Abstract
Background
Neuregulin-1 (Nrg1) is a pleiotropic signaling molecule that regulates neural development and mutation of Nrg1 is a risk factor for schizophrenia. Cleavage of type I β1 Nrg1 isoform by BACE1 releases a secreted N-terminal fragment (Nrg1-ntfβ), which can bind to a cognate ErbB receptor to activate the specific signaling cascade. This study aimed to determine whether increased expression of Nrg1 is beneficial for brain development and functions.
Methods
We generated transgenic mice overexpressing this fragment under the control of a tetracycline-inducible promoter and examined functional and behavioral changes in mice upon reversible expression of the transgene.
Results
Increased expression of full length Nrg1 in mouse neurons has been previously shown to enhance myelination in the central nervous system. Overexpressing Nrg1-ntfβ enhanced the expression of myelin proteins, consistent with the expected activation of the Nrg1 signaling pathway by Nrg1-ntfβ. Contrary to expectations, overexpressing Nrg1-ntfβ transgene caused schizophrenia-like behaviors in transgenic mice and these abnormal behaviors were reversible if the expression of the Nrg1-ntfβ transgene was turned off. Our molecular assay suggests that protein levels of NMDA receptors (NMDARs) are reduced in this transgenic mouse model, which may underlie the observed social and cognitive behavioral impairments.
Conclusion
Our results indicate that overexpressing the secreted form of Nrg1 is sufficient to cause schizophrenia-like behaviors in a mouse model, meaning the effect is independent of the transmembrane and C-terminal domains of Nrg1. Hence, genetic gain-of-function mutations of Nrg1 are also risk factors for schizophrenia.
Keywords: BACE1, neuregulin, schizophrenia, NMDA receptor, transgenic mice, tetracycline control expression
INTRODUCTION
Mouse genetic studies have shown that Neuregulin-1 (Nrg1), a pleiotropic signaling molecule, controls developmental myelination [1] and cardiac development [2]. Human genetic studies have also identified the Nrg1 gene as a risk factor for schizophrenia [3]. Thus the mechanism(s) by which Nrg1 exerts its functional roles in development and disease pathogenesis has attracted great attention. The Nrg1 gene encodes 33 spliced isoforms in six topology types due to alternative splicing [4,5]. Types I and II transmembrane Nrg1 have N-terminal Ig- and epidermal growth factor (EGF)-like domains, while type III Nrg1 has the same transmembrane domain and another hydrophobic Cys-rich domain (CRD). All Nrg1 isoforms share an EGF-like domain, which is indispensable for initiating Nrg1 signaling via binding to its cognate ErbB receptors [6].
To exert signaling function, transmembrane Nrg1 undergoes proteolytic cleavage to allow binding of the N-terminal domain to an ErbB receptor. Enzymatic mapping shows that this cleavage of Nrg1 is mediated by BACE1 (between EF and ME) or ADAM10/ADAM17 (multiple adjacent sites) at the juxtamembrane region [7–9]. After this ectodomain shedding, type I Nrg1 releases its N-terminal fragment (Nrg1-ntf) to the extracellular space, where it binds to ErbB receptors on nearby cells in a paracrine fashion, while type III Nrg1-ntf, which remains tethered on the lipid bilayer by the hydrophobic CRD, signals to adjacent cells in a juxtacrine fashion. The distinct modes of signaling via isoform-specific Nrg1-ntfs appear to target specific in vivo functions [10].
In BACE1-null mice, full length Nrg1 is increased because cleavage of Nrg1 by BACE1 is abolished. Due to a reduction in the availability of Nrg1 signaling fragments, BACE1-null mice exhibit hypomyelination during early development [11,12] and delayed remyelination in adulthood [7], consistent with an important role of Nrg1 in the control of myelination [1]. Haplo-insufficient Nrg1 in mice also causes schizophrenia-like behaviors [3]. Indeed, BACE1-null mice exhibit schizophrenia-like phenotypes [13], further suggesting Nrg1 hypo-function upon BACE1 deletion.
Our previous biochemical studies show that expression of type I Nrg1-ntfβ in ErbB-expressing MCF-7 cells activates the Nrg1-ErbB pathway by enhancing phosphorylation of the downstream signaling molecules Akt and Erk [8]. In this study, we used mouse models to investigate whether an increase in the expression of BACE1-cleaved Nrg1-ntf (termed as Nrg1-ntfβ) would have beneficial effects on brain development and functions. For this purpose, we generated transgenic mice overexpressing Nrg1-ntfβ under the control of tetracycline (Tet) responsive element (Tet-Off promoter). We found that increased expression of the Nrg1-ntfβ transgene in mouse forebrain is sufficient to increase expression of myelin proteins, consistent with activation of the Nrg1-ErbB pathway. Unexpectedly, these mice also developed schizophrenia-like behaviors, which were reversed if transgene expression was turned off. Hence, our results suggest that Nrg1 levels should be finely balanced and that sustained high levels of soluble Nrg1 can cause schizophrenia-like behaviors.
Methods and Materials
Generation of human N1β transgenic mice
BACE1-cleaved N-terminal fragment of human NRG1 β1a (N1β) was subcloned into the BamHI and NotI sites of pTRE2hyg vector (Clontech Laboratories Inc., Mountain View, CA). A linearized NheI fragment containing the transgene was used for transgenic mouse production. Five TRE-N1β founders in the C57BL/6-CBA(J) background were identified by PCR with primers (forward CATCGTGGAATCAAACGAGA; reverse TTTGCCCCCTCCATATAACA) and further confirmed by Southern blotting. Tg-N1β mice were backcrossed with C57BL/6J mice for six generations before crossing with CaMK2α-tTA mice (Jackson Lab, stock number 007004). Mice were housed in designated animal rooms at 23 °C on a 12 h light/dark cycle with food and water available ad libitum. For Dox (Sigma-Aldrich, St. Louis, MO) treatment, the drug was added to drinking water at 0.5 mg/ml, supplemented with 2% sucrose. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the Lerner Research Institute in compliance with the guidelines established by the Public Health Service Guide for the Care and Use of Laboratory Animals.
Western blotting and antibodies
Mouse tissues were freshly dissected and proteins were extracted using modified RIPA buffer (50mM Tris-HCl, pH 7.4, 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1mM EDTA, 1 mM sodium vanadate, protease inhibitors). At least two mice from each group were used for western blot analysis. Equal amounts of protein (40 µg) were resolved on a NuPAGE Bis-Tris gel (Invitrogen, Carlsbad, CA) and transferred onto a nitrocellulose membrane (Invitrogen). After protein transfer, blots were incubated with the specified antibodies: Neuregulin 1 (H210), SNAP25, Complexin, and VAMP (Santa Cruz Biotechnology Inc., Santa Cruz, CA); p-ErbB4 (sc-33040, Santa Cruz, CA), Akt and p–Akt (S473) (Cell Signaling Technology, Danvers, MA, USA); MBP (Sternberger Monoclonal Inc., Lutherville, MD, USA); Actin (Sigma-Aldrich, St. Louis, MO); ErbB4, NR1, NR2A/B, GABARα1, and Parvalbumin (EMD Millipore, Billerica, MA); Synaptophysin and GAP43 (Sigma-Aldrich, St. Louis, MO). The antibody to PLP was previously obtained as a gift from Dr. Pfeiffer at the University of Connecticut.
Behavioral testing
All testing except for PPI was performed at 23–24°C in an isolated behavior room of the Rodent Behavioral Core at the Lerner Research Institute, Cleveland Clinic. Mice were taken to the testing room 1 h before testing began to acclimate to the environment. All behavioral tasks were video-tracked and analyzed by the Ethovision XT software system (Noldus Information Technology, Leesburg, VA).
Y-maze test
The Y maze is composed of black plastic with 3 identical arms positioned 120 degrees apart. Mice were placed in the center of the maze and allowed to explore for 5 minutes, during which time a video camera was used to record the activity of the animal in the maze. The number of spontaneous alternations was used to assess spatial working memory. Mice were used for the Y-maze test first and then half of the mice were used for the social behavioral test and the other half were used for the contextual fear conditioning test.
Social behavior test
The sociability apparatus is a rectangular, three-chamber box (Stoelting Co., Wood Dale, IL). Each chamber measures 20 cm (length) × 40.5 cm (width) × 22 cm (height). Dividing walls are made from clear Plexiglas, with small openings (10-cm width × 5-cm height) that allow free access into each chamber. Photobeams are embedded across each doorway. An automated photobeam detector registers time spent in each chamber and the number of transitions. After mice were habituated in the center chamber for 5 min, their social interactions were assessed by evaluating the amount of time (during a 10 min period) the animal spent investigating an unfamiliar ‘stranger’ mouse inside a wire cage located in one of the side chambers as compared to the time spent investigating an identical but empty wire cage contained in the other side chamber. In the second phase of testing, a second unfamiliar mouse was introduced into the previously empty chamber. The preference for social novelty was then tested for 10 min by measuring the amount of time the test mouse spent investigating the new, unfamiliar ‘stranger’ mouse (also restricted in a wire cage) as compared to the time spent with the now familiar mouse.
Open field test
The open field arena (41 cm×41 cm) was equipped with a 16×16 grid of photo beam sensors 2.54 cm apart (San Diego Instruments, San Diego, CA, USA). An additional 16 photo beam sensors were used to measure rearing behaviors. In each trial, mice were placed into the center of the arena and allowed to explore freely. Beam breaks were recorded at 1 minute intervals throughout the trial and converted to directionally specific movements. After 20 min initial habituation in the arena, mice were injected with MK-801 (0.3mg/kg in PBS, i.p.; Sigma-Aldrich, St. Louis, MO) and their locomotion was recorded for 90 min. Locomotor activity was measured as total distance travelled.
Contextual Fear Conditioning Test
This test consisted of 3 daily trials. On the first day, the conditioning period, the mouse was placed in the conditioning chamber (Med Associates, St. Albans, VT) for 3 min (phase A) before the onset of a sound at 2800 Hz and 85 dB for 30 sec (phase B, conditioning stimulus). The last 2 sec of the conditioning stimulus was coupled with a 0.7 mA continuous foot shock (phase C, unconditioned stimulus). After resting an additional 30 sec in the chamber, phases B and C were repeated once and the mouse was returned to its home cage after resting in the chamber for another 30 sec. On the second day, mice were tested for their contextual memory in the same chamber for 3 min without either sound or foot shock. On the third day, mice were tested for their cue-induced memory in a different chamber environment with the sound, but no foot shock. Fear learning ability of the mice was measured as the percentage of freezing, which was defined as the percentage of time completely lacking movement, except for respiration, in intervals of 5 sec.
Statistical Analysis
Statistical analysis was performed using Sigmastat 3.5 (Systat Software, Inc., IL.). All data are expressed as mean ± SEM. Social behavior tests were analyzed by two-way ANOVA with Tukey’s post hoc tests. Data from other experiments with 3 or more groups were analyzed by one-way ANOVA with Tukey’s post hoc tests. Two-tailed Student’s t- tests were used to analyze data from experiments with 2 groups. Significant p values are denoted by the use of asterisks in the text and figures (*: p<0.05; **: p < 0.01; ***: p < 0.001).
RESULTS
Generation of transgenic mice expressing Nrg1-ntfβ transgene
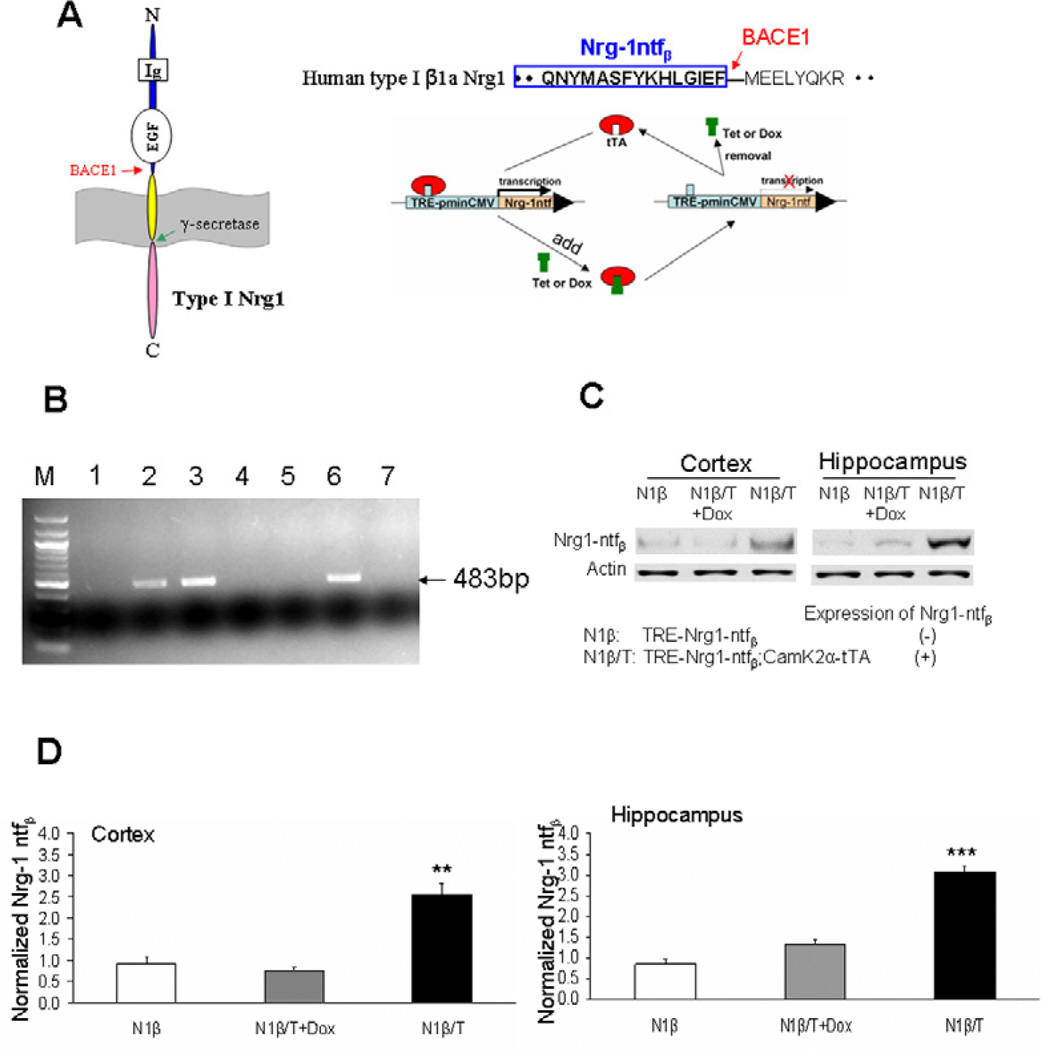
We have previously mapped BACE1 cleavage of Nrg1 to the site between amino acids F237 and M238, which is located 10 amino acids upstream of the transmembrane domain of the Nrg1 β1 isoform [7]. This has been confirmed in separate studies [9,14]. To generate transgenic mice overexpressing BACE1-cleaved Nrg1 β1 isoform (Nrg1-ntfβ), we subcloned the corresponding fragment into a vector under the control of an inducible tetracycline responsive element (TRE) (Figure 1A). The assembled construct was then linearized by enzymatic digestions and the gel-purified plasmid DNA was injected into mouse pronuclei (B6C3F1 strain). After screening 26 pups, we recovered 5 positive founder mice, which were verified by both PCR genotyping (examples in Figure 1B) and Southern blotting (data not shown). Most of the 5 founder lines of transgenic mice had similar levels of the transgene integrated into the mouse genome (data not shown). Therefore, two lines of mice were chosen to further breed with transgenic mice expressing tetracycline-controlled trans-activator protein (tTA) driven by CaMK2α promoter (Tg-CaMK2α-tTA) to verify Nrg1-ntfβ protein levels. After biochemical characterizations, one line was eventually chosen for most of the studies as described below.
Figure 1. Generation of transgenic mice expressing BACE1-cleaved soluble Nrg1 N-terminal fragment.
(A) Schematic illustration of the transgene construct. Type I Nrg1 β1a isoform is a single pass transmembrane protein and contains a BACE1 cleavage site between F–M. The N-terminal fragment (Nrg1-ntfβ) ending at residue F was subcloned into a vector under the control of tetracycline responsive element (TRE) coupled with a minimal CMV promoter. The driver line expresses tetracycline transactivator (tTA). In this case, CaMK2α promoter drives the expression of tTA in forebrain neurons; treatment with tetracycline (Tet) or doxycycline (Dox) turns off transgene expression. (B) Representative example of genotyping PCR showing three positive lines with the expected size of 483bp. (C) Two-month-old transgenic mice, designated as Tg-N1β/T for double transgenic mice or Tg-N1β for single non-transgene-expressing mice, were tested for the expression of transgene. Increased expression of transgene was detected and Dox treatment for one month switched off this expression. (D). Bar graphs show normalized transgene expression levels in the three groups of mice tested in (C) (n=3 per group; **: p<0.01, ***:p<0.001, one-way ANOVA with Tukey’s post hoc test).
The CaMK2α promoter was previously shown to drive transgene expression in forebrain [15]. Therefore, cortical and hippocampal lysates from Tg-Nrg1-ntfβ/CaMK2α-tTA transgenic mice (expressing transgene and abbreviated as Tg-N1β/T hereafter in the text and figures) and Tg-Nrg1-ntfβ transgenic mice (expressing no transgene due to the lack of transactivator protein and abbreviated as Tg-N1β) of different ages were prepared for western blot assays. Representative assays are shown in Figure 1C, in which we validated that Nrg1-ntfβ levels, detected by an antibody specific to the N-terminus of Nrg1, were clearly elevated in two-month-old N1β/T transgenic mice. This elevation was reversed if N1β/T mice were pre-treated with Doxycycline (Dox) for one month. The elevation of transgene expression in the cortex and hippocampus appeared to be similar (Figure 1D). Overall, N1β/T transgenic mice are viable and fertile with no easily discernible phenotype in the two founder lines that were examined.
Increased expression of Nrg1-ntfβ transgene in mouse brains activates the Nrg1-ErbB pathway
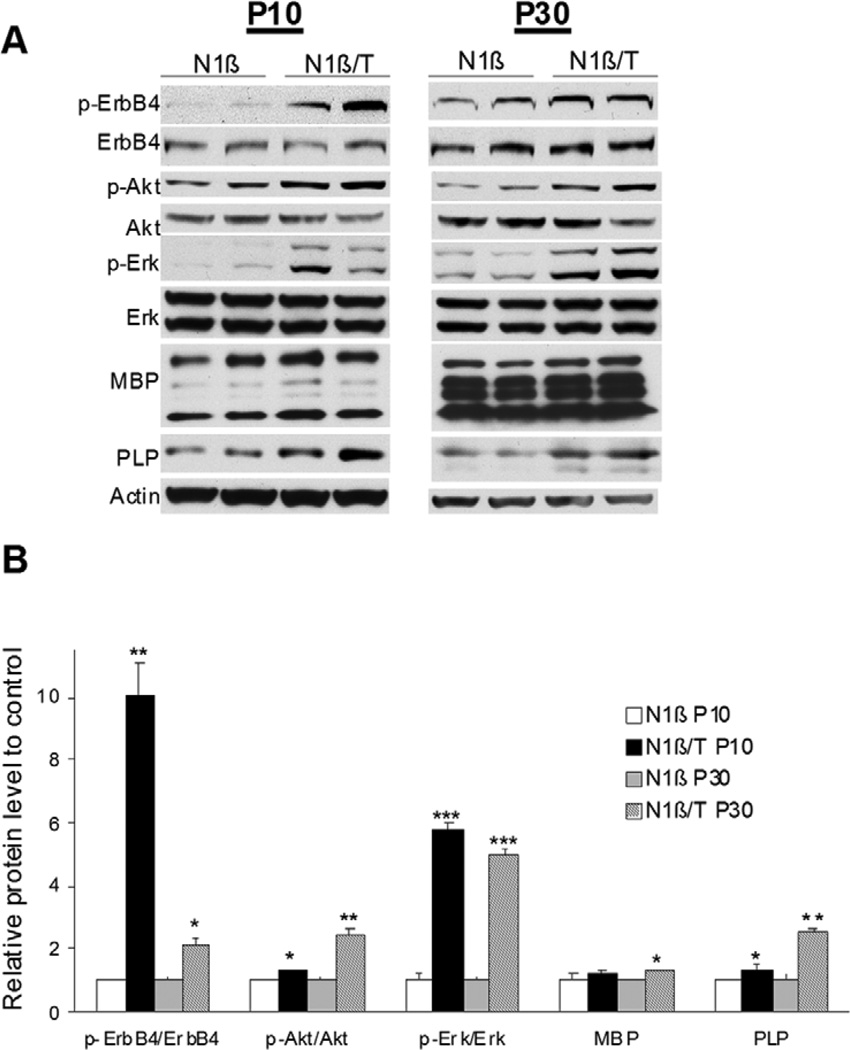
To determine whether expression of Nrg1-ntfβ activates the Nrg1-ErbB signaling pathway similar to full length Nrg1, we first examined the phosphorylation levels of ErbB4 and found a significant elevation of phosphorylated ErbB4 (normalized to total protein) in N1β/T transgenic mice (expressing the Nrg1-ntfβ transgene) at postnatal day 10 (P10) and P30 compared to N1β transgenic littermate controls with no transgene expression (Figure 2A–B). Phosphorylation of Akt and Erk, two known downstream signaling molecules in the Nrg1-ErbB pathway, was similarly examined. As shown, phosphorylated Akt and Erk were significantly increased (Figure 2). Since enhanced expression of Nrg1 in mice results in increased expression of myelin genes [16], we also examined the protein levels of myelin basic protein (MBP) and proteolipid protein (PLP). Both MBP and PLP were increased in N1β/T transgenic mice (Figure 2A–B), with the increase in PLP being more pronounced during early development. Prior studies have shown that increased Akt activity is sufficient to enhance expression of myelin proteins [17,18]. Hence, our results demonstrate an activation of the Nrg1-ErbB pathway by the secreted Nrg1-ntfβ transgene expression in mice.
Figure 2. Overexpressing Nrg1-ntfβ in mice activates the Nrg1-ErbB pathway.
(A) Transgenic mice at postnatal day 10 (P10) and 30 (P30) were examined for changes in the indicated proteins. Increased phosphorylation of Akt (p-Akt) and Erk (p-Erk) was observed in Tg-N1β/T compared to control Tg-N1β mice. There was a significant increase in levels of the myelin protein PLP, while MBP levels were slightly elevated in Tg-N1β/T mice of both ages. (B). Bar graphs show normalized changes in p-Akt/Akt and p-Erk/Erk ratios, as well as MBP and PLP levels (n=3 per group; *:p<0.05, **:p<0.01, ***:p<0.001, Student’s t-test).
Increased expression of Nrg1-ntfβ transgene in mouse brains results in schizophrenia-like behaviors
Since genetic mutations of the Nrg1 gene or disease-associated single polymorphisms are closely associated with schizophrenia [3,19,20], we examined whether increased expression of type I Nrg1-ntfβ in mice affects their behaviors. To address this question, we conducted a battery of behavioral tests that are commonly used to assess schizophrenia-like behaviors in rodents [21].
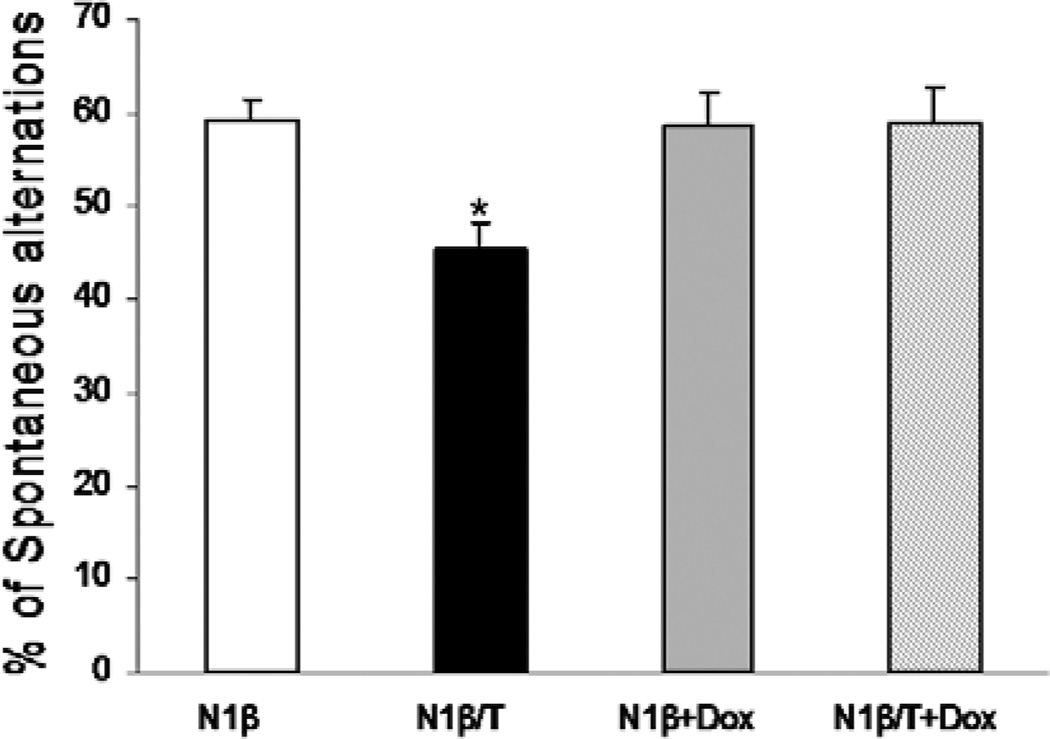
Schizophrenia patients exhibit significant and widespread cognitive deficits [22]. We therefore conducted a Y-maze test using both Tg-N1β/T and Tg-N1β mice, as this behavioral paradigm measures spatial working memory. Tg-N1β/T mice showed a significant reduction in spontaneous alternations compared to control Tg-N1β mice (n=24 animals in each group, **P<0.01; Figure 3). When these mice were treated with Dox for one month to switch off the expression of Nrg1-ntfβ, the percentage of spontaneous alternations between the two genotypes of mice was similar (Figure 3, +Dox groups, n= 24 animals in each group), indicating that this impaired spatial working memory was dependent on the expression of the transgene and was reversible.
Figure 3. Testing of Tg-N1β/T and Tg-N1β mice in the Y-maze.
Three-month-old Tg-N1β/T and Tg-N1β mice were subjected to a standard Y-maze test. Two separate groups of mice were treated with Dox at two months of age for one month and subjected to the same Y-maze test. Only Tg-N1β/T mice showed a significant reduction in spontaneous alternations (n=24 per group; *:p<0.05, one-way ANOVA with Tukey’s post hoc test).
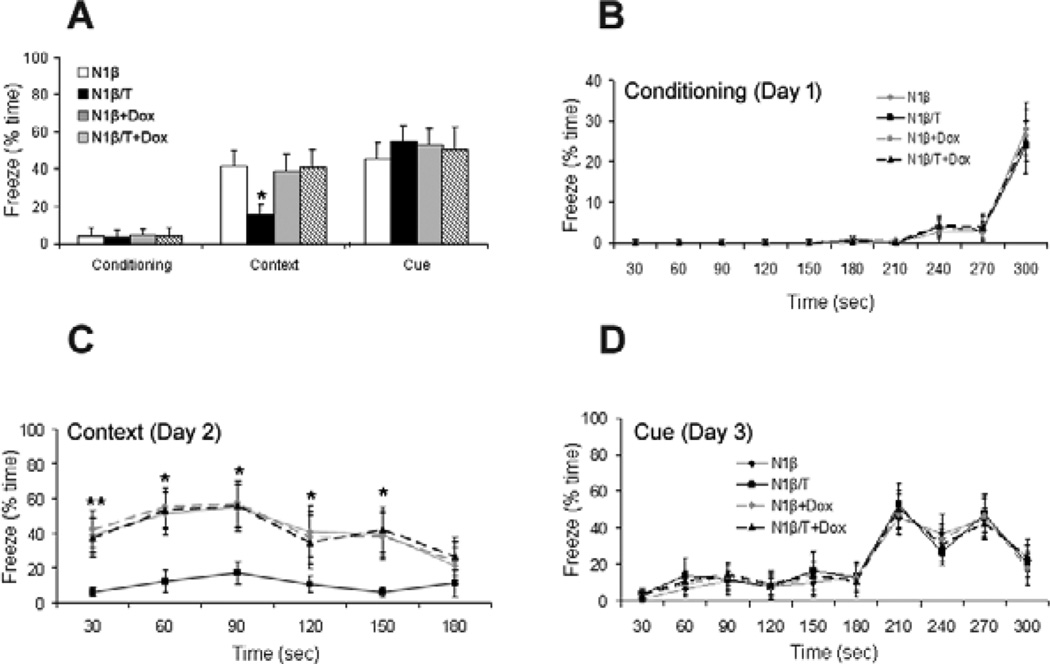
Another commonly used cognitive test in rodents is the contextual fear conditioning task [15,23] and animals from models of schizophrenia exhibit impairments in this task [24]. During this test, the associative learning of a cue (sound) or a context (environment) with a brief aversive stimulus (electric shock) was measured by analyzing the freezing response of mice. Mice with or without Dox treatment for one month were subjected to the test when they reached the age of 3 months. On day one, mice were placed in the fear conditioning chamber and exposed to a sound followed by a foot shock. During the first-day conditioning test, both Tg-N1β/T and Tg-N1β mice had similar levels of freezing (Figure 4A–B). Context-dependent freezing was recorded on day two by placing mice back in the same chamber but without exposure to the sound or shock. Only the Tg-N1β/T mice exhibited a lower freezing time (Figure 4A, C), indicating that Nrg1-ntfβ transgene overexpression impaired contextual fear learning. This impairment was not observed in Tg-N1β/T mice that were pre-treated with Dox for one month, again suggesting that this is a phenotype dependent on transgene overexpression. On test day three, which mainly assesses amygdala function [25], the freezing time of mice in response to the tone (sound) in a context-altered chamber was indistinguishable among all groups of mice (Figure 4D), suggesting hippocampal dysfunction as the underlying cause of the contextual fear learning deficits of Tg- N1β/T mice.
Figure 4. Tg-N1β/T and Tg-N1β mice exhibited impaired contextual fear learning.
(A) The fear conditioning assay included a 3 day testing procedure in a fear conditioning chamber. There were no differences in the percentage of freeze time during the day 1 conditioning test. On day 2, contextual fear learning of the mice was analyzed. Percentage of total freeze time of mice on Day 2 was recorded as an indicator of contextual learning ability. The percentage of total freeze time was significantly reduced from 41.3±8.8% in Tg-N1β mice to 15.5±5.8% in Tg-N1β/T mice. Day 3 measured cued memory by comparing total freeze time during the presentation of tones in a different chamber, which is primarily related to amygdala function. The Tg-N1β/T group showed a similar percentage of total freezing time compared to the control and Dox-treated groups. Detailed percentages of freeze time at 30-sec intervals during the entire recording period for conditioning (B), context (C) and cue (D) are plotted. Statistics were analyzed by one-way ANOVA with Tukey’s post hoc test (n=12 per group; *:p<0.05, **:p<0.01).
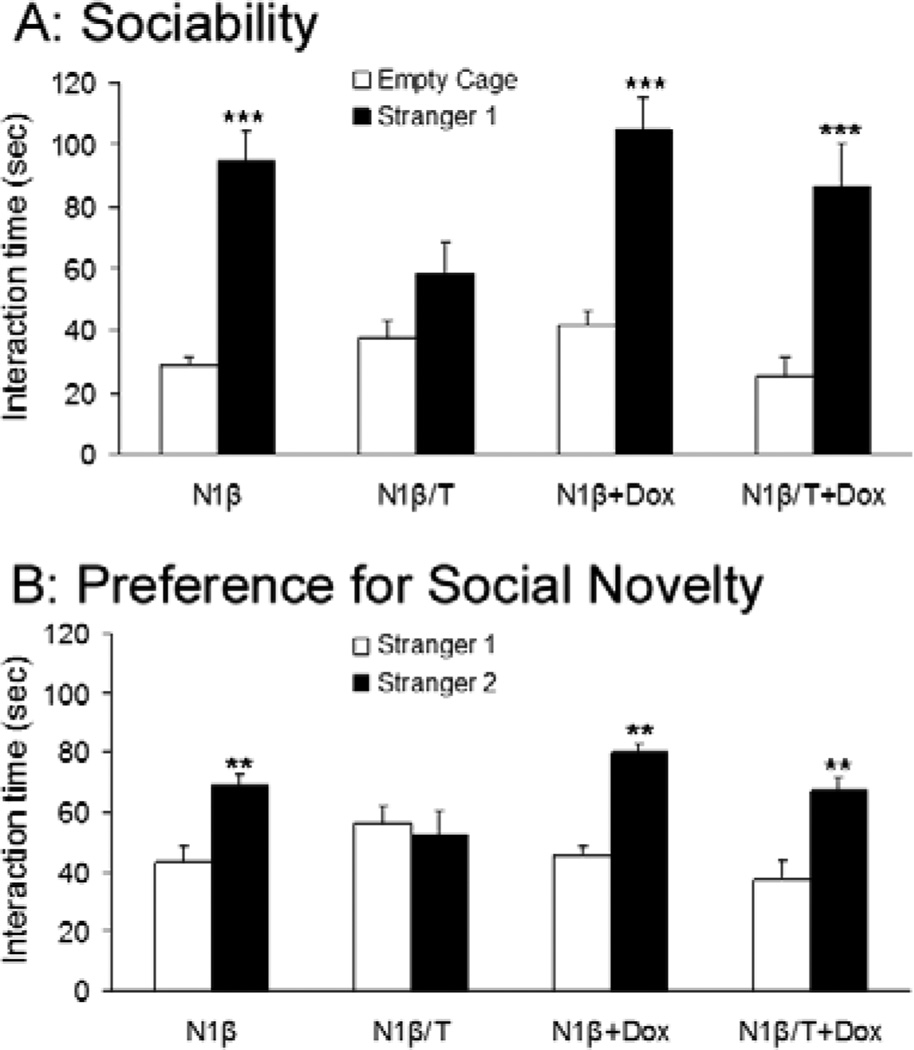
We also conducted a social behavioral test, as most schizophrenia models exhibit impaired social interactions [26]. In the first test, we measured the sociability of mice by using an encounter with a novel mouse in the testing chamber; a mouse will normally actively engage with a novel mouse. Tg-N1β/T mice spent significantly less time investigating the novel juvenile mouse (Stranger 1) compared to Tg-N1β mice; moreover, Tg-N1β/T mice spent a similar amount of time investigating the novel mouse as the empty cage. These results suggest impaired sociability of Tg-N1β/T mice (Figure 5A). In further testing, the test mouse was exposed to the previously encountered Stranger 1 and a new novel mouse (Stranger 2) in the same chamber on a subsequent day. This paradigm tests the preference for social novelty (i.e., social memory) of the test mouse; previous studies have established that a mouse naturally tends to spend more time interacting with Stranger 2 than with Stranger 1 [27]. Tg-N1β/T mice failed to distinguish between Stranger 1 and Stranger 2 and spent an essentially equal amount of time with each (Figure 5B), indicating social memory deficits in these mice. These social impairments were clearly reversible in animals treated with Dox for one month to switch off the expression of Nrg1-ntfβ, as the social behaviors of Tg-N1β/T+Dox mice were similar to control mice in both tests (Figure 5A–B).
Figure 5. Impaired social interaction of Tg-N1β/T mice.
(A) The sociability test was conducted by comparing the time a test mouse (Tg-N1β/T or Tg-N1β mice) spent investigating a novel juvenile mouse (Stranger 1) versus an empty cage in the testing chamber. Tg-N1β/T spent same amount of time investigating Stranger 1 and the empty cage, whereas the Tg-N1β control group spent significantly more time with Stranger 1 than with the empty cage. After the test, the same groups of mice were treated with Dox for one month before being retested in the same paradigm and showed similar sociability as the control group. (B) The test of preference for social novelty was conducted by placing the test mouse in a cage with the previously encountered mouse (Stranger 1) and a novel mouse (Stranger 2) in the same testing chamber. The time spent exploring Stranger 1 and Stranger 2 was recorded and Tg-N1β/T mice spent an equal amount of time with both strangers, while the Tg-N1β control group preferentially interacted with Stranger 2. The Dox-treated groups showed similar preferences as the control group (n=12 mice in each group, **: p<0.01, ***: p<0.001; two-way ANOVA with Tukey’s post hoc test).
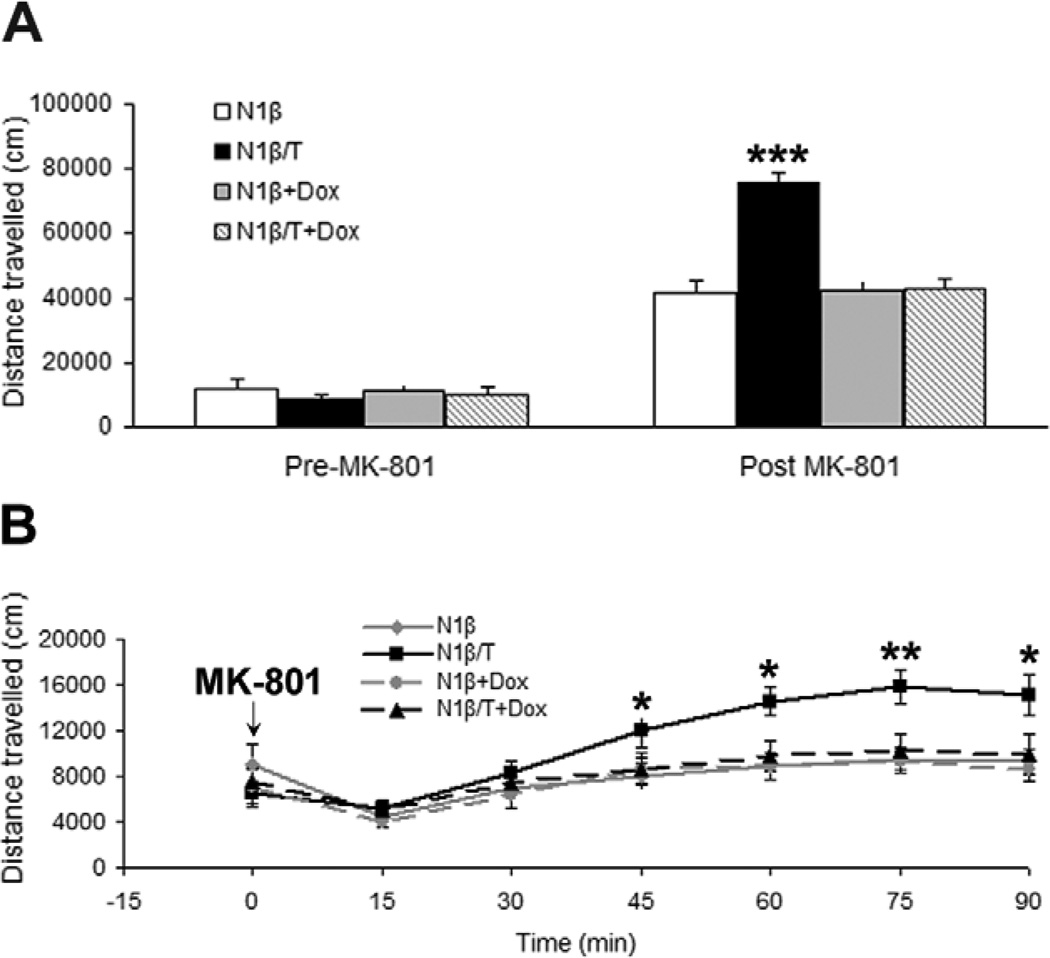
Mice are also often subjected to an open field task to examine their exploratory activity in schizophrenia models [28,29]. Our paradigm was designed to investigate the effects of acute administration of MK-801 (0.3 mg/kg), a noncompetitive NMDA receptor antagonist that induces the release of glutamate and ascorbic acid, on freely moving mice. We showed that both Tg-N1β/T and control Tg-N1β mice showed similar basal open field behaviors, as they traveled similar distances without acute administration of MK-801 (Figure 6A). While MK-801 treatment resulted in hyperactivity in all groups of mice, Tg-N1β/T mice appeared to be supersensitive to the drug, as indicated by travelling almost double the distance in the open field compared to the Tg-N1β control group (Figure 6A). During the 90-min recording period, supersensitivity of Tg-N1β/T mice to MK-801 became evident 45 min after administration of the drug (Figure 6B). Again, this supersensitivity to MK-801 was reversed if expression of Nrg1-ntfβ transgene in N1β/T transgenic mice was turned off by Dox treatment for one month (Figure 6B), indicating a transgene-dependent and reversible effect.
Figure 6. Tg-N1β/T mice showed hyperactivity in an open field test.
(A) Prior to injection with MK-801, a noncompetitive antagonist of the NMDA receptor, all four groups of mice (with or without Dox treatment for one month) showed no significant differences in exploratory activity in the open field. After the injection, all groups of mice became hyperactive compared to the time before the injection. Tg-N1β/T mice showed significantly higher levels of exploratory activity compared to the control groups. (B) Detailed time series plots show that hyperactivity was significant beginning at 45 min after the injection and reached a peak at 75 min after the injection. Statistics were analyzed by one-way ANOVA followed by Tukey’s post hoc test (n=10 per group; *:p<0.05, **:p<0.01, ***:p<0.001).
Collectively, this battery of behavioral tests shows that Tg-N1β/T mice display schizophrenia-like phenotypes in response to overexpressed Nrg1-ntfβ and that this behavioral phenotype was reversible by turning off the transgene.
Increased expression of Nrg1-ntfβ transgene in mouse brains reduces levels of glutamate receptor proteins
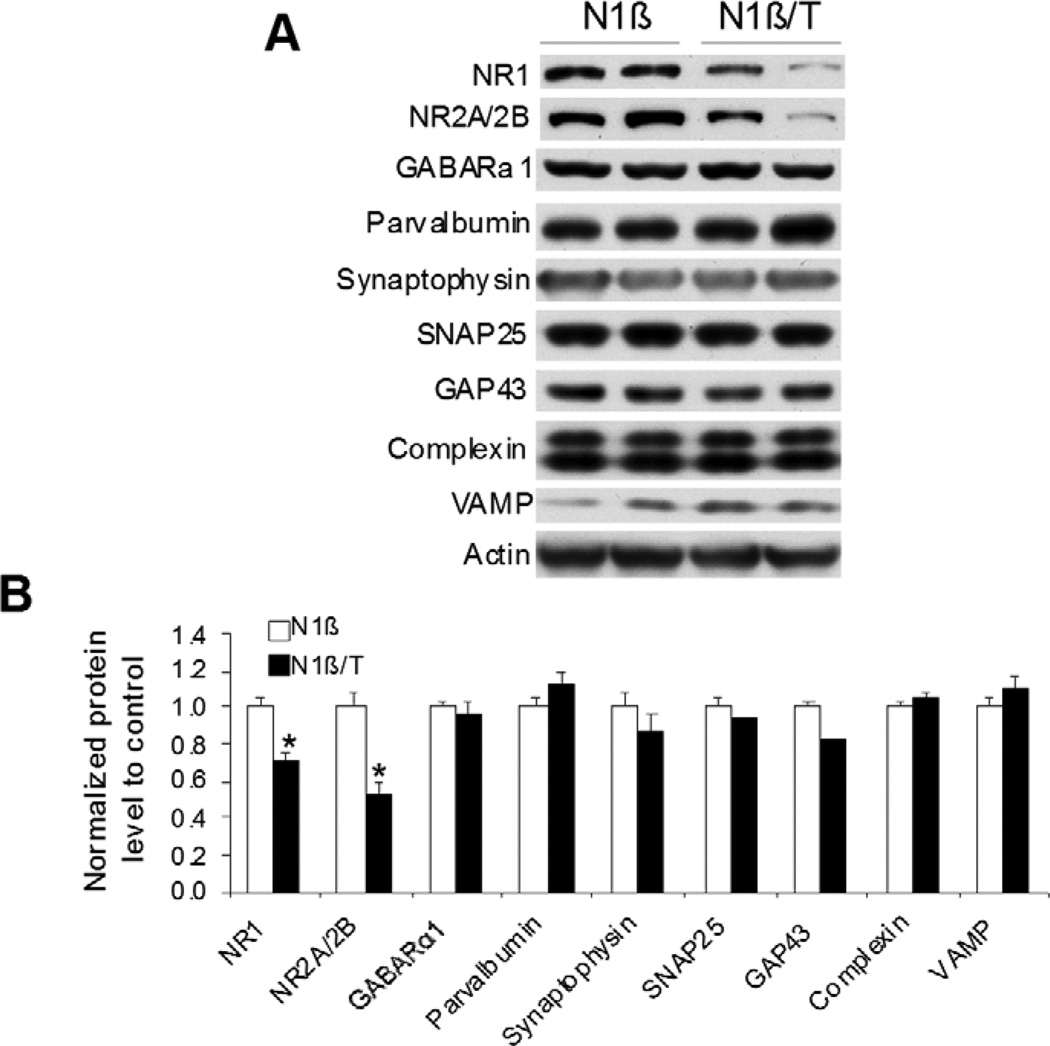
To explore the molecular mechanism underlying the impairment of cognition and social behavior in Tg-N1β/T mice, we used hippocampal protein lysates to examine a panel of proteins important for synapse formation or synaptic plasticity. From the selected list of proteins, NMDA receptor proteins NR1 and NR2A/2B were significantly reduced (Figure 7). NMDA receptors (NMDARs), composed of two NR1 and two NR2 subunits (NR2A or NR2B), are essential for learning and memory [30]. Proteins important for synapse formation such as synaptophysin, SNAP25, complexin and VAMP were not significantly altered (Figure 7). Changes in parvalbumin, GAP43 and GABAR proteins were also not significant (Figure 7). Hence, we observed a reduction in NMDARs that may contribute to the observed behavioral abnormalities of Tg-N1β/T mice, as hypofunction of NMDARs is linked to impairments in cognition and sociability in schizophrenia [31–34].
Figure 7. Reduced levels of NMDA receptor proteins in Tg-N1β/T mice.
(A) Protein extracts from 3-month-old mouse hippocampus were examined by Western blotting using antibodies specific to the indicated proteins. Two pairs of mice were used for protein extractions. (B) Bar graphs are shown with the indicated protein levels normalized to actin (n=3 per genotype; *:p<0.05; Student’s t-test.).
Discussion
Nrg1 is an indispensable signaling molecule for the control of neural development and neuronal functions [35–37]. Nrg1 initiates its signaling activity by binding to its cognate tyrosine kinase receptor, consisting of an ErbB2/ErbB3 heterodimer or an ErbB4 homodimer, which induces a cascade of signaling events including phosphorylation of the downstream molecules Akt and Erk. Membrane-bound pro-Nrg1 protein seems to be inactive as the EGF-like domain within the N-terminal region is not readily accessible to the ErbB receptor. For effective binding to its receptor, membrane-anchored Nrg1 undergoes proteolytic cleavage to release either soluble Type I N-terminal fragment or membrane-tethered Type III N-terminal fragment, both of which have a high affinity for the ErbB receptor. Clinically, recombinant soluble Nrg1 β1 isoform has been subcutaneously administered to patients to treat Chronic Systolic Heart Failure [38] and has also been considered for therapeutic application in schizophrenia patients.
In this report, we demonstrate that constitutive overexpression of soluble Nrg1 (Nrg1-ntfβ) in mouse forebrain results in cognitive deficits. The difference between the aforementioned recombinant soluble type I Nrg1 β1 isoform and this Nrg1-ntfβ is that Nrg1-ntfβ lacks the extra 10 amino acids (MEAEELYQKR) located between the BACE1 cleavage site and the transmembrane domain of Nrg1; this Nrg1-ntfβ should be normally secreted in vivo. We showed that Tg-N1β/T mice exhibited impairments in both cognition and social behavior, and that these impairments were dependent on the expression of Nrg1-ntfβ transgene, as demonstrated in several behavioral tests including the Y-maze, contextual fear conditioning, an open field arena and social recognition. We have also conducted prepulse inhibition (PPI) tests using these mice. Although we did not observe significant differences between Tg-N1β/T transgenic mice and control Tg-N1β mice, the Tg-N1β/T group showed a trend towards impairment, indicated by a decreased percentage of PPI at all three prepulse intensities tested (at 73 dB, N1β/T: 15.10±4.34%, N1β: 17.19±7.63%, p=0.1731; at 76 dB, N1β/T: 22.89±4.62, N1β: 29.32±8.08, p=0.1584; at 82 dB, N1β/T: 49.98±5.86, N1β: 58.96±6.33, p=0.076; n=12 mice in each group). Small or weak changes in PPI have also been found in other schizophrenia mouse models [26].
How the elevated expression of soluble Nrg1-ntfβ leads to schizophrenia-like behaviors in animals is an intriguing question. Haplo-insufficiency of Nrg1 or ErbB4 has been suggested to cause schizophrenia [4,39,40]. Consistent with this notion, reduced activity of the Akt/GSK3β pathway has also been linked to schizophrenia [41]. Therefore, schizophrenia-associated Nrg1 mutations are logically considered as loss of function [3]. In this study, we observed increased Akt phosphorylation in transgene-expressing mice during early development (Figure 2). Since Erk, another signaling molecule of the Nrg1/ErbB pathway, is also increased during early development, it is likely that overexpressed Nrg1-ntfβ induces activation of the Nrg1-ErbB pathway in early developing mouse brains. Our results also indicate that high Nrg1-ErbB signaling activity can disrupt neural circuitry and lead to schizophrenia-like behaviors in animals. Consistent with this observation, transgenic mice with constitutive overexpression of type I full length Nrg1 also exhibit schizophrenia-like behaviors [42]. More recently, an inducible mouse model with overexpression of full-length type I Nrg1 was also shown to develop schizophrenia-like behaviors, and this study also showed reversible effects of the expressed transgene [43]. The difference between our study and the aforementioned studies is that we expressed only the secreted form of Nrg1. We demonstrate that increased expression of the soluble EGF domain-containing N-terminal fragment is sufficient to cause schizophrenia-like behaviors in mice. Since Nrg1 has a relatively large C-terminal fragment (355 amino acids) and this C-terminal fragment is suggested to have a back signaling activity [44], our study could not exclude the contributory effect of this region on schizophrenia-like behaviors by an additional mechanism [43].
Nrg1 stimulation suppresses NMDA receptor activation in the human prefrontal cortex, as previously reported in the rodent cortex [3,45,46]. Nrg1-induced suppression of NMDA receptor activation was more pronounced in schizophrenia subjects than in controls, consistent with enhanced Nrg1-ErbB4 signaling seen in this disorder [47]. In our biochemical assays, we detected a significant reduction in NMDR proteins in adult animals, and this reduction is likely related to the altered Nrg1-ErbB signaling, although the detailed mechanism remains to be established. Although Nrg1 is known to alter GABAergic circuitry [48–51], we did not observe any significant changes in GARARα1 levels in our transgenic mouse model (Figure 7). This may be related to the lack of the transmembrane and C-terminal domains of Nrg1 in our mouse model. Consistent with this hypothesis, Yin et al. showed a reduction in GARARα1 mRNA and protein levels in mice overexpressing full length Nrg1 [43].
In summary, we demonstrate here that increased expression of soluble Nrg1 in mouse forebrains beginning at the neonatal stage reduces NMDA function and leads to schizophrenia-like behaviors in animals. Our data suggest that gain-of-function mutations in Nrg1 are also potential risk factors for schizophrenia. A finely balanced level of Nrg1 is clearly required for normal cognitive and social behaviors and overexpressed Nrg1-ntfβ in mice clearly disrupts this balance.
ACKNOWLEDGEMENTS
Authors want to thank Ms. Xiaoying Tang and Mr. Alvaro Alvarado for their assistance in behavioral assays. We also thank Dr. Youran Fan (Department of Quantitative Health Science at Cleveland Clinic) for his support on the statistical analyses and Dr. Chris Nelson for critical reading and helpful editing of the manuscript. This study was supported by a grant from the National Institute of Health to R Yan (NS074256). The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Nave KA, Salzer JL. Axonal regulation of myelination by neuregulin 1. Curr. Opin. Neurobiol. 2006;16:492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 3.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, et al. Neuregulin 1 and susceptibility to schizophrenia. Am. J. Hum. Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp. Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 6.Holmes WE, Sliwkowski MX, Akita RW, Henzel WJ, Lee J, Park JW, Yansura D, Abadi N, Raab H, Lewis GD, et al. Identification of heregulin, a specific activator of p185erbB2. Science. 1992;256:1205–1210. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- 7.Hu X, He W, Diaconu C, Tang X, Kidd GJ, Macklin WB, Trapp BD, Yan R. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J. 2008;22:2970–2980. doi: 10.1096/fj.08-106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo X, Prior M, He W, Hu X, Tang X, Shen W, Yadav S, Kiryu-Seo S, Miller R, Trapp BD, et al. Cleavage of neuregulin-1 by BACE1 or ADAM10 protein produces differential effects on myelination. J. Biol. Chem. 2011;286:23967–23974. doi: 10.1074/jbc.M111.251538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleck D, van BF, Colombo A, Galante C, Schwenk BM, Rabe L, Hampel H, Novak B, Kremmer E, Tahirovic S, et al. Dual Cleavage of Neuregulin 1 Type III by BACE1 and ADAM17 Liberates Its EGF-Like Domain and Allows Paracrine Signaling. J. Neurosci. 2013;33:7856–7869. doi: 10.1523/JNEUROSCI.3372-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Britsch S. The neuregulin-I/ErbB signaling system in development and disease. Adv. Anat. Embryol. Cell Biol. 2007;190:1–65. [PubMed] [Google Scholar]
- 11.Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, Yan R. Bace1 modulates myelination in the central and peripheral nervous system. Nat. Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 12.Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 13.Savonenko AV, Melnikova T, Laird FM, Stewart KA, Price DL, Wong PC. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5585–5590. doi: 10.1073/pnas.0710373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.La MR, Cerri F, Horiuchi K, Bachi A, Feltri ML, Wrabetz L, Blobel CP, Quattrini A, Salzer JL, Taveggia C. TACE (ADAM17) inhibits Schwann cell myelination. Nat. Neurosci. 2011 doi: 10.1038/nn.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bach ME, Hawkins RD, Osman M, Kandel ER, Mayford M. Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal LTP in the range of the theta frequency. Cell. 1995;81:905–915. doi: 10.1016/0092-8674(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 16.Brinkmann BG, Agarwal A, Sereda MW, Garratt AN, Muller T, Wende H, Stassart RM, Nawaz S, Humml C, Velanac V, et al. Neuregulin-1/ErbB Signaling Serves Distinct Functions in Myelination of the Peripheral and Central Nervous System. Neuron. 2008;59:581–595. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB. Constitutively active Akt induces enhanced myelination in the CNS. J. Neurosci. 2008;28:7174–7183. doi: 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu X, Schlanger R, He W, Macklin WB, Yan R. Reversing hypomyelination in BACE1-null mice with Akt-DD overexpression. FASEB J. 2013 doi: 10.1096/fj.12-224212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall J, Whalley HC, Job DE, Baig BJ, McIntosh AM, Evans KL, Thomson PA, Porteous DJ, Cunningham-Owens DG, Johnstone EC, et al. A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nat. Neurosci. 2006;9:1477–1478. doi: 10.1038/nn1795. [DOI] [PubMed] [Google Scholar]
- 20.Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, Harrison PJ, Kleinman JE, Weinberger DR. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5' SNPs associated with the disease. Proc. Natl. Acad. Sci. U. S. A. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takao K, Miyakawa T. Investigating gene-to-behavior pathways in psychiatric disorders: the use of a comprehensive behavioral test battery on genetically engineered mice. Ann. N. Y. Acad. Sci. 2006;1086:144–159. doi: 10.1196/annals.1377.008. [DOI] [PubMed] [Google Scholar]
- 22.Kellendonk C, Simpson EH, Kandel ER. Modeling cognitive endophenotypes of schizophrenia in mice. Trends Neurosci. 2009;32:347–358. doi: 10.1016/j.tins.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov. J. Biol. Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 24.Arguello PA, Gogos JA. Cognition in mouse models of schizophrenia susceptibility genes. Schizophr. Bull. 2010;36:289–300. doi: 10.1093/schbul/sbp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 26.Jones CA, Watson DJ, Fone KC. Animal models of schizophrenia. Br. J. Pharmacol. 2011;164:1162–1194. doi: 10.1111/j.1476-5381.2011.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winslow JT. Mouse social recognition and preference. Curr. Protoc. Neurosci. 2003;Chapter 8(Unit) doi: 10.1002/0471142301.ns0816s22. [DOI] [PubMed] [Google Scholar]
- 28.Daenen EW, Wolterink G, Gerrits MA, Van Ree JM. Amygdala or ventral hippocampal lesions at two early stages of life differentially affect open field behaviour later in life; an animal model of neurodevelopmental psychopathological disorders. Behav. Brain Res. 2002;131:67–78. doi: 10.1016/s0166-4328(01)00350-3. [DOI] [PubMed] [Google Scholar]
- 29.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 30.Hunt DL, Castillo PE. Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Curr. Opin. Neurobiol. 2012;22:496–508. doi: 10.1016/j.conb.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilmour G, Dix S, Fellini L, Gastambide F, Plath N, Steckler T, Talpos J, Tricklebank M. NMDA receptors, cognition and schizophrenia--testing the validity of the NMDA receptor hypofunction hypothesis. Neuropharmacology. 2012;62:1401–1412. doi: 10.1016/j.neuropharm.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Coyle JT. NMDA receptor and schizophrenia: a brief history. Schizophr. Bull. 2012;38:920–926. doi: 10.1093/schbul/sbs076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snyder MA, Gao WJ. NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Front Cell Neurosci. 2013;7:31. doi: 10.3389/fncel.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson G, Maes M. Schizophrenia: linking prenatal infection to cytokines, the tryptophan catabolite (TRYCAT) pathway, NMDA receptor hypofunction, neurodevelopment and neuroprogression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;42:5–19. doi: 10.1016/j.pnpbp.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Wen D, Peles E, Cupples R, Suggs SV, Bacus SS, Luo Y, Trail G, Hu S, Silbiger SM, Levy RB, et al. Neu differentiation factor: a transmembrane glycoprotein containing an EGF domain and an immunoglobulin homology unit. Cell. 1992;69:559–572. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- 36.Burgess TL, Ross SL, Qian YX, Brankow D, Hu S. Biosynthetic processing of neu differentiation factor. Glycosylation trafficking, and regulated cleavage from the cell surface. J. Biol. Chem. 1995;270:19188–19196. doi: 10.1074/jbc.270.32.19188. [DOI] [PubMed] [Google Scholar]
- 37.Kramer R, Bucay N, Kane DJ, Martin LE, Tarpley JE, Theill LE. Neuregulins with an Ig-like domain are essential for mouse myocardial and neuronal development. Proc. Natl. Acad. Sci. U. S. A. 1996;93:4833–4838. doi: 10.1073/pnas.93.10.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawyer DB, Caggiano A. Neuregulin-1beta for the treatment of systolic heart failure. J. Mol. Cell Cardiol. 2011;51:501–505. doi: 10.1016/j.yjmcc.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat. Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- 40.Buonanno A, Kwon OB, Yan L, Gonzalez C, Longart M, Hoffman D, Vullhorst D. Neuregulins and neuronal plasticity: possible relevance in schizophrenia. Novartis. Found. Symp. 2008;289:165–177. doi: 10.1002/9780470751251.ch13. [DOI] [PubMed] [Google Scholar]
- 41.Emamian ES. AKT/GSK3 signaling pathway and schizophrenia. Front Mol. Neurosci. 2012;5:33. doi: 10.3389/fnmol.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kato T, Kasai A, Mizuno M, Fengyi L, Shintani N, Maeda S, Yokoyama M, Ozaki M, Nawa H. Phenotypic characterization of transgenic mice overexpressing neuregulin-1. PLoS. One. 2010;5:e14185. doi: 10.1371/journal.pone.0014185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin DM, Chen YJ, Lu YS, Bean JC, Sathyamurthy A, Shen C, Liu X, Lin TW, Smith CA, Xiong WC, et al. Reversal of behavioral deficits and synaptic dysfunction in mice overexpressing neuregulin 1. Neuron. 2013;78:644–657. doi: 10.1016/j.neuron.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bao J, Wolpowitz D, Role LW, Talmage DA. Back signaling by the Nrg-1 intracellular domain. J. Cell Biol. 2003;161:1133–1141. doi: 10.1083/jcb.200212085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bjarnadottir M, Misner DL, Haverfield-Gross S, Bruun S, Helgason VG, Stefansson H, Sigmundsson A, Firth DR, Nielsen B, Stefansdottir R, et al. Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1+/− knock-outs compared with wild-type mice. J. Neurosci. 2007;27:4519–4529. doi: 10.1523/JNEUROSCI.4314-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bennett M. Positive and negative symptoms in schizophrenia: the NMDA receptor hypofunction hypothesis, neuregulin/ErbB4 and synapse regression. Aust. N. Z. J. Psychiatry. 2009;43:711–721. doi: 10.1080/00048670903001943. [DOI] [PubMed] [Google Scholar]
- 47.Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat. Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Ford B, Mann MA, Fischbach GD. Neuregulins increase alpha7 nicotinic acetylcholine receptors and enhance excitatory synaptic transmission in GABAergic interneurons of the hippocampus. J. Neurosci. 2001;21:5660–5669. doi: 10.1523/JNEUROSCI.21-15-05660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, Lai C, Rubenstein JL, Marin O. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 50.Okada M, Corfas G. Neuregulin1 downregulates postsynaptic GABAA receptors at the hippocampal inhibitory synapse. Hippocampus. 2004;14:337–344. doi: 10.1002/hipo.10185. [DOI] [PubMed] [Google Scholar]
- 51.Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, Neiswender H, Dong XP, Wu J, Gassmann M, et al. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]