Abstract
Objective. Hypertension is one of the most common cardiovascular disorders with high mortality. Here we explored the antihypertension effects of Huanglian Jiedu Decoction (HJD) on thoracic aorta gene expression in spontaneous hypertensive rats. Methods. A rat model of spontaneous hypertension was used. The gene change profile of thoracic aorta after JHD treatment was assessed by GeneChip(GC) analysis using the Agilent Whole Rat Genome Oligo Microarray. Results. Hypertension induced 441 genes upregulated and 417 genes downregulated compared with the normal control group. Treatment of HJD resulted in 76 genes downregulated and 20 genes upregulated. GC data analysis showed that the majority of change genes were involved in immune system process, developmental process, and cell death. Conclusion. Hypertension altered expression of many genes that regulate various biological functions. HJD significantly reduced hypertension and altered the gene expression profiles of SHR rats. These changing genes were involved in many cellular functions such as regulating smooth muscle contraction, Ca(2+) homeostasis, and NO pathway. This study provides the potential novel insights into hypertension and antihypertension effects of HJD.
1. Introduction
Essential hypertension (primary hypertension or idiopathic hypertension) is the most common type of hypertension, affecting 95% of hypertensive patients. The total number of adults with hypertension in 2000 was 972 million, and the number of adults with hypertension in 2025 was predicted to increase by about 60% to a total of 1.56 billion (29.2% of the world's adult population) [1]. The etiology of primary hypertension is not fully understood. Treatment of hypertension is facing the challenge because effective therapies of hypertension are limited by availability, cost, and adverse effects. Chinese traditional medicine provides a potential option to overcome current challenge of antihypertension therapy. Chinese medicine, a system of ancient medical practice that differs in substance, methodology, and philosophy to modern medicine, plays key role in health maintenance and has become more frequently used.
CM has been used to treat symptoms related to hypertension for more than 2500 years. JHD is one of the effective formulas to combat hypertension in clinic. HJD is an ancient Chinese prescription, first reported in the text Zh u Hîu Bèi Jí Fāng (Emergency Standby Remedies) by Gě Hïng (281–341 CE, Jìn dynasty). This formula is commonly used for all types of Fire Toxin obstructing the three burners with high fever, irritability, and dry mouth and throat. Pharmacologic Researches of this formula show that it has antisepticised dephlogisticate and enhances the immune system functions. Our previous studies show that HJD has antihypertension effects via regulating the inflammatory factors and altering NO and SOD expression [2]. Here, this report is to study the pathogenic mechanism of hypertension and its effects on thoracic aorta gene expression.
2. Materials and Methods
2.1. Animals Models and Treatment
The cohorts of 6-week-old male Spontaneous Hypertensive Rats (SHRs) were randomly placed into HJD group and SHR model group. There are eight rats in each group. All rats were from Beijing Vital River Laboratory Animal Technology Co., Ltd., license Number is SCXK2006-0009, SPF, weight 160 ± 10 g. Eight 6-week-old male normotensive Wistar-Kyoto(WKY) rats were used as the normal control group that were from Experimental Animal Center of Guangxi Medical University, license Number is SCXK2006-0003, SPF, weight 160 ± 10 g.
The animals were housed in a room with 20–24°C 30–40% humidity. Rats were ad lib to food and water. This study was performed following the Guide for the Care and Use of Laboratory Animals of the P. R. China. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Guangxi University of Chinese Medicine.
HJD was consisted of Rhizoma Coptidis, Phellodendron amurense Rupr, Radix Scutellariae, and Gardenia jasminoides Ellisin the ratio of 3 : 2 : 2 : 3. All the herbs were provided by Ruikang Affiliated Hospital to Guangxi University of Chinese Medicine who bought from Yulin Herb Market in unify. The purity of each component was determined to be above 98% by high-performance liquid chromatography (HPLC) analysis. HPLC-grade reagents, methanol, acetonitrile, and water were obtained from J. T. Baker (Phillipsburg, NJ, USA). These four herbs were dried in 24 h and grinded into coarse powder. The herbs were cooked into concentrated juice (1 mL juice = 2.2 g raw herb). The rats were given HJD juice at 5.4 g/(kg·d) by gavage. The control group was given 0.9% saline with the same volume. All the treatments were administrated by gavage for 6 successive weeks.
2.2. Measurement of Blood Pressure
Noninvasive BP of rats was measured using the Nexfin monitor (BMEYE B.V, Amsterdam, and The Netherlands) with the latest implementation of the caudal artery method. Rats were measured for BP at day 0, 7, 14, 28, and 42 after HJD treatment each rat was measured 3 times to obtain average BP value.
2.3. RNA Isolation and Microarray Experiment
After the last HJD administration, all rats were sacrificed and then the thoracic aortas were quickly dissected and stored in the freezer tube in −80°C for RNA extraction. Rat tissues were homogenized in Trizol (Invitrogen, Carlsbad, CA), and the total RNA was extracted using chloroform and isopropyl alcohol and purified using the RNeasy Mini Kit and RNase-free DNase Set (Qiagen, Valencia, CA) according to the manufacturers' protocols. The quality of the extracted total RNA was measured using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and the Nano-Chip-protocol before microarray experiments. Gene Chip analysis was performed using the Agilent Whole Rat Genome Oligo Microarray. Labeling and hybridization were performed at Shanghai Biotechnology Corporation.
2.4. Data Analysis
Microarray data were analyzed using the SBC Analysis system (: http://sas.ebioservice.com/index.jsp).
Gene expression profiles of SHR were compared to those from WKY or HJD treatment group. The changing was selected by the criteria: P < 0.05 and Fold Changes ≥2.
3. Results
3.1. HJD Reduced Blood Pressure of SHR Rats
The rat in SHR group had significantly higher blood pressures compared with normal control group before HJD treatment (P < 0.05). After one week administration, the BP of HJD group began to decrease than SHR group. After 6 weeks of treatment, the rats in HJD group had almost similar blood pressure to the rats in control group (Figure 1).
Figure 1.
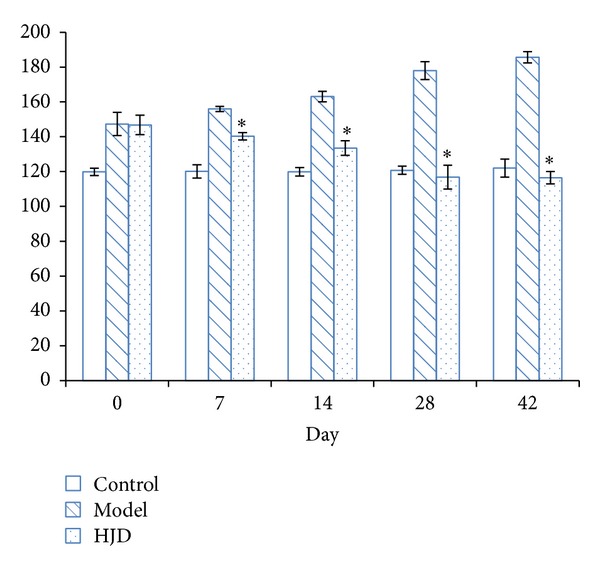
Effects of HJD on blood pressure in rats. BP of rat was measured with mmHg unit. There were 8 rats in each group. *P < 0.05 versus SHR group.
3.2. Hypertension and HJD Treatment Caused Gene Expression Changes
Volcano plot that was drawn by P value and Fold Change checked by t-test showed the significant difference of sample data between chipset groups. (Figures 2 and 3 described by Gene-Spring 11.0: X-axis for Log2 (Fold Change), Y-axis for—Log10 (P value); X-axis parallel line: P = 0.05, Y-axis parallel line: Fold Change = 2.0; Red Zone: P < 0.05; and Fold Change ≥2.0).
Figure 2.
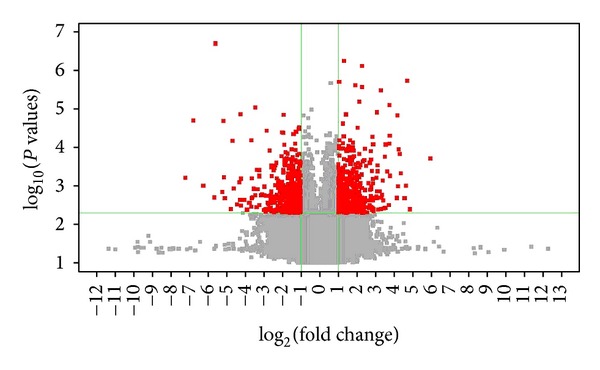
Hypertension induced gene changes compared with normal control rats. Note: model = spontaneous hypertension rats (SHR) model group, and control = normotensive Wistar-Kyoto rats control group.
Figure 3.
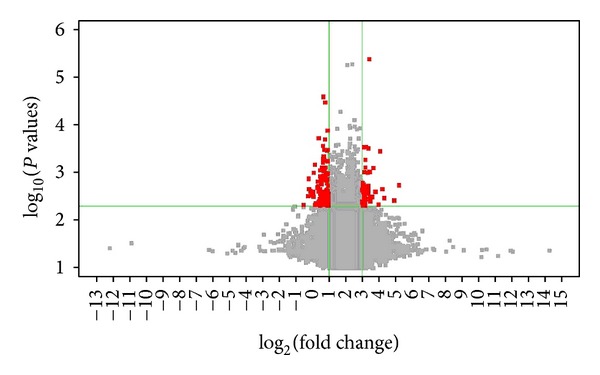
HJD treatment caused gene changes compared with untreated SHR rats versus SHR. Note: model = spontaneous hypertension rats (SHR) model group; medication = HJD treatment group.
Gene microarray analysis showed that there were 858 genes that alter in SHR group compared with normal control groups (Figure 2). There were 417 downregulated (Table 1) and there were 441 upregulated (Table 2).
Table 1.
Downregulated genes in hypertension rats.
| Gene | Abbr. | Gene Id | Chro. | FC | P |
|---|---|---|---|---|---|
| DRABUB01 rat DRG library Rattus norvegicus cDNA clone DRABUB01 5′ | — | NA | 9 | 0.097 | 3.86E − 03 |
| Similar to RIKEN cDNA 1700001F09 (LOC289957) | — | NA | 15 | 0.092 | 9.47E − 05 |
| Mitochondrial ribosomal protein S2 | Mrps2 | 362094 | 3 | 0.081 | 6.49E − 03 |
| Q99LR9_MOUSE (Q99LR9) Riok3 protein (fragment), partial (13%) | — | NA | 18 | 0.079 | 6.79E − 04 |
| AGENCOURT_26625193 NIH_MGC_253 Rattus norvegicus cDNA clone IMAGE:7304073 5′ | — | NA | 5 | 0.054 | 6.58E − 03 |
| Similar to 60S ribosomal protein L19 (LOC316856) | — | NA | Un | 0.053 | 1.40E − 04 |
| Solute carrier family 4 (anion exchanger), member 1 | Slc4a1 | 24779 | 10 | 0.051 | 8.24E − 03 |
| Cell division cycle 25 homolog A (S. pombe) | Cdc25a | 171102 | 8 | 0.039 | 6.88E − 04 |
| Synuclein, alpha (non-A4 component of amyloid precursor) | Snca | 29219 | 4 | 0.029 | 6.00E − 03 |
| Unknown | — | NA | 7 | 0.028 | 2.12E − 04 |
| Coproporphyrinogen oxidase | Cpox | 304024 | 11 | 0.021 | 2.11E − 06 |
| AGENCOURT_70342463 NIH_MGC_368 Rattus norvegicus cDNA clone IMAGE:8376762 5′ | — | NA | 1 | 0.013 | 9.98E − 03 |
| Q8FIR6_ECOL6 (Q8FIR6) cytochrome b561 homolog 2, partial (5%) | — | NA | 9 | 0.009 | 2.05E − 04 |
| UI-R-C3-sf-g-04-0-UI | — | NA | 1 | 0.007 | 6.28E − 03 |
Note. Abbr.: gene abbreviation, Chro.: chromosome, Gene Id represents the identity (ID) of the gene in GenBank; FC = SHR model group gene expression level/normotensive WKY control group expression level. “—/NA/Un” indicates unknown.
Table 2.
Upregulated genes (model versus control).
| Gene | Abbr. | Gene Id | Chro. | FC | P |
|---|---|---|---|---|---|
| Bone gamma-carboxyglutamate (gla) protein | Bglap | 25295 | 2 | 61.402 | 1.96E − 03 |
| Uncharacterized protein | — | NA | 2 | 25.856 | 1.96E − 05 |
| Unknown | — | NA | 13 | 24.949 | 9.99E − 03 |
| Cellular retinoic acid binding protein 2 | Crabp2 | 29563 | 2 | 20.657 | 4.73E − 03 |
| Major histocompatibility complex, assembled from 40 BACs, strain Brown Norway (BN/ssNHsd), RT1n haplotype; segment 2/11 | — | NA | 20 | 19.648 | 1.52E − 03 |
| V-set domain containing T cell activation inhibitor 1 | Vtcn1 | 295322 | 2 | 18.951 | 1.15E − 03 |
| Interferon-induced protein with tetratricopeptide repeats 1 | Ifit1 | 56824 | 1 | 17.991 | 1.51E − 04 |
| Unknown | — | NA | 10 | 13.875 | 5.54E − 03 |
| Uncharacterized protein | — | NA | 1 | 13.621 | 5.06E − 04 |
| Similar to SMAD-interacting zinc finger protein 2 | LOC679126 | 679126 | 13.572 | 8.21E − 05 | |
| Transient receptor potential cation channel, subfamily C, member 4 | Trpc4 | 84494 | 2 | 13.374 | 8.20E − 04 |
| Q3X0T1_9ACTN (Q3X0T1) 60 kDa innermembrane protein, partial (6%) | — | NA | 19 | 12.417 | 4.35E − 03 |
| CJ032_MOUSE (Q9CRC6) protein C10orf32 homolog, partial (15%) | — | NA | 1 | 11.726 | 8.63E − 04 |
| TL0ACA45YL24 | — | NA | X | 11.407 | 1.80E − 03 |
Note. Abbr.: gene abbreviation, Chro.: chromosome, Gene Id represents the identity (ID) of the gene in GenBank; FC = SHR model group gene expression level/normotensive WKY control group expression level. “—/NA/Un” indicates unknown.
JHD treatment altered 96 gene expressions pared with untreated SHR group; P < 0.05 and Fold Change ≥ 2.0 were identified between the HJD treatment group and the SHR model groups. Of these, 76 genes were downregulated and 20 genes were upregulated (Table 3).
Table 3.
The upregulated and downregulated genes after the treatment of HLJDD.
| Gene | Abbr. | Gene Id | Chro. | FC | P |
|---|---|---|---|---|---|
| Solute carrier family 4 (anion exchanger), member 1 | Slc4a1 | 24779 | 10 | 3.871 | 6.74E − 03 |
| Protein phosphatase 1, regulatory subunit 3D | Ppp1r3d | 689995 | 3 | 2.566 | 2.78E − 03 |
| Ribonucleotide reductase M2 | Rrm2 | 362720 | 6 | 2.551 | 6.30E − 03 |
| pim-1 oncogene | Pim1 | 24649 | 20 | 2.529 | 5.12E − 03 |
| Spectrin, beta, erythrocytic | Sptb | 314251 | 6 | 2.470 | 4.66E − 03 |
| Transmembrane and coiled-coil domain family 2 | Tmcc2 | 305095 | 13 | 2.444 | 3.38E − 03 |
| Unknown | — | NA | 3 | 2.264 | 7.87E − 03 |
| UI-R-C4-alc-g-09-0-UI | — | NA | Un | 0.235 | 3.60E − 03 |
| UI-R-BJ2-bql-b-07-0-UI | — | NA | 13 | 0.385 | 3.14E − 03 |
| Potassium voltage-gated channel, subfamily H (eag-related), member 1 | Kcnh1 | 65198 | 13 | 0.393 | 9.98E − 03 |
| Nuclear receptor subfamily 4 group A member 3 | Nr4a3 | 58853 | 5 | 0.456 | 9.87E − 03 |
Note. Abbr.: gene abbreviation, Chro.: chromosome, Gene Id represents the identity (ID) of the gene in GenBank; FC = HLJDT treatment group/SHR model group gene expression level. “—/NA/Un” indicates unknown.
3.3. HJD Induced Gene Changes and Their Biological Consequences
The genes that were altered after HJD treatment mainly belong to 12 classifications (Table 4) including genes that regulate immune system, cellular functions, and metabolic and developmental functions. There were 33 genes in the category of immune system development, leukocyte activation, regulation of immune system process, cell activation and proliferation, regulation of immune system process, positive regulation of biological process, and so forth (P < 0.05) (Table 5).
Table 4.
Functional catalogers of hypertension-induced gene change profiles.
| GO Id | Term | Hit genes | P |
|---|---|---|---|
| GO:0032501 | Multicellular organismal process | ||
| GO:0051239 | Regulation of multicellular organismal process | 56 | 0.0033 |
| GO:0048771 | Tissue remodeling | 14 | 0.0068 |
| GO:0001816 | Cytokine production | 15 | 0.0243 |
| GO:0003008 | System process | 77 | 0.0485 |
| GO:0032502 | Developmental process | ||
| GO:0001503 | Ossification | 18 | 0.0005 |
| GO:0050793 | Regulation of developmental process | 66 | 0.0045 |
| GO:0051704 | Multiorganism process | ||
| GO:0051707 | Response to other organism | 23 | 0.0026 |
| GO:0044419 | Interspecies interaction between organisms | 7 | 0.0059 |
| GO:0050896 | Response to stimulus | ||
| GO:0009607 | Response to biotic stimulus | 24 | 0.0074 |
| GO:0042221 | Response to chemical stimulus | 97 | 0.0214 |
| GO:0010926 | Anatomical structure formation | ||
| GO:0022607 | Cellular component assembly | 41 | 0.042 |
| GO:0050789 | Regulation of biological process | ||
| GO:0051239 | Regulation of multicellular organismal process | 56 | 0.0033 |
| GO:0050793 | Regulation of developmental process | 66 | 0.0045 |
| GO:0048518 | Positive regulation of biological process | 86 | 0.0361 |
Table 5.
Functional category of HJD-induced gene change profiles.
| GO Id | Term | Hit genes | P |
|---|---|---|---|
| GO:0002376 | Immune system process | ||
| GO:0002520 | Immune system development | 10 | 0.0001 |
| GO:0045321 | Leukocyte activation | 12 | 0.0001 |
| GO:0002682 | Regulation of immune system process | 12 | 0.0001 |
| GO:0006955 | Immune response | 10 | 0.0003 |
| GO:0002684 | Positive regulation of immune system process | 7 | 0.0006 |
| GO:0045058 | T cell selection | 2 | 0.0046 |
| GO:0002253 | Activation of immune response | 3 | 0.026 |
| GO:0009987 | Cellular process | ||
| GO:0001775 | Cell activation | 13 | 0.0001 |
| GO:0008283 | Cell proliferation | 15 | 0.0001 |
| GO:0048522 | Positive regulation of cellular process | 22 | 0.0002 |
| GO:0008219 | Cell death | 13 | 0.0016 |
| GO:0048523 | Negative regulation of cellular process | 16 | 0.0086 |
| GO:0016043 | Cellular component organization | 19 | 0.0181 |
| GO:0007049 | Cell cycle | 7 | 0.0453 |
| GO:0008152 | Metabolic process | ||
| GO:0009058 | Biosynthetic process | 28 | 0.0005 |
| GO:0009893 | Positive regulation of metabolic process | 11 | 0.0361 |
| GO:0006807 | Nitrogen compound metabolic process | 5 | 0.0413 |
| GO:0019748 | Secondary metabolic process | 2 | 0.0423 |
| GO:0032502 | Developmental process | ||
| GO:0050793 | Regulation of developmental process | 17 | 0.0008 |
| GO:0051093 | Negative regulation of developmental process | 10 | 0.0026 |
| GO:0048856 | Anatomical structure development | 21 | 0.0086 |
| GO:0009790 | Embryonic development | 8 | 0.0154 |
| GO:0007275 | Multicellular organismal development | 20 | 0.0249 |
| GO:0001503 | Ossification | 4 | 0.0251 |
| GO:0009653 | Anatomical structure morphogenesis | 12 | 0.0409 |
| GO:0016265 | Death | ||
| GO:0008219 | Cell death | 13 | 0.0016 |
| GO:0032501 | Multicellular organismal process | ||
| GO:0001816 | Cytokine production | 7 | 0.0003 |
| GO:0051240 | Positive regulation of multicellular organismal process | 7 | 0.0017 |
| GO:0051239 | Regulation of multicellular organismal process | 14 | 0.0021 |
| GO:0007275 | Multicellular organismal development | 20 | 0.0249 |
| GO:0050896 | Response to stimulus | ||
| GO:0006955 | Immune response | 10 | 0.0003 |
| GO:0009605 | Response to external stimulus | 11 | 0.0084 |
| GO:0009607 | Response to biotic stimulus | 6 | 0.0201 |
| GO:0048584 | Positive regulation of response to stimulus | 5 | 0.0209 |
| GO:0048585 | Negative regulation of response to stimulus | 3 | 0.0445 |
| GO:0050789 | Regulation of biological process | ||
| GO:0002682 | Regulation of immune system process | 12 | 0.0001 |
| GO:0048518 | Positive regulation of biological process | 24 | 0.0001 |
| GO:0050793 | Regulation of developmental process | 17 | 0.0008 |
| GO:0051239 | Regulation of multicellular organismal process | 14 | 0.0021 |
| GO:0048519 | Negative regulation of biological process | 18 | 0.0045 |
| GO:0032844 | Regulation of homeostatic process | 3 | 0.045 |
| GO:0048518 | Positive regulation of biological process | 24 | 0.0001 |
| GO:0048519 | Negative regulation of biological process | 18 | 0.0045 |
| GO:0010926 | Anatomical structure formation | 12 | 0.0254 |
Further functional pathway analysis showed that hypertension altered genes were involved in 22 KEGG pathways (Table 6), while HJD altered genes were mainly related to 10 KEGG pathways (P < 0.05) (Table 7).
Table 6.
Hypertension altered genes in 22 KEGG pathways.
| KEGG pathway | Hit genes | P |
|---|---|---|
| Olfactory transduction | 29 | 0 |
| Neuroactive ligand-receptor interaction | 13 | 0.0002 |
| Cytokine-cytokine receptor interaction | 10 | 0.0009 |
| Focal adhesion | 9 | 0.0009 |
| Tight junction | 6 | 0.0061 |
| RIG-I-like receptor signaling pathway | 4 | 0.0081 |
| Ether lipid metabolism | 3 | 0.0083 |
| Calcium signaling pathway | 7 | 0.0086 |
| Leishmania infection | 4 | 0.0089 |
| Amyotrophic lateral sclerosis (ALS) | 4 | 0.0113 |
| Arrhythmogenic right ventricular cardiomyopathy (ARVC) | 4 | 0.014 |
| ABC transporters | 3 | 0.0171 |
| Aldosterone-regulated sodium reabsorption | 3 | 0.0213 |
| Hypertrophic cardiomyopathy (HCM) | 4 | 0.0231 |
| Dilated cardiomyopathy | 4 | 0.0247 |
| Glycosphingolipid biosynthesis—ganglio series | 2 | 0.0265 |
| Glutathione metabolism | 3 | 0.0284 |
| Apoptosis | 4 | 0.032 |
| Wnt signaling pathway | 5 | 0.0324 |
| Natural killer cell mediated cytotoxicity | 5 | 0.0339 |
| Maturity onset diabetes of the young | 2 | 0.0415 |
| MAPK signaling pathway | 7 | 0.047 |
Table 7.
HJD altered genes in 10 KEGG pathways.
| KEGG pathway | Hit genes | P |
|---|---|---|
| Hematopoietic cell lineage | 3 | 0.0004 |
| Metabolic pathways | 8 | 0.0016 |
| Natural killer cell mediated cytotoxicity | 3 | 0.0027 |
| NOD-like receptor signaling pathway | 2 | 0.0066 |
| Cytokine-cytokine receptor interaction | 3 | 0.0105 |
| Pyrimidine metabolism | 2 | 0.0148 |
| T cell receptor signaling pathway | 2 | 0.0192 |
| Axon guidance | 2 | 0.0254 |
| Jak-STAT signaling pathway | 2 | 0.0308 |
| Purine metabolism | 2 | 0.0379 |
4. Discussion and Conclusions
prehypertension criterion is systolic pressure 120~139 mmHg (1 mmHg = 0.133 kPa) and/or diastolic pressure 80~89 mmHg. As the important pathogenic risk factor of many cardiovascular and cerebrovascular diseases, prehypertension can affect the structure and function of heart, brain, and liver compared to the ideal blood pressure. During prehypertension, the elasticity of large artery trunks was already obviously damaged, and the relaxation function of left ventricle was slightly damaged, which are the damage symbol of target organs in hypertension [3]. Our previous data showed that SHR arterial elasticity and endothelial functions in SHR rats were impaired in early stage [3]. And the gene array studies coincidences showed that multiple genes in aorta thoracic in young SHR were changed in transcription level compared with normotensive WKY. In particular, expression genes related to smooth muscles shrink or cytoskeletons were significantly increased or decreased. These genes were Chrm3, MRPS2, TRPC4, P2rx4, Slc8a3, Htr5a, Chp, and so forth. The muscarinic cholinergic receptor 3, also known as Chrm3 (FC = 4.343), controls smooth muscle contraction and its stimulation causes secretion of glandular tissue [4]. MRPS2 (FC = 0.081) encodes mitochondrial ribosomal protein S2 [5, 6]. Genes related to Calcium signaling pathway, like TRPC4(FC = 13.374), P2rx2 (FC = 4.075), and P2rx4 (FC = 3.329) expression, increased, whereas Slc8a3 (FC = 0.313), Htr5a (FC = 0.332), and Chp (FC = 0.398) expression decreased, which will cause the imbalance of Ca(2+) in endothelial cells. TRPC4 is well recognized as a prominent Cation channel in the vascular endothelium, which suggested to serve stimulated Ca(2+) entry in a specific endothelial state during the transition from a proliferating to a quiescent phenotype. Thus, TRPC4 may adopt divergent, as yet unappreciated functions in endothelial Ca(2+) homeostasis and emerges as a potential key player in endothelial phenotype switching and tuning of cellular growth factor signaling [7]. P2X receptors are ATP activated channels that allow the passage of ions across cell membranes and participate in regulating renal microvascular function, autoregulation, and hypertension-associated renal vascular injury [8].
The ATP-gated P2X4 ion channel, expressed on endothelial cells and encoded by P2rx4 gene, is crucial to flow-sensitive mechanisms that regulate blood pressure and vascular remodeling. P2rx4 (−/−) mice do not have normal endothelial cell responses to flow, such as influx of Ca(2+) and subsequent production of the potent vasodilator nitric oxide (NO). Additionally, vessel dilation induced by acute increases in blood flow is markedly suppressed in P2rx4 (−/−) mice. Furthermore, P2rx4 (−/−) mice have higher blood pressure and excrete smaller amounts of NO products in their urine than wild-type mice do. Moreover, no adaptive vascular remodeling, that is, a decrease in vessel size in response to a chronic decrease in blood flow, was observed in P2rx4 (−/−) mice [9]. SLC8A3, solute carrier family 8 (sodium/calcium exchanger) members 3, encodes a member of the sodium/calcium exchanger integral membrane protein family. Three mammalian isoforms in family 8 (SLC8A1, SLC8A2, and SLC8A3) and their splice variants are expressed in a tissue-specific manner to mediate Ca(2+) fluxes across the cell membrane and, thus, significantly contribute to maintain Ca(2+) homeostasis in a wide variety of cell types [10]. The gene described in this record is a member of 5-hydroxytryptamine receptor family and encodes a multipass membrane protein that functions as a receptor for 5-hydroxytryptamine and couples to G proteins, negatively influencing cAMP levels via GI and Go [11]. This protein has been shown to function in part through the regulation of intracellular Ca(2+) mobilization [12]. Calcium binding protein p22 (Chp), which belongs to the EF-hand superfamily of Ca(2+)-binding proteins, may act by transducing cellular Ca(2+) signals to downstream effectors in many cell types [13]. It is also known to be an endogenous inhibitor of calcineurin activity [14] and thus inactivates the T cells of the immune system. Prehypertension showed the symptoms of dizziness, headache, anxiety, and irritability [15]. 300 cases of prehypertension were investigated [16] on TCM syndrome on the criterion of Subhealth of TCM clinical guidelines which was announced in 2006. The results showed that most of the cases were belonging to patterns of depression of the liver generating pathogenic fire, stagnation of liver qi and spleen deficiency, and interior disturbance of phlegm heat.
HJD were first reported in Gehong' book of Handbook of Prescription for Emergency, but it did not give the name of this prescription; however, the first name SLC8A3, solute carrier family 8 (sodium/calcium exchanger) members 3, encodes a member of the sodium/calcium exchanger integral membrane protein family. Three mammalian isoforms in family 8 (SLC8A1, SLC8A2, and SLC8A3) and their splice variants are expressed in a tissue-specific manner to mediate Ca(2+) fluxes across the cell membrane and, thus, significantly contribute to maintain Ca(2+) homeostasis in a wide variety of cell types [10]. The gene described in this record is a member of 5-hydroxytryptamine receptor family and encodes a multipass membrane protein that functions as a receptor for 5-hydroxytryptamine and couples to G proteins, negatively influencing cAMP levels via GI and Go [11]. This protein has been shown to function in part through the regulation of intracellular Ca(2+) mobilization [12]. Calcium binding protein p22 (Chp), which belongs to the EF-hand superfamily of Ca(2+) binding proteins, may act by transducing cellular Ca(2+) signals to downstream effectors in many cell types [13]. It is also known to be an endogenous inhibitor of calcineurin activity [14] and thus inactivates the T cells of the immune system. Prehypertension showed the symptoms of dizziness, headache, anxiety, and irritability [15]. 300 cases of prehypertension were investigated [16] on TCM syndrome on the criterion of Subhealth of TCM clinical guidelines which was announced in 2006. The results showed that most of the cases were belonging to patterns of depression of the liver generating pathogenic fire, stagnation of liver qi and spleen deficiency, and interior disturbance of phlegm heat.
HJD were first reported in Gehong' book of Handbook of Prescription for Emergency, but it did not give the name of this prescription; however, the first name occurred in Wangtao' book of Waitaimiyao. This prescription was made up of Rhizoma Coptidis, Phellodendron amurense Rupr, Radix Scutellariae, and Gardenia jasminoides Ellis in proportion. Rhizoma Coptidis exerts as the sovereign to purge heart fire and middle energizers fire; Phellodendron amurense Rupr works as the minister to clear away lung heat and the upper energizers fire. Radix Scutellariae clears away the lower energizers fire and Gardenia jasminoides Ellis clears away the triple energizers fire. They work together to remove the toxin. HJD have played more and more important role in cardiovascular diseases that have already used HJD in treatment of hypertension in clinic for many years.
Hypertension induces endothelial dysfunction resulting in impairment of endothelium-dependent vasodilatation and widespread abnormalities in endothelial integrity and homeostasis [17]. Endothelial dysfunction exhibits several pathological conditions, including altered anticoagulant and anti-inflammatory properties of the endothelium, impaired modulation of vascular growth, and dysregulation of vascular remodeling. The major cause of impairment of endothelium-dependent vasorelaxation is loss of nitric oxide (NO) bioactivity in the vessel wall [18].
Our previous studies showed that' spontaneous hypertension rats' blood pressure increases at six-week-old age, and the tail-arterial-systolic-pressure increase at 12-weeksof age. Hypertension decreases plasma NO and SOD, and increase of MDA and Von Wille brand factor (vWF) at the 12 weeks of age. HJD treatment significantly reversed hypertension-induced NO and SOD [2].
HJD treatment altered many genes related to cell response processes, hyperplasia, and immune response, such as IL1b(FC = 2.519), Klrk1(FC = 2.001), Snca(FC = 2.665), and Gch1(FC = 2.164). It has been reported that interleukin (IL)-1 beta induces the synthesis of both nitric oxide (NO) and prostaglandin (PG)E2 in cultures of dispersed ovarian cells and exerts cytotoxic effects on these cells [19, 20]. Furthermore, IL-1 beta can interact with NO and PGE2 during steroid genesis [21]. Klrk1 is involved in positive regulation of nitrogen compound metabolic process (Gene Ontology: 0051173). Alpha-synuclein (ASN), encoded by Snca gene, is a small soluble acidic protein and plays an important role in the regulation of synaptic nerve terminal function. It has been shown that ASN stimulated NOS activity and NO release in the rat brain [22]. GTP cyclohydrolase 1 (GCH1) is the first and rate-limiting enzyme in the synthesis of tetrahydrobiopterin (BH4), an essential cofactor for tyrosine hydroxylase (TH) and NOS in the catecholamine and NO production pathways, respectively, thus playing a crucial role in the sympathoadrenal and endogenous nitro vasodilator systems [23].
According to the GO analyses, compared with normotensive WKY, the biological procedure which SHR differential expression genes attended mainly focused on 6 items of secondary classification, such as multicellular organismal process, developmental process, multiorganism process, response to stimulus, anatomical structure formation, and regulation of biological process, mainly involving 12 items of third classification, for example, ossification, response to other organism, regulation of multicellular organismal process, regulation of developmental process, interspecies interaction between organisms, tissue remodeling, response to biotic stimulus, and so froth (P < 0.05) (Table 5).
HJD can upregulate NO expression in the blood plasma [4]. Here HJD also altered the NO pathway related genes including IL1b, Klrk1, Snca, and Gch1. Taking together, these findings suggest that HJD may reduce hypertension by regulating NO pathways.
In conclusion, HJD can significantly reduce hypertension and alter the gene profiles in aorta thoracic in rats. These results provide the potential gene changes and pathways underlying hypertension and antihypertension effects of HJD in treatment of hypertension.
Acknowledgments
The authors are supported by Chinese Nature Science Foundation Grant (no. 81060294), Talents in Guangxi Colleges and Universities supported plans (J11064), and The key project of Guangxi University of Chinese Medicine Affiliated Ruikang Hospital (RKZ201015).
Conflict of Interests
The authors declare no conflict of interests regarding the publication of this paper.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. The Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Yue h G, Zhang w Z, Meng y Z. Effect of Huanglian Jiedu Decoction on endothelial function in younger spontaneously hypertensive rats. Chinese Journal of Basic Medicine in Traditional Chinese Medicine. 2010;(4):303–305. [Google Scholar]
- 3.Erdogan D, Yildirim I, Ciftci O, et al. Effects of normal blood pressure, prehypertension, and hypertension on coronary microvascular function. Circulation. 2007;115(5):593–599. doi: 10.1161/CIRCULATIONAHA.106.650747. [DOI] [PubMed] [Google Scholar]
- 4.Onodera S, Chiba T, Sugai T, Habano W. A genetic association between β3-aderenoceptor and cholinergic receptor muscarinic 3 polymorphisms in irritable bowel syndrome. Hepato-Gastroenterology. 2011;58(110-111):1474–1478. doi: 10.5754/hge10153. [DOI] [PubMed] [Google Scholar]
- 5.Alim MA, Hossain MS, Arima K, et al. Tubulin seeds α-synuclein fibril formation. Journal of Biological Chemistry. 2002;277(3):2112–2117. doi: 10.1074/jbc.M102981200. [DOI] [PubMed] [Google Scholar]
- 6.Alim MA, Ma Q-L, Takeda K, et al. Demonstration of a role for α-synuclein as a functional microtubule-associated protein. Journal of Alzheimer’s Disease. 2004;6(4):435–442. doi: 10.3233/jad-2004-6412. [DOI] [PubMed] [Google Scholar]
- 7.Graziani A, Poteser M, Heupel W-M, et al. Cell-cell contact formation governs Ca2+ signaling by TRPC4 in the vascular endothelium: evidence for a regulatory TRPC4-β-catenin interaction. Journal of Biological Chemistry. 2010;285(6):4213–4223. doi: 10.1074/jbc.M109.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan Z, Inscho EW. Role of adenosine 5′-triphosphate in regulating renal microvascular function and in hypertension. Hypertension. 2011;58(3):333–340. doi: 10.1161/HYPERTENSIONAHA.110.155952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto K, Sokabe T, Matsumoto T, et al. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nature Medicine. 2006;12(1):133–137. doi: 10.1038/nm1338. [DOI] [PubMed] [Google Scholar]
- 10.Khananshvili D. The SLC8 gene family of sodium-calcium exchangers (NCX) - structure, function, and regulation in health and disease. Molecular Aspects of Medicine. 2013;34(2-3):220–235. doi: 10.1016/j.mam.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Francken BJB, Jurzak M, Vanhauwe JFM, Luyten WHML, Leysen JE. The human 5-ht(5A) receptor couples to G(i)/G(o) proteins and inhibits adenylate cyclase in HEK 293 cells. European Journal of Pharmacology. 1998;361(2-3):299–309. doi: 10.1016/s0014-2999(98)00744-4. [DOI] [PubMed] [Google Scholar]
- 12.Schanen NC, Scherer SW, Tsui L-C, Francke U. Assignment of the 5-hydroxytryptamine (serotonin) receptor 5A gene (HTR5A) to human chromosome band 7q36.1. Cytogenetics and Cell Genetics. 1996;72(2-3):187–188. doi: 10.1159/000134184. [DOI] [PubMed] [Google Scholar]
- 13.Barroso MR, Bernd KK, DeWitt ND, Chang A, Mills K, Sztul ES. A novel Ca2+-binding protein, p22, is required for constitutive membrane traffic. Journal of Biological Chemistry. 1996;271(17):10183–10187. doi: 10.1074/jbc.271.17.10183. [DOI] [PubMed] [Google Scholar]
- 14.Jiménez-Vidal M, Srivastava J, Putney LK, Barber DL. Nuclear-localized calcineurin homologous protein CHP1 interacts with upstream binding factor and inhibits ribosomal RNA synthesis. Journal of Biological Chemistry. 2010;285(47):36260–36266. doi: 10.1074/jbc.M110.165555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong WJ. The application of the classical formula in the treatment of hypertension. The Journal of Traditional Chinese Medicine. 2013;11(20):1836–1839. [Google Scholar]
- 16.Gu H, Chen J, Wang J. The invertigate of the risk factors for the patients with prehypertension and cardiovascular disease. Chinese Orthodox Medicine Journal. 2012;1:33–35. [Google Scholar]
- 17.Brunner H, Cockcroft JR, Deanfield J, et al. Endothelial function and dysfunction. Part II: association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. Journal of Hypertension. 2005;23(2):233–246. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circulation Research. 2000;87(10):840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 19.Ellman C, Corbett JA, Misko TP, McDaniel M, Beckerman KP. Nitric oxide mediates interleukin-1-induced cellular cytotoxicity in the rat ovary: a potential role for nitric oxide in the ovulatory process. Journal of Clinical Investigation. 1993;92(6):3053–3056. doi: 10.1172/JCI116930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ben-Shlomo I, Adashi EY, Payne DW. The morphogenic/cytotoxic and prostaglandin-stimulating activities of interleukin-1β in the rat ovary are nitric oxide independent. Journal of Clinical Investigation. 1994;94(4):1463–1469. doi: 10.1172/JCI117484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahsan S, Lacey M, Whitehead SA. Interactions between interleukin-1(β), nitric oxide and prostaglandin E2 in the rat ovary: effects on steroidogenesis. European Journal of Endocrinology. 1997;137(3):293–300. doi: 10.1530/eje.0.1370293. [DOI] [PubMed] [Google Scholar]
- 22.Adamczyk A, Kaźmierczak A, Strosznajder JB. α-Synuclein and its neurotoxic fragment inhibit dopamine uptake into rat striatal synaptosomes: relationship to nitric oxide. Neurochemistry International. 2006;49(4):407–412. doi: 10.1016/j.neuint.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Rao F, Zhang K, et al. Discovery of common human genetic variants of GTP cyclohydrolase 1 (GCH1) governing nitric oxide, autonomic activity, and cardiovascular risk. Journal of Clinical Investigation. 2007;117(9):2658–2671. doi: 10.1172/JCI31093. [DOI] [PMC free article] [PubMed] [Google Scholar]


