Abstract
Tactile defensiveness, characterized by behavioral hyperresponsiveness and negative emotional responses to touch, is a common manifestation of aberrant sensory processing in autism spectrum disorders (ASD) and other developmental disabilities (DD). Variations in tactile defensiveness with the properties of the stimulus and the bodily site of stimulation have been addressed in adults with self-report of perceived tactile pleasantness, but not in children. We presented three materials (pleasant, unpleasant, social) at three bodily sites and measured both examiner-coded defensiveness and self-reported pleasantness from a group of children with ASD and two comparison groups (one with DD, one with typical development (TD)). The main findings were: 1) children with ASD and DD showed significantly more defensiveness reactions and lower pleasantness ratings than the TD group, with higher variability, 2) there was a double dissociation for the effects of material and bodily site of stimulation: while bodily site predicted behavioral defensiveness, material predicted pleasantness rating. Additionally, it was noted that 3) the most pleasant material and the social touch conditions best distinguished ASD and DD from TD on defensiveness, and 4) within the ASD group, social impairment and defensiveness in bodily sites associated with social touch were positively correlated, suggesting a clinically relevant distinction between social and discriminative touch in ASD.
Keywords: touch, tactile, affective, defensiveness, pleasantness, self-report
Many sources of evidence—including clinical, anecdotal, and experimental—converge to suggest that individuals with autism spectrum disorder (ASD) experience simple sensory stimuli differently than their typically-developing peers (Baranek et al., 2006; Rogers et al., 2005; Jones et al., 2003; Mottron et al., 2006). Experimental study of sensory differences has been dominated by studies of the visual and auditory modalities, perhaps in part because of the prominent role of visual and auditory processing in the verbal and nonverbal communication abilities that are impacted by ASD. However, the onset of ASD in the first few years of life points to the importance of pre-verbal socio-communicative development, for which the sense of touch plays a pivotal role.
The skin—the body's largest sensory organ—and its system of receptors develop early in utero, in preparation for the use of tactile sensation for rooting and sucking reflexes during nursing immediately after birth (Muir, 2002). Touch is a primary mode of communication in the first year of life (Field, 2001), when physical contact with a caregiver lays the foundation for social interaction and bonding (Monatgu 1986), and is associated with nourishment and comfort, providing the infant's earliest experiences of social reward. Maternal touch in early infancy (4 months) critically influences later secure attachment (Weiss et al., 2000; Beebe et al., 2012), and the absence of maternal touch has been associated with deleterious effects on behavior (Harlow & Harlow, 1962; Hertenstein, 2002). Lack of ventral-ventral contact (in which infants are held against a caregiver's chest) early in infancy is strongly associated with aberrant behavior including repetitive behaviors such as echolalia and stereotypies (Main & Stadtman, 1981), and the animal literature links abnormal or absent early maternal touch to decreased cognitive abilities, novelty exploration, and attachment (Hertenstein et al., 2006). If the perception of touch in these foundational early interactions differs for infants with ASD, these differences may contribute to the aberrant development of social, communication, and repetitive behaviors that characterize individuals on the autism spectrum.
The study of human affective response to touch in typical adult populations has utilized psychophysical magnitude estimation or scaling to determine what properties of tactile stimuli (e.g. texture, velocity, social context, force, bodily site of stimulation, etc.) contribute to the perceived pleasantness or unpleasantness of touch (Essick et al., 1999). Although interactions among these properties are complex, it is clear that soft or smooth surfaces are generally rated as more pleasant than stiff or rough textures (Essick et al., 2010). It is also clear that more intense affective responses result from passive stimulation with a material rather than active haptic exploration of the material (Guest et al., 2011).
This effect of passive versus active stimulus presentation on perceived pleasantness may be partly mediated by bodily site of stimulation. A specialized class of peripheral C-class afferents known as C-touch (CT) fibers (Vallbo et al., 1999; Olausson et al., 2002; Loken et al., 2009) is present in the hairy skin and the face, but absent from the glabrous skin of the palms (used for active exploration). The CT fibers respond preferentially to slow, stroking touch and project directly to the posterior insular cortex rather than primary somatosensory cortex, suggesting an afferent system that is specialized for social stimuli and is distinct from the fast-conducting, myelinated A-beta fibers that innervate cutaneous receptors in the palm and mediate discriminative touch (Sewards & Sewards, 2002; McGlone et al., 2007).
Previous studies used pleasantness ratings for a range of passively received textures in adults with ASD and found patterns of hedonic (pleasantness) ratings that were strikingly similar to typical adults for unpleasant, neutral, and pleasant textures (Cascio et al., 2008; 2012). In spite of this similarity in self-reported hedonic experience of the textures, global brain response to each of the textures was diminished in the ASD group, particularly for pleasant and neutral textures. For the most unpleasant texture, some affective processing brain regions such as the insula were more responsive in the ASD group (Cascio et al., 2012), suggesting a heightened negative affective response, despite rating the material similarly to controls in its unpleasantness. This discrepancy between hedonic ratings and neural response may reflect an effect of experience in adults: perhaps initially more intense negative behavioral responses to touch are dampened over time through repeated exposure and greater awareness of social norms, while the neural response continues to reveal aberrant affective processing of touch. To begin to investigate this possibility, we set out to measure hedonic behavioral response to touch in young children with ASD.
Despite developmental challenges associated with obtaining reliable psychophysical ratings from children, researchers and clinicians who specialize in pain have developed several effective tools for overcoming these challenges and have validated them to ensure that they produce accurate self-report of affective perceptual experience from children. A common and well-validated tool is an ordinal scale with cartoon faces as anchor points, such as the Wong-Baker FACES scale (Wong and Baker, 1988; Keck et al., 1996; Garra et al., 2010) for assessing perceived pain in children. Ordinal scales using descriptive words as anchor points have also been used successfully in self-report (Keck et al., 1996). In the current study, our aim was to investigate the effects of tactile material and bodily site of stimulation (sites chosen for differential innervation by CT-afferents) on hedonic responses to passively received touch in young children with ASD compared to both a typically developing group (TD) and a group with generalized developmental disability (DD). Further, we wished to facilitate comparison with adult studies that utilize self-report. Thus, we obtained both self-reported hedonic ratings by training participants to use an adaptation of the FACES scale, and recorded observational measures of tactile defensiveness behavior using a manualized coding system developed for the Tactile Defensiveness and Discrimination Test-Revised (Baranek et al., 1997; Foss-Feig et al., 2012).
Method
Participants
Participants included 33 children with ASD, 20 children with other DD, and 56 children with TD, all of whom were a subset of participants from a larger federally funded longitudinal study about sensory experiences in ASD. Table 1 provides information regarding participant demographics. Families were recruited through strategies including e-mail listservs, brochures, a university-based research registry, parent support groups, advocacy groups, and local conferences. The university's institutional review board approved measures and procedures in advance; parents gave consent for participation and were provided with monetary compensation for their participation in the larger study ($25-75, depending on the extent of their involvement). Participants were screened following enrollment to confirm that both their vision and hearing were within normal limits.
Table 1.
Participant demographics. The ASD and DD groups differed significantly on mental age (t51=2.8, p=0.004) but were matched on chronological age, while the ASD and TD groups differed on chronological age (t87=2.92, p=0.005) but were matched on mental age. ADI-R subscale scores represent diagnostic algorithm.
| Group (n) | Chronological age in months (SD) | Mental age in months (SD) | ADI-R A (Social) | ADI-R Bv (Verbal Communication) | ADI-R C (Repetitive Behavior) |
|---|---|---|---|---|---|
| ASD (33) | 98.6 (28.4) | 78.1 (46.1) | 21.3 (5) | 18.6 (3.2) | 7.5 (2.2) |
| DD (20) | 100.2 (30.3) | 44.7 (34.4) | . | . | . |
| TD (56) | 80.6 (27.7) | 73.5 (54.4) | . | . | . |
Participants with ASD had received a diagnosis of Autistic Disorder, Asperger's Disorder, or Pervasive Developmental Disorder – Not Otherwise Specified by an independent physician or licensed psychologist prior to their involvement in the study. All ASD participants met criteria for autism spectrum disorder on the Autism Diagnostic Interview – Revised (ADI-R; LeCouteur, Lord, & Rutter, 2003) and/or the Autism Diagnostic Observation Schedule (ADOS; Lord, Rutter, DiLavore, & Risi, 1999), and met criteria for a DSM-IV diagnosis of autistic disorder based on expert clinical impression. All ADOS and ADI-R assessors had achieved research reliability and were supervised by an experienced clinical coordinator, who reviewed all assessment data and community records and derived an expert clinical judgment for each case.
The DD group was comprised of participants with either a known genetic syndrome associated with intellectual disability (e.g., Down syndrome; n = 11), a nonspecific developmental delay based on a cognitive assessment with IQ >2 standard deviations below the mean or significant delays (>1.5 standard deviations below the mean) in two developmental domains (e.g., expressive language, receptive language, visual reception, fine or gross motor, adaptive behavior; n = 7), or developmental delay associated with prematurity (n = 2). Potential participants were excluded from the DD group if they met criteria for autism on the ADOS or on the Childhood Autism Rating Scale (Schopler, Reichler, & Renner, 1998). The group with TD was comprised of children who had no history of learning or developmental problems, and did not include children who received special education services.
For all three groups (i.e., ASD, DD, TD), exclusionary criteria were as follows: diagnosis of any genetic condition associated with autism, seizure disorder, mental age (MA) below 6 months, or receipt of psychopharmacological treatments (e.g., antipsychotics) that might alter typical sensory responses.
Procedures
The primary task of interest involved passive touch from three different textures (unpleasant: plastic mesh; pleasant: soft fleece; social: experimenter's finger) at three different sites on the participant's upper body with various levels of CT afferent innervation (perioral face; dorsal forearm; thenar palm). A trained experimenter administered each texture x site combination twice for a total of 18 trials. Participants who were successful in completing training prior to the task (detailed in the following section), used a 5-point Likert scale with child-friendly “smiley face” icons (modeled after Wong and Baker, 1988; see Figure 1) to self-rate the pleasantness or unpleasantness of the touch (1 = really bad; 2 = pretty bad; 3 = okay; 4 = pretty good; 5 = really good). In addition to affective rating from the participant, the examiner also coded the participant's immediate reaction after each trial for displays of defensiveness, using a 0-3 scale (0 = no negative reaction within 5 seconds; 1 = slight negative reaction; 2 = definite negative reaction; 3 = severe negative reaction). The experimenter randomly assigned each participant one of three different orders of trials.
Figure 1.

5-point scale used for affective ratings of tactile stimuli.
Prior to the aforementioned task, the experimenter provided brief instruction regarding the Likert scale (“smiley face”) affective rating procedures to each participant. Training with various textured stimuli or cards depicting various foods were used to practice assigning hedonic ratings; the consistency of responses during this practice session was the basis upon which the examiner determined whether the participant understood the pleasant/unpleasant self-report task. If the experimenter determined after the instruction that the participant did not understand the pleasant/unpleasant self-report portion of the task, the experimental procedure (18 trials) proceeded with only examiner defensiveness ratings and did not include participant self-report ratings. This resulted in the exclusion of self-report ratings for 5 children in the ASD group, 5 in the DD group, and 4 in the TD group.
In addition to the aforementioned screening and diagnostic measures, study participation also consisted of cognitive testing and completion of a caregiver questionnaire.
Measures
Cognitive measures
Each participant's nonverbal MA was determined based on either the Stanford-Binet Intelligence Scales, Fifth Edition (SB5; Roid, 2003) Nonverbal IQ score (n=97), or the Mullen Scales of Early Learning (MSEL; Mullen, 1995) age equivalent of the Visual Reception (VR) scale (n=12). The SB5 is a standardized measure of cognitive functioning appropriate for individuals 2 years of age and older, while the MSEL is a standardized measure of cognitive functioning for children from birth to 68 months of age.
Sensory measure
Additional information regarding participants' sensory experiences and features was gathered using the Sensory Experiences Questionnaire, version 2.1 (SEQ; Baranek, 1999—unpublished manuscript; Baranek, David, Poe, Stone, & Watson, 2006). The SEQ is a 43-item (5-point, Likert-type scale) parent-report questionnaire that measures sensory features across different modalities categorized by three sensory response patterns (i.e., hyporesponsiveness, hyperresponsiveness, and sensory seeking) in children between the ages of 6 months and 12 years. The items elicit information regarding tactile, auditory, visual, vestibular-proprioceptive, and gustatory-olfactory experiences. Previous research has demonstrated excellent reliability and internal consistency of the SEQ (see Little et al., 2011). The SEQ has been used previously with clinical populations, including children with ASD and DD (Baranek et al., 2006; Boyd et al., 2010; Watson et al., 2010). The mean of the items addressing tactile response in the SEQ was calculated and utilized in correlation analyses.
Data Analysis
Scores across the two trials for each combination of material and site were averaged for each participant. Separate models were run for average self-report pleasantness ratings and examiner-coded defensiveness scores. Because both outcome variables were ordinal, with fewer than 10 levels, proportional odds models (Walker & Duncan, 1967; McCullagh, 1980) were used, with either self-report pleasantness rating or examiner rating of defensiveness as the outcome variable, and group, material, and bodily site as predictor variables. A robust covariance matrix estimate was used in each model to correct for correlated responses (4 participants had data from two time points). Because sensory symptoms have been shown to be related to mental age (MA), MA was included as a covariate in both models.
Correlation of self- ratings and examiner ratings with the mean of tactile items on the SEQ and clinical scores were computed using Spearman's rho across the sample and separately by group.
Results
Affective ratings
Both diagnostic group and material affected affective ratings. In the model examining the affective ratings given by the participants, there was a significant main effect of group (χ2(10)=20.84, p=0.022) and material ((χ2(6)=52.62, p<0.0001). The effect of site was not significant, and there were no significant interactions between independent variables, although the interaction between group and material approached statistical significance ((χ2(4)=8.93, p=0.0630). There was no significant main effect of MA ((χ2(1)=0.43, p=0.5109). Because the distribution of the rating data violated the assumption of normality, the nonparametric Mann-Whitney U test was used to explore these effects further. This analysis demonstrated that the ASD and DD group did not differ significantly from each other in their ratings of any of the materials. The two clinical groups both gave lower ratings for the fleece (pleasant) and skin (social touch) materials (Fleece: ASD vs. TD: (p=0.004), DD vs. TD: p=0.056; Skin: ASD vs. TD: p=0.059, DD vs. TD (p=0.041)), but their ratings of the unpleasant mesh material did not differ from the TD group (Figure 2). The main effect of material is also apparent in Figure 2.
Figure 2.
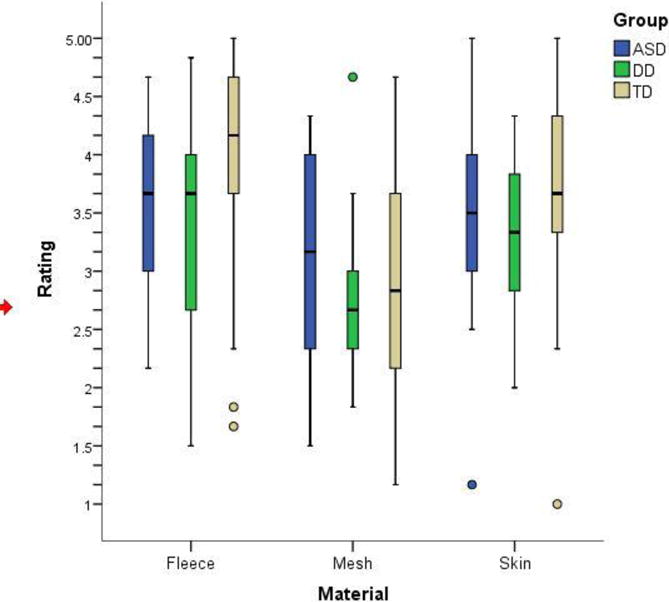
Box plots of affective ratings of pleasantness for each material in the three groups.
Defensiveness scores
Both diagnostic group and bodily site affected defensiveness scores. In the model examining the defensiveness scores given by the examiner, there was again a significant main effect of group (χ2(10)=26.51, p=0.0031), and a main effect of bodily site ((χ2(6)=16.85, p=0.0099). Neither the effect of material nor any interactions between independent variables were statistically significant. There was, however, a significant main effect of MA ((χ2(1)=5.39, p=0.0202).
The distribution of the defensiveness data also violated the assumption of normality, thus the Mann-Whitney U test was used to explore these effects as well. This analysis demonstrated that neither the ASD and DD group nor the DD and TD group differed significantly from each other in their defensiveness reactions for any of the bodily sites. As is evident from Figure 3, the variability in the clinical groups was quite high compared to the TD group, and both groups had higher defensiveness scores overall than the TD group.
Figure 3.
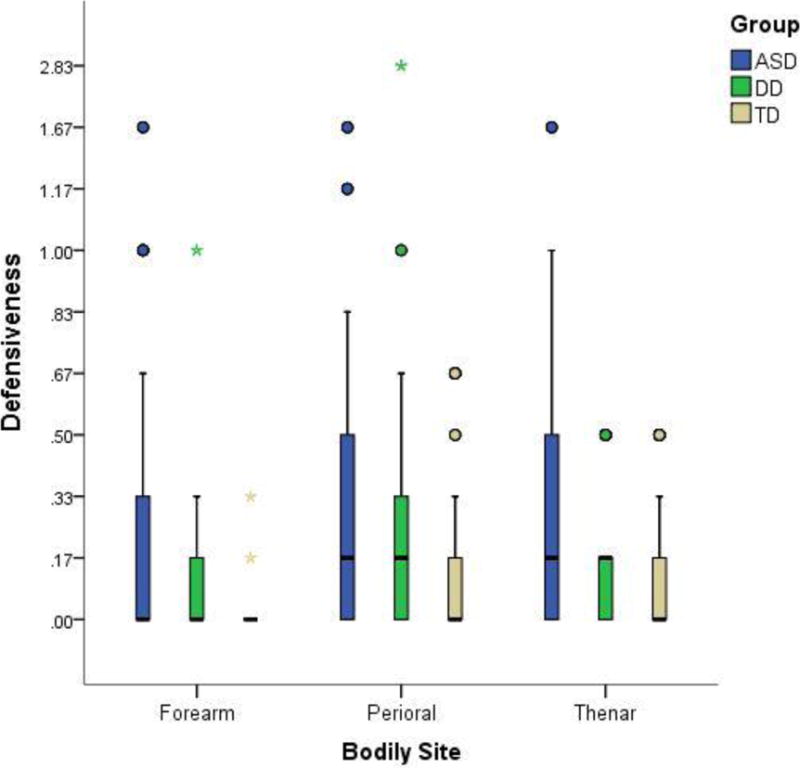
Box plots of examiner-coded rating of defensiveness for each bodily site in the three groups.
The ASD group had statistically significantly higher defensiveness reactions than the TD group at the two bodily sites innervated by CT fibers (perioral: p=0.024, forearm: p=0.014) and showed a trend in the direction of demonstrating higher defensiveness reactions than the TD group at the bodily site that is not innervated by CT afferents (thenar: p=0.063). While the DD group also exhibited more defensiveness than the TD group at all three sites, these differences did not reach statistical significance.
Correlations between ratings and defensiveness
Because it was expected that defensiveness scores and pleasantness ratings would be negatively correlated, we sought to verify this using Spearman's rho correlation coefficients performed both across the sample and separately for each group for each of the materials and bodily sites. As expected, there were no significant positive correlations, and most correlations were negative (i.e., high levels of defensiveness were associated with low pleasantness ratings, see Table 4). The fleece material and the thenar and perioral bodily sites showed statistically significant negative correlations across groups; the patterns of correlation strengths across sites and materials differed between the three groups.
Table 4.
Spearman's rho correlation coefficients for ratings (self-report) and observed tactile defensiveness for each material and bodily site tested. *: p value of correlation coefficient < .05.
| Material | All groups | ASD | DD | TD |
|---|---|---|---|---|
| Fleece | -.324* | -.184 | -.258 | -.340* |
| Mesh | .059 | -.009 | .378 | -.029 |
| Skin | -.192 | -.288 | -.232 | -.015 |
|
| ||||
| Site | ||||
|
| ||||
| Thenar | -.210* | -.337 | .046 | -.223 |
| Forearm | -.157 | .077 | -.137 | -.221 |
| Perioral | -.250* | -.215 | -.422 | -.136 |
Correlations with parent report measures
Spearman's rho partial correlation coefficients were computed with MA for each group separately to assess relation between affective rating or defensiveness score and the tactile subscale of the SEQ. For the tactile subscale of the SEQ, there were no significant relations between parent report of tactile symptoms and ratings for the ASD and TD groups. The DD group exhibited significant positive correlations between parent report of tactile symptoms and ratings for fleece (ρ=0.649, p=.031) and overall ratings across the three textures (ρ =0.738, p=.01). In other words, in this group, parent report of more unusual tactile responses was associated with higher pleasantness ratings. There were no significant relations between parent report of tactile symptoms and experimenter score of tactile defensiveness for the TD and DD groups. The ASD group showed significant positive correlations between parent report of tactile symptoms and defensiveness at the thenar site (ρ =0.363, p=.045) and overall defensiveness score (ρ =0.360, p=.047); in this group, parent report of more unusual higher tactile responses was related to higher levels of defensiveness. While these relations all remained significant when MA was partialled out, none of them remained after correction for multiple comparisons.
For the ASD group, we examined correlation between the self-report rating and examiner-coded defensiveness scores and the subscales of the ADI-R for current behavior, using nonparametric correlations as above. There was a significant negative correlation between rating for the fleece material and nonverbal communication impairment (ρ =− 0.560, p=.02). In other words, higher pleasantness ratings were associated with less impaired communication. There was also a trend for a positive correlation between rating for the mesh material and social impairment (ρ =0.459, p=.055); higher pleasantness ratings for mesh were associated with more severe social impairment. Similarly, there were significant correlations between defensiveness at the forearm (ρ = 0.444, p=.05) and perioral (ρ =0.502, p=.024) sites and current social impairment. In other words, at the forearm and perioral sites, increased defensiveness was associated with more severe social impairment. When MA was partialled out, the relations between fleece rating and nonverbal communication impairment and defensiveness for the perioral site and social impairment remained, although none of the correlations remained after correction for multiple comparisons.
Discussion
Group effects
Overall, both clinical groups gave lower self-ratings and exhibited higher levels of examiner-coded tactile defensiveness relative to the TD group. The clinical groups gave lower self-report ratings for the pleasant and social materials (fleece and skin), but did not differ from the TD group in their ratings of the unpleasant material (mesh), suggesting that all three groups found the mesh similarly unpleasant. The ASD group differed significantly from the TD group in examiner-scored defensiveness at the perioral and forearm sites, while the DD group did not differ significantly from either group. While this may indicate specificity of defensiveness at these sites for children on the autism spectrum, and thus implicate the CT afferent system in ASD, the lack of group differences between the DD and other groups should be interpreted in the context of the small size, heterogeneous makeup, and large variability of defensiveness responses in the DD group.
The group results are in accord with previous findings that sensory defensiveness is generally heightened in children with developmental disabilities relative to their typically-developing peers (Rogers et al., 2005; Baranek et al., 2006). To the body of literature that has previously assessed sensory defensiveness with parent report and observed defensiveness reactions, the current study adds self-report hedonic (pleasantness) ratings as a novel way to assess affective response to sensory stimuli in young children with disabilities.
Concordance between self-rating of pleasantness and examiner-coded defensiveness
Correlations between defensiveness score and pleasantness rating were consistently negative, as expected, reflecting higher observed defensiveness for stimuli that elicited lower pleasantness ratings. Although nearly all materials and bodily sites showed at least a slight negative correlation, the pattern of correlations suggests that children in all groups may have had differential ability to ascribe affective ratings that reflected their defensiveness behaviors across materials and bodily sites. The mesh material elicited the least consistency between affective ratings and defensiveness response, particularly in the DD group, which actually showed relatively strong correlation in the opposite direction (higher ratings associated with more defensiveness reactions). Because most other correlations for the DD group were in the expected direction, we do not attribute this surprising result to a lack of comprehension for the rating task. A more plausible interpretation is that children with DD may have had a stronger desire to please the experimenter by giving higher-than-expected ratings for this material when in fact their behavior revealed more negative affect. Nonverbal responses that are subjected to a rigorous coding structure as in the present study are considered more reliable and less susceptible to dissimulation than verbal report in the pain field (Craig & Prkachin, 1983); this should be a consideration when applying this self-report method to young clinical samples in the future, particularly for stimuli that are emotionally negative.
In contrast, high levels of concordance between examiner-coded defensiveness and self-ratings were seen for the fleece material and the perioral site. Although the concordance was present for all three groups, it reached statistical significance only for the TD group. This is likely attributable to the combination of higher ratings given by the TD group relative to both clinical groups, and the very low levels of defensiveness in the TD group. We speculate that the unusual experience of contact from a stranger on the perioral region may have been disarming and particularly robust against the kind of contextually-motivated discrepancy between behavior and verbal report described above for the mesh material.
Effect of material on rating and site on defensiveness
Aside from group effects, it was interesting to note the double dissociation (two predictor variables that each predict only one of two outcome variables, having no effect on the other) that emerged from the ratings and defensiveness variables: material was a significant predictor for rating but not defensiveness, whereas bodily site was a significant predictor for defensiveness, but not rating. These findings are consistent with previous studies both in typical adults (Essick et al., 2010) and adults with autism (Cascio et al., 2008) measuring self-report of pleasantness: material strongly affects pleasantness ratings but the effects of bodily site of stimulation are weak or absent. What our combined approach allowed us to demonstrate is that, in the current sample, another method (observation of defensiveness reactions) of probing affective response to touch does reveal an effect of bodily site. Although it is unknown what the source of the double dissociation is, one could speculate that overt verbal responses are biased toward evaluation the properties of materials that could be described verbally (e.g., soft, rough, smooth, etc.), and therefore focus attention on the material itself when providing pleasantness ratings. In contrast, the observable, nonverbal defensiveness behaviors that we quantified (e.g., change in facial expression, wincing, withdrawal from stimulus), are likely to be mediated by more automatic processes that would be more tightly linked to bodily location than external stimulus properties. It would be interesting, albeit challenging given the tendency for sensory defensiveness responses to decline with developmental maturity, to explore the development of a behavioral coding system to assess tactile defensiveness reactions in adolescents and adults. Because of the differential innervation of glabrous and hairy skin with CT mechanoreceptors that respond to slow, stroking touch that is generally perceived as pleasant (Vallbo et al., 1999), a paradigm that unmasks potential effects of bodily site in the broader population could provide further support for the role of this afferent system in pleasant touch.
Correlations with parent report of sensory symptoms
We noted significant correlations between parent report of sensory symptoms and ratings for the DD group, and between parent report and defensiveness for the ASD group. Somewhat surprisingly, these were the only significant relations found with parent report of sensory symptoms. It should be noted that tactile experience in everyday life and in a laboratory setting are quite different. The predictable, repeated, stimulation administered by an experimenter is limited in its ecological validity, and introduces many factors that could differentiate it from defensiveness or verbal report of affective response in daily life. These include the ability to anticipate and prepare for a series of controlled stimuli in the laboratory in contrast to unexpected encounters with touch in daily life; the social component of all stimuli being administered by an unfamiliar adult in the laboratory in contrast to a combination of nonsocial tactile experiences, as well as social with both familiar and unfamiliar people in daily life; and the entirely passive nature of the stimulation in the laboratory as opposed to a combination of active tactile exploration and passive touch in daily life. Additionally, there may have been differences in parental criteria for endorsing defensiveness behaviors as compared to the structured observational coding system and self-report paradigms used in the laboratory.
Correlations with parent report of current autism symptoms
We noted several relations between ratings or defensiveness scores to touch and current clinical symptoms in the ASD group, which is consistent with prior reports of relation between autism symptoms and sensory processing difficulties (Boyd et al., 2010; Foss-Feig et al., 2012; Robertson and Simmons, 2012). Of note was that social impairment was associated with defensiveness to the passively-presented materials at the two sites that are innervated by CT afferents (perioral and forearm) but not at the thenar palm site (Vallbo et al., 1999). This association provides some rationale for continued investigation of possible abnormalities of the CT “social touch” system in ASD.
There was a lack of association between either tactile defensiveness behaviors or ratings and repetitive behaviors as assessed by parent report using the ADI-R. Previous work suggests a link between generalized sensory defensiveness (i.e., hyperresponsiveness) and repetitive behaviors (Boyd et al., 2010), but a study focusing solely on tactile defensiveness did not show such a link (Foss-Feig et al., 2012), which may indicate that sensory modality plays a role in the link between hyperresponsiveness and repetitive behaviors. Given the disparate patterns of sensory response across sensory modalities in individuals who exhibit hyperresponsiveness (Leekam et al. 2007; Ben-Sasson et al., 2009), it may be useful in future experimental studies to focus on a single sensory modality.
Study limitations and conclusions
This study had several strengths: a relatively large and well-characterized sample, a unique combination of verbal report and observed behavior, and a focus on an understudied sensory modality that is likely to have a substantial impact on early social and communication. The study also had some limitations: our DD comparison group was heterogenous and included children with DD of known genetic etiology (e.g., Down syndrome), for whom the developmental trajectory and behavioral symptoms are known to differ from ASD and which may limit generalizability of findings to the broader population of children with DD. Both outcome variables were ordinal and thus were inherently limited in variability, which may have decreased power to detect subtler group differences between the ASD and DD groups. In spite of these limitations, this study uncovered important new information about tactile response in ASD. Unlike adults with ASD (Cascio et al., 2012), children with ASD (and DD) do differ significantly from typically developing children in their affective ratings of various textured materials, rating them as less pleasant, particularly for social touch (skin) and a material typically judged as pleasant (fleece). This supports the observation that the lack of perceptual differences in adults with ASD as measured by self report is paradoxically accompanied by substantial differences in the cortical response to these tactile experiences, including diminished response to pleasant and neutral textures and heightened response of limbic regions to unpleasant textures (Cascio et al., 2012). Taken together, these two studies suggest that the similar ratings given by adults with and without ASD may reflect a developmental process of learning to inhibit negative affective response to touch in individuals with ASD.
We validated the self-report ratings by also measuring examiner coded behavioral defensiveness to each stimulus. The expected inverse relationship between defensiveness behavioral scores and pleasantness ratings suggest that the careful use of a self-report measure could be a helpful way to measure sensory responses in children with ASD that would enable better comparisons with adults. Our combined approach also allowed us to uncover a double dissociation in the effects of stimulus material and bodily site on self-reported pleasantness and behavioral scores of defensiveness reactions. While the material itself predicted self-report ratings but not behavioral defensiveness scores, the bodily site of stimulation predicted behavioral defensiveness scores but not self-report ratings of pleasantness.
Finally, we noted significant correlation between current social impairment and behavioral coding of defensiveness at the two bodily sites innervated by the CT afferent system. This somatic specificity of the relation between defensiveness and social impairment suggests that further investigation of CT afferent (or “social touch”) dysfunction in ASD is warranted.
Table 2.
Model output for pleasantness (self-report) ratings. The model yielded a significant main effect of group and material, with no significant effect of site of stimulation and no significant interactions among predictor variables (although there was a trend for an interaction between material and group).
| Factor | χ2 | d.f. | p |
|---|---|---|---|
| Group (factor + higher order factors) | 20.84 | 10 | 0.022 |
| All interactions | 13.36 | 8 | 0.1000 |
| MA | 0.43 | 1 | 0.5109 |
| Material (factor + higher order factors) | 52.62 | 6 | <0.0001 |
| All interactions | 7.86 | 4 | 0.0968 |
| Site (factor + higher order factors) | 7.53 | 6 | 0.2749 |
| All interactions | 5.08 | 4 | 0.2789 |
| Group x material (factor + higher order factors) | 7.86 | 4 | 0.0968 |
| Group x site (factor + higher order factors) | 5.08 | 4 | 0.2789 |
| Total interaction | 13.36 | 8 | 0.1000 |
| Total | 78.54 | 15 | <0.0001 |
Table 3.
Model output for defensiveness ratings. The model yielded a significant main effect of group and site of stimulation, with no significant effect of material and no significant interactions among predictor variables.
| Factor | χ2 | d.f. | p |
|---|---|---|---|
| Group (factor + higher order factors) | 26.51 | 10 | 0.0031 |
| All interactions | 12.75 | 8 | 0.1206 |
| MA | 5.39 | 1 | 0.0202 |
| Material (factor + higher order factors) | 9.16 | 6 | 0.1648 |
| All interactions | 5.46 | 4 | 0.2436 |
| Site (factor + higher order factors) | 16.85 | 6 | 0.0099 |
| All interactions | 4.14 | 4 | 0.3873 |
| Group x material (factor + higher order factors) | 5.46 | 4 | 0.2436 |
| Group x site (factor + higher order factors) | 4.14 | 4 | 0.3873 |
| Total interaction | 12.75 | 8 | 0.1206 |
| Total | 46.85 | 15 | <0.0001 |
Acknowledgments
This study was supported in part by a grant from NICHD (R01 HD42168) and CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences. CJC's effort is supported by NIMH (K01 MH090232). We would like to acknowledge John Bulluck for his assistance with data processing, and Jeanne Lovmo, Melissa Furlong, Heather Miller, Beth Schultz, and Amanda Lert for their assistance with recruitment and data collection.
References
- Baranek GT, Foster LG, Berkson G. Tactile defensiveness and stereotyped behaviors. American Journal of Occupational Therapy. 1997;51:91–95. doi: 10.5014/ajot.51.2.91. [DOI] [PubMed] [Google Scholar]
- Baranek GT, David FJ, Poe MD, Stone WL, Watson LR. The Sensory Experiences Questionnaire: Discriminating response patterns in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry. 2006;47:591–601. doi: 10.1111/j.1469-7610.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Beebe B, Lachmann F, Marksee S, Buck KA, Bahrick LE, Chen H, Cohen P, Andrews H, Feldstein S, Jaffe J. On the origins of disorganized attachment and internal working models: Paper II. An empirical microanalysis of 4-month mother-infant interaction. Psychoanalytic Dialogues. 2012;22:352–74. doi: 10.1080/10481885.2012.679606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, Gal E. A meta-analysis of sensory modulation symptoms on individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;29:1–11. doi: 10.1007/s10803-008-0593-3. [DOI] [PubMed] [Google Scholar]
- Boyd BA, Baranek GT, Sideris J, Poe MD, Watson LR, Patten E, et al. Sensory features and repetitive behaviors in children with autism and developmental delays. Autism Research. 2010;3(2):78–87. doi: 10.1002/aur.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio C, McGlone F, Folger S, Tannan V, Baranek G, Pelphrey KA, Essick G. Tactile perception in adults with autism: a multidimensional psychophysical study. Journal of Autism and Developmental Disorders. 2008;38:127–37. doi: 10.1007/s10803-007-0370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CJ, Moana-Filho EJ, Guest S, Nebel MB, Weisner J, Baranek GT, Essick GK. Perceptual and neural response to affective tactile texture stimulation in adults with autism spectrum disorders. Autism Research. 2012;5:231–44. doi: 10.1002/aur.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig KD, Prkachin KM. Nonverbal measures of pain. In: Melzac R, editor. Pain measurement and assessment. New York: Raven Press; 1983. pp. 173–182. [Google Scholar]
- Essick GK, James A, McGlone FP. Psychophysical assessment of the affective components of non-painful touch. Neuroreport. 1999;10(10):2083–7. doi: 10.1097/00001756-199907130-00017. [DOI] [PubMed] [Google Scholar]
- Essick GK, McGlone F, Dancer C, Fabricant D, Ragin Y, Phillips N, Jones T, Guest S. Quantitative assessment of pleasant touch. Neuroscience and Biobehavioral Reviews. 2010;34:192–203. doi: 10.1016/j.neubiorev.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Field T. Touch. MIT Press; Cambridge, MA: 2001. 2001. [Google Scholar]
- Foss-Feig JH, Heacock J, Cascio CJ. Tactile responsiveness patterns and their association with core features in autism spectrum disorders. Research in Autism Spectrum Disorders. 2012;6:337–44. doi: 10.1016/j.rasd.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garra G, Singer AJ, Taira BR, Chohan J, Cardoz H, Chisena E, Thode HC. Validation of the Wong-Baker FACES pain rating scales in pediatric emergency department patients. Academy of Emergency Medicine. 2010;17:50–4. doi: 10.1111/j.1553-2712.2009.00620.x. [DOI] [PubMed] [Google Scholar]
- Guest S, Dessierier JM, Mehrabyan A, McGlone F, Essick G, Gescheider G, Fontana A, Xiong R, Ackerly R, Blot K. The development and validation of sensory and emotional scales of touch perception. Attention Perception and Psychophysics. 2011;73:531–50. doi: 10.3758/s13414-010-0037-y. [DOI] [PubMed] [Google Scholar]
- Harlow HF, Harlow MK. The effect of rearing conditions on behavior. Bulletin of the Menninger Clinic. 1962;26:213–224. [PubMed] [Google Scholar]
- Hertenstein MJ. Touch: Its communicative functions in infancy. Human Development. 2002;45:70–94. [Google Scholar]
- Hertenstein MJ, Verkamp JM, Kerestes AM, Homes RM. The communicative function of touch in humans, nonhuman primates, and rats: a review and synthesis of the empirical research. Genetic, Social and General Psychology Monographs. 2006;132:5–94. doi: 10.3200/mono.132.1.5-94. [DOI] [PubMed] [Google Scholar]
- Jones R, Quigney C, Huws J. First-hand accounts of sensory perceptual experiences in autism: a qualitative analysis. J Intellectual and Developmental Disability. 2003;28:112–121. [Google Scholar]
- Keck JF, Gerkensmeyer JE, Joyce BA, Schade JG. Reliability and validity of the faces and word descriptor scales to measure procedural pain. Journal of Pediatric Nursing. 1996;11:368–74. doi: 10.1016/S0882-5963(96)80081-9. [DOI] [PubMed] [Google Scholar]
- Kientz MA, Dunn W. A comparison of the performance of children with and without autism on the Sensory Profile. American Journal of Occupational Therapy. 1997;51:530–537. doi: 10.5014/ajot.51.7.530. [DOI] [PubMed] [Google Scholar]
- Leekam SR, Nieto C, Libby SJ, Wing L, Gould J. Describing the sensory abnormalities of children and adults with autism. Journal of Autism and Developmental Disorders. 2007;37:894–910. doi: 10.1007/s10803-006-0218-7. [DOI] [PubMed] [Google Scholar]
- LeCouteur A, Lord C, Rutter M. Autism Diagnostic Interview – Revised (ADI-R) Los Angeles, CA: Western Psychological Corporation; 2003. [Google Scholar]
- Little LM, Freuler AC, Houser MB, Guckian L, Carbine K, David FJ, Baranek GT. Psychometric validation of the Sensory Experiences Questionnaire. American Journal of Occupational Therapy. 2011;65:207–210. doi: 10.5014/ajot.2011.000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nature Neuroscience. 2009;12:547–8. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Dilavore P, Risi S. The Autism Diagnostic Observation Schedule (ADOS) Los Angeles, CA: Western Psychological Corporation; 1999. [Google Scholar]
- Main M, Stadtman J. Infant response to rejection of physical contact by the mother: Aggression, avoidance, and conflict. Journal of the American Academy of Child Psychiatry. 1981;20:292–307. doi: 10.1016/s0002-7138(09)60990-0. 1981. [DOI] [PubMed] [Google Scholar]
- McCullagh P. Regression models for ordinal data. Journal of the Royal Statistical Society B. 1980;42:109–142. [Google Scholar]
- McGlone F, Vallbo AB, Olausson H, Loken L, Wessberg J. Discriminative touch and emotional touch. Canadian Journal of Experimental Psychology. 2007;61:173–83. doi: 10.1037/cjep2007019. [DOI] [PubMed] [Google Scholar]
- Montagu A. Touching: The human significance of the skin. Perennial Library; New York: 1986. [Google Scholar]
- Mottron L, Dawson M, Soulieres I, Hubert B, Burack J. Enhanced perceptual, functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord. 2006;36:27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Muir DW. Adult communications with infants through touch: The forgotten sense. Human Development. 2002;45:95–99. [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning (AGS Edition) Los Angeles, CA: Western Psychological Services; 1995. [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, Ekholm S, Strigo I, Worsley K, Vallbo AB, Bushnell MC. Unmyelinated tactile afferents signal touch and project to insular cortex. Nature Neuroscience. 2002;5(9):900–4. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Robertson AE, Simmons DR. The relationship between sensory sensitivity and autistic traits in the general population. Journal of Autism and Developmental Disorders. 2012 Jul 26; doi: 10.1007/s10803-012-1608-7. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Ozonoff S. Annotation: what do we know about sensory dysfunction in autism? A critical review of the empirical evidence. J Child Psychol Psychiatry. 2005;46(12):1255–68. doi: 10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- Roid GH. Stanford-Binet Intelligence Scales, Fifth Edition (SB5) Itasca, IL: Riverside Publishing; 2003. [Google Scholar]
- Schopler E, Reichler RJ, Renner BR. The Childhood Autism Rating Scale. Los Angeles, CA: Western Psychological Services; 1988. [Google Scholar]
- Sewards TV, Sewards M. Separate, parallel sensory and hedonic pathways in the mammalian somatosensory system. Brain Research Bulletin. 2002;58:243–60. doi: 10.1016/s0361-9230(02)00783-9. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales. Circle Pines, MN: AGS; 1984. [Google Scholar]
- Vallbo AB, Olausson H, Wessberg J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. Journal of Neurophysiology. 1999;81(6):2753–63. doi: 10.1152/jn.1999.81.6.2753. [DOI] [PubMed] [Google Scholar]
- Walker SH, Duncan DB. Estimation of the probability of an event as a function of several independent variables. Biometrika. 1967;54:167–79. [PubMed] [Google Scholar]
- Watson L, Patten E, Baranek G, Poe M, Boyd B, Freuler A, Lorenzi J. Differential associations between sensory response patterns and language, social, and communication measures in children with autism or other developmental disorders. Journal of Speech, Language & Hearing Research. 2011;54:1562–1576. doi: 10.1044/1092-4388(2011/10-0029). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SJ, Wilson P, Hertenstein MJ, Campos RG. The tactile context of a mother's caregiving” implications for attachment of low birth weight infants. Infant Behavior and Development. 2000;23:91–111. [Google Scholar]
- Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatric Nursing. 1988;14:9–17. [PubMed] [Google Scholar]


