1.03 | |||
| TT | 235 (40.7) | 9.12 ± 0.96 | 4.35 ± 0.98 | |||
|
| ||||||
| rs712853 | CC | 56 (9.7) | 8.99 ± 1.27 | 0.2356 | 4.47 ± 1.06 | 0.3133 |
| CT | 238 (41.4) | 9.16 ± 0.87 | 4.30 ± 1.12 | |||
| TT | 281 (48.9) | 9.20 ± 0.68 | 4.24 ± 0.92 | |||
*Significant (P < 0.05) values are in bold. aMeans ± SD.
4. Discussion
We systematically investigated five ORAI1 tSNPs (rs12313273, rs6486795, rs7135617, rs12320939, and rs712853) in CKD patients. None of the tSNPs of ORAI1 were associated with the risk of CKD. However, rs12313273 was found to be significantly associated with increased serum calcium levels. Patients with CC genotype showed higher serum calcium levels than those with other genotypes. Impaired calcium and phosphate homeostasis have been reported in the early stages of CKD. We frequently used calcium-based or non-calcium-based phosphate binder to manage hyperphosphatemia, yet calcium-based binders often result in hypercalcemia [25]. Recent studies showed that CKD patients with high serum calcium levels (>2.75 mmol/L) have a higher risk of death than patients with low serum calcium levels do [26, 27]. Moreover, high calcium-phosphate product is associated with increased risk of vascular calcification and cardiovascular mortality [28, 29]. Our findings showed that patients with CC genotype of rs12313273 were associated with higher calcium levels. Therefore, we may take ORAI1 polymorphism into account when prescribing calcium or non-calcium-based phosphate binder to CKD patients with hyperphosphatemia.
ORAI1-mediated calcium signaling plays critical roles in inflammatory diseases. Chang et al. identified several polymorphisms in ORAI1 from Taiwanese and Japanese atopic dermatitis patients [30]. In addition, the CC genotype of rs12313273 in ORAI1 was strongly associated with the risk and recurrence of calcium nephrolithiasis [31]. Furthermore, the ORAI1 haplotypes (rs12313273 and rs7135617) are associated with the risk of HLA-B27-positive ankylosing spondylitis [32]. Consistent with the findings of previous studies, our results confirm the functional role of ORAI1 polymorphism rs12313273 in modulating the serum calcium concentration.
The calcium-dependent pathway is involved in multiple physiological and cellular functions such as modulation of immune responses, activation of inflammation, and enzyme metabolism [33, 34]. Inflammation is an important mediator of CKD progression and is a contributing factor in malnutrition and increased risk of cardiovascular morbidity [35]. A vast body of evidence supports the important role of calcium in kidney disease. Mutations in transient receptor potential canonical 6 (TRPC6) channels and polycystin-2, a prototypical member of a subfamily of the TRPC channel superfamily, have been reported to cause familial focal segmental glomerulosclerosis and autosomal dominant polycystic kidney disease, respectively [36–41].
Recently, Lu et al. demonstrated a significant correlation between TPRC1, ORAI1, STIM1, and parathyroid cells [42]. PTH plays a key role in serum calcium regulation. PTH itself is also regulated by extracellular calcium through stimulating the calcium-sensing receptor (CaSR) expressed on the surface of parathyroid cells [43]. CaSR, a G-protein PLC-linked receptor, has been shown to be involved in the TRPC1-mediated transient calcium oscillation in human embryonic kidney cells [44]. Our results suggest that the genetic polymorphisms of ORAI1 may alter ORAI1 gene expression in store-operated calcium channels, which in turn may affect PTH secretion and thereby serum calcium levels.
This study has several limitations. First, we did not consider several factors that are known to influence calcium levels, such as concomitant drug usage and underlying disease. Second, the underlying comorbidities were not identified in this study, and a possible relationship between the different comorbidities and the tSNPs of ORAI1 cannot be ruled out. Our results showed that the genotype of ORAI1 was not associated with CKD susceptibility. However, owing to the moderate size of our cohort, our analyses may not have sufficient power for detecting minor genetic effects. Therefore, we cannot exclude rare causal genetic polymorphisms in ORAI1. Direct ORAI1 sequencing using larger samples may be useful for identifying new SNPs in the ORAI1 gene and for clarifying the association of ORAI1 polymorphisms with CKD susceptibility. Further investigation on other variants of the genes of the SOC pathway and of the genes involved in calcium homeostasis are needed to fully understand CKD susceptibility and progression.
In conclusion, our results showed that the ORAI1 polymorphism rs12313273 is associated with higher serum calcium levels in Taiwanese CKD patients. To have a better management of serum calcium, ORAI1 polymorphism might be used as a potential biomarker for initiating non-calcium-based phosphate binder in CKD patients in the future.
Acknowledgments
The publication of this work was supported by the funding from the Department of Pharmacy and Clinical Research Center, Taipei Medical University Hospital and the Taiwan National Science Council (NSC 102-2314-B-038-008-MY3 (1-3)).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Author's Contribution
Daw-Yang Hwang and Shu-Chen Chien contribute equally to the paper.
References
- 1.Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives—a position statement from Kidney disease improving global outcomes. Kidney International. 2007;72(3):247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 2.US Renal Data System (USRDS) Annual Data Report. Bethesda, Md, USA: National Institute of Health, National Institute of Diabetes and Digestive and Kidney Disease; 2009. [Google Scholar]
- 3.Hwang S-J, Tsai J-C, Chen H-C. Epidemiology, impact and preventive care of chronic kidney disease in Taiwan. Nephrology. 2010;15(supplement 2):3–9. doi: 10.1111/j.1440-1797.2010.01304.x. [DOI] [PubMed] [Google Scholar]
- 4.Landray MJ, Wheeler DC, Lip GYH, et al. Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the Chronic Renal Impairment in Birmingham (CRIB) Study. American Journal of Kidney Diseases. 2004;43(2):244–253. doi: 10.1053/j.ajkd.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 5.Pereira BJG, Shapiro L, King AJ, Falagas ME, Strom JA, Dinarello CA. Plasma levels of IL-1β, TNFα and their specific inhibitors in undialyzed chronic renal failure, CAPD and hemodialysis patients. Kidney International. 1994;45(3):890–896. doi: 10.1038/ki.1994.117. [DOI] [PubMed] [Google Scholar]
- 6.Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney International. 2005;68(2):766–772. doi: 10.1111/j.1523-1755.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- 7.Herbelin A, Urena P, Nguyen AT, Zingraff J, Descamps-Latscha B. Elevated circulating levels of interleukin-6 in patients with chronic renal failure. Kidney International. 1991;39(5):954–960. doi: 10.1038/ki.1991.120. [DOI] [PubMed] [Google Scholar]
- 8.Knight EL, Rimm EB, Pai JK, et al. Kidney dysfunction, inflammation, and coronary events: a prospective study. Journal of the American Society of Nephrology. 2004;15(7):1897–1903. doi: 10.1097/01.asn.0000128966.55133.69. [DOI] [PubMed] [Google Scholar]
- 9.Yeun JY, Levine RA, Mantadilok V, Kaysen GA. C-reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. American Journal of Kidney Diseases. 2000;35(3):469–476. doi: 10.1016/s0272-6386(00)70200-9. [DOI] [PubMed] [Google Scholar]
- 10.Berridge MJ, Bootman MD, Lipp P. Calcium—a life and death signal. Nature. 1998;395(6703):645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 11.Baba Y, Matsumoto M, Kurosaki T. Calcium signaling in B cells: regulation of cytosolic Ca increase and its sensor molecules, STIM1 and STIM2. Molecular Immunology. 2013 doi: 10.1016/j.molimm.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Shaw PJ, Feske S. Regulation of lymphocyte function by ORAI and STIM proteins in infection and autoimmunity. The Journal of Physiology. 2012;590(part 17):4157–4167. doi: 10.1113/jphysiol.2012.233221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Ramachandran S, Oh-hora M, Rao A, Hogan PG. Pore architecture of the ORAI1 store-operated calcium channel. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(11):4896–4901. doi: 10.1073/pnas.1001169107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vig M, DeHaven WI, Bird GS, et al. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nature Immunology. 2008;9(1):89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Seaghdha CM, Fox CS. Genetics of chronic kidney disease. Nephron - Clinical Practice. 2010;118(1):c55–c63. doi: 10.1159/000320905. [DOI] [PubMed] [Google Scholar]
- 16.Kottgen A, Glazer NL, Dehghan A, et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nature Genetics. 2009;41(6):712–717. doi: 10.1038/ng.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Annals of Internal Medicine. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American Journal of Kidney Diseases. 2002;39(2, supplement 1):S1–S266. [PubMed] [Google Scholar]
- 20.Levey AS, De Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney International. 2011;80(1):17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 21.Stevens PE, Levin A. Kidney disease: improving global outcomes chronic Kidney disease guideline development work group M. evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Annals of Internal Medicine. 2013;158(11):825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 22.Ma LC, Chang HJ, Liu YM, et al. The relationship between health-promoting behaviors and resilience in patients with chronic kidney disease. The Scientific World Journal. 2013;2013:7 pages. doi: 10.1155/2013/124973.124973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalantar-Zadeh K, Shah A, Duong U, Hechter RC, Dukkipati R, Kovesdy CP. Kidney bone disease and mortality in CKD: revisiting the role of vitamin D, calcimimetics, alkaline phosphatase, and minerals. Kidney International. 2010;(117):S10–S21. doi: 10.1038/ki.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz S, Trivedi BK, Kalantar-Zadeh K, Kovesdy CP. Association of disorders in mineral metabolism with progression of chronic kidney disease. Clinical Journal of the American Society of Nephrology. 2006;1(4):825–831. doi: 10.2215/CJN.02101205. [DOI] [PubMed] [Google Scholar]
- 25.Ketteler M, Biggar PH. Use of phosphate binders in chronic kidney disease. Current Opinion in Nephrology and Hypertension. 2013;22(4):413–420. doi: 10.1097/MNH.0b013e32836214d4. [DOI] [PubMed] [Google Scholar]
- 26.Floege J, Kim J, Ireland E, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrology Dialysis Transplantation. 2011;26(6):1948–1955. doi: 10.1093/ndt/gfq219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naves-Daz M, Passlick-Deetjen J, Guinsburg A, et al. Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. the CORES Study. Nephrology Dialysis Transplantation. 2011;26(6):1938–1947. doi: 10.1093/ndt/gfq304. [DOI] [PubMed] [Google Scholar]
- 28.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. American Journal of Kidney Diseases. 1998;31(4):607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 29.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. Journal of the American Society of Nephrology. 2004;15(8):2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 30.Chang W-C, Lee C-H, Hirota T, et al. ORAI1 genetic polymorphisms associated with the susceptibility of atopic dermatitis in Japanese and Taiwanese populations. PLoS ONE. 2012;7(1) doi: 10.1371/journal.pone.0029387.e29387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou Y-H, Juo S-HH, Chiu Y-C, et al. A polymorphism of the ORAI1 gene is associated with the risk and recurrence of calcium nephrolithiasis. Journal of Urology. 2011;185(5):1742–1746. doi: 10.1016/j.juro.2010.12.094. [DOI] [PubMed] [Google Scholar]
- 32.Wei JC-C, Yen J-H, Juo S-HH, et al. Association of ORAI1 haplotypes with the risk of HLA-B27 positive ankylosing spondylitis. PLoS ONE. 2011;6(6) doi: 10.1371/journal.pone.0020426.e20426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature Reviews Molecular Cell Biology. 2003;4(7):517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 34.Verkhratsky A. Calcium and cell death. Sub-Cellular Biochemistry. 2007;45:465–480. doi: 10.1007/978-1-4020-6191-2_17. [DOI] [PubMed] [Google Scholar]
- 35.Silverstein DM. Inflammation in chronic kidney disease: role in the progression of renal and cardiovascular disease. Pediatric Nephrology. 2009;24(8):1445–1452. doi: 10.1007/s00467-008-1046-0. [DOI] [PubMed] [Google Scholar]
- 36.Mochizuki T, Wu G, Hayashi T, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272(5266):1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 37.Winn MP, Conlon PJ, Lynn KL, et al. Medicine: a mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308(5729):1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 38.Reiser J, Polu KR, Möller CC, et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nature Genetics. 2005;37(7):739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dryer SE, Reiser J. TRPC6 channels and their binding partners in podocytes: role in glomerular filtration and pathophysiology. American Journal of Physiology. 2010;299(4):F689–F701. doi: 10.1152/ajprenal.00298.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian D, Jacobo SMP, Billing D, et al. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels (Science Signaling (2010)) Science Signaling. 2010;3(147, article er11) doi: 10.1126/scisignal.2001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greka A, Mundel P. Balancing calcium signals through TRPC5 and TRPC6 in podocytes. Journal of the American Society of Nephrology. 2011;22(11):1969–1980. doi: 10.1681/ASN.2011040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu M, Bränström R, Berglund E, et al. Expression and association of TRPC subtypes with ORAI1 and STIM1 in human parathyroid. Journal of Molecular Endocrinology. 2010;44(5):285–294. doi: 10.1677/JME-09-0138. [DOI] [PubMed] [Google Scholar]
- 43.Sherwood LM, Herrman I, Bassett CA. Parathyroid hormone secretion in vitro: regulation by calcium and magnesium ions. Nature. 1970;225(5237):1056–1058. doi: 10.1038/2251056a0. [DOI] [PubMed] [Google Scholar]
- 44.Rey O, Young SH, Papazyan R, Shapiro MS, Rozengurt E. Requirement of the TRPC1 cation channel in the generation of transient Ca2+ oscillations by the calcium-sensing receptor. The Journal of Biological Chemistry. 2006;281(50):38730–38737. doi: 10.1074/jbc.M605956200. [DOI] [PubMed] [Google Scholar]


