Abstract
Background
Alzheimer’s disease (AD) profoundly affects the end-of-life experience. Yet counts of deaths attributable to AD understate this burden of AD in the population. Therefore, we estimated the annual number of deaths in the US among older adults with AD from 2010–2050.
Methods
We calculated probabilities of AD incidence and mortality from a longitudinal, population-based study of 10,800 participants. From this population, 1,913 previously disease-free individuals, selected via stratified random sampling, underwent 2,577 detailed clinical evaluations. Over the course of follow-up, 990 participants died. We computed age-sex-race- and education-specific AD incidence and education-adjusted AD mortality proportions specific to age-sex-race group. We then combined these probabilities with US-wide census, education, and mortality data.
Results
In 2010, approximately 600,000 deaths occurred among individuals age 65 or older with AD, comprising 32% of all older adult deaths. By 2050, this number is projected to be 1.6 million, 43% of all older adult deaths.
Conclusion
Individuals with AD comprise a substantial number of older adult deaths in the US, a number expected to rise considerably in coming decades.
Keywords: Alzheimer’s disease, dementia, mortality, United States, epidemiology, forecasting, longitudinal studies, censuses, aged, population surveillance
1. Introduction
Over the past century, common causes of death in the United States have shifted from a portfolio in which acute, communicable diseases played a prominent role to one dominated by chronic diseases most of which have less obvious involvement with infectious agents.1 Accompanying this shift has been a dramatic reduction infant and childhood mortality—by about 90% between 1935 and 20102— and a substantial lengthening of life expectancy.3 Even in the modern era of chronic, degenerative diseases of old age, the “face of death” has been changing. The rate of cardiovascular deaths has been falling since the 1960s.2 Moreover, from 2000 to 2010, declines have occurred in the number of deaths attributed, via death certificate, to cardiovascular disease, as well as some other chronic diseases such as HIV infection and prostate and colorectal cancer.4–5 By contrast, during the same period the number of deaths attributed to Alzheimer’s disease dementia (AD) has increased by more than two thirds. This increase is likely due, in part, to improved awareness of and diagnosis of the condition. The changing age structure of the population likely underlies the increase as well: between 2000 and 2010, the number of adults age 65 or older in the US grew by 15%, and the number of adults age 85 or older, the age of greatest AD risk, grew even more—by 30%.6
The more frequent appearance of AD in the “face of death” has critical implications for the end-of-life experience of an increasing number of affected individuals and their families. For example, many individuals with advanced cognitive impairment or dementia are likely to live in a nursing home toward the end of their lives.7 Even if care in that setting is appropriate, it is not covered by Medicare, the most common form of medical insurance among older adults. Rather, patients pay out of pocket, use long-term care insurance, or, if their assets are sufficiently meager, use Medicaid coverage. The quality of end-of-life care for persons with AD or other dementias varies substantially, as well,8–9 and is not necessarily shifting away from less intervention: over the past decade, the rate of health care transitions at the end of life among people with dementia has increased slightly, involving more hospice care but also more days in critical care.7 These shortcomings in the end-of-life experiences of individuals with AD may be grossly magnified over the coming decades unless effective treatments emerge or effective approaches for managing AD are more widely disseminated.
Death certificates likely undercount the number of deaths among people with AD, irrespective of whether AD was deemed to be the underlying cause of a particular death. Furthermore, so long as there is no effective treatment for AD, the expected upsurge of AD cases in the population10 means that, in the coming decades, even more individuals will be suffering from it when they die. Estimating the current and future number of deaths among people with AD contributes to fuller appreciation of the needs to develop and broadly implement effective and humane methods for managing AD and supporting informal caregivers—needs that are consistent with the goals of the National Plan to Address Alzheimer’s Disease. Therefore, we combined data from a population-based study, in which participants were followed longitudinally and systematically evaluated for AD, along with national population and mortality data, to estimate the number of deaths in the US among older adults with AD from 2010 to 2050. In essence, we extrapolated the AD mortality experience of this well-characterized cohort to the US at large, accounting for age, sex, race and education.
2. Methods
As part of our previous estimation of prevalence of AD,10 we estimated the number of people who die each year with AD. To estimate the number of deaths, we used: AD incidence and mortality information from the Chicago Health and Aging Project (CHAP); US census data on the population, projected population, and projected mortality; and data on past mortality from the National Center for Health Statistics.
2.1. Assessment of Alzheimer’s Disease in CHAP
We obtained data on incidence of AD and relative risk of mortality (comparing persons with AD versus those without) from the Chicago Health and Aging Project (CHAP),11–12 a longitudinal, population-based study in a geographically defined area of Chicago. The study began in 1993 with a census of individuals age 65 or older. Of those identified, 6,158 (79%) participated in a home interview. Additional people enrolled as they turned age 65, for a total of 10,802 participants through 2011. Participants were re-interviewed in 3-year cycles. Each data-collection cycle consisted of in-home interview of all participants; a stratified, Bernoulli sample of participants underwent detailed clinical evaluation for AD. The sampling strategy for this clinical evaluation has previously been described in detail.11–14 Briefly, from the surviving cohort determined to be free of AD at the previous cycle, sampling for clinical evaluation of incident AD in cycles 2 to 6 was stratified by age, race, sex, and change in cognitive function from the previous home interview, with persons randomly selected for evaluation from all levels of cognitive change. Between 1997 and 2010, 402 cases of incident AD were identified in 2577 evaluations among 1913 individuals determined to be free of AD at the previous cycle, an average interval of 4.0 years earlier. These 1913 participants were 38% male and 54% black. Their mean age was 77.1 years and mean years of education was 12.9. All participants examined received identical structured clinical evaluations by examiners blinded to population-interview cognitive testing and sampling category. Diagnosis of dementia required the study neurologist’s or geriatrician’s assessment of loss of cognitive function and impairment in at least two areas on cognitive performance testing. Criteria for AD were those of the working group of the National Institute of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association for probable AD.15 Persons who met these AD criteria and had another condition impairing cognition also were classified as AD cases. Nearly all dementia cases diagnosed in CHAP (93%) met minimum clinical criteria for AD or AD mixed with another dementia.
2.2. Alzheimer’s Disease Incidence and Mortality in CHAP
We computed risk of AD incidence using weighted logistic regression models with predictors consisting of age at time of evaluation (truncated at 90), age squared (truncated), an indicator for age> 90, sex, race (black/other), years of education in 3 groups (<12, 12, >12) and interval between disease-free determination and clinical evaluation. (The age truncation and a group indictor were used because there were not enough observations over age 90 to reliably indicate trend. Education was grouped in a way that gave the best distribution to categories used by the 2006–2008 US Bureau of Census Current Population Survey.)
Among the same people, we estimated risk of mortality among persons with AD versus those without AD, using weighted Cox proportional hazards regression models, adjusting for age, sex, race and education. There were 990 deaths and mean follow-up of 6.1 years. There were no significant interactions among predictors.
We adjusted the sampling weights for participation in the clinical evaluation using Iterative Proportional Fitting.16 Using these weights in the computation of our incidence and mortality models provided estimates pertaining to the full CHAP population. Including loss to death since the most recent home interview, 68% of sampled individuals participated in the clinical evaluation.
2.3. Extrapolation to the U.S. Population of Adults, Ages 65 and Older
We calculated separate incidence rate estimates (for each evaluation and calendar year) for 432 groups defined by single year of age, sex, two race groups and three education groups. We then generated estimates for each age-sex-race group that were collapsed across the education groups, by computing a weighted average of the individual education cells. The weights in these calculations were based on the 2006–2008 level of education from the US Bureau of Census Current Population Survey. We obtained the educational status of populations beyond 2010 (e.g., 2011, 2012, etc.) by aging the population in the 2010 data so that the education distribution of people age 65 in 2010 would be the same distribution used for those age 75 in 2020.
For each calendar year, we estimated the prevalence of AD in each US subpopulation jointly defined by sex, race, and year of age, beginning with age 65. Each estimate incorporated information on the AD and mortality (with or without AD) experience of the corresponding birth cohort in previous years. For example, the prevalence estimate for African-American women aged 69 in 2013 incorporated information on African-American women aged 68 in 2012, which in turn incorporated information from African-American women aged 67 in 2011, and so on. We used life-table estimates of number of people alive (of a theoretical birth cohort of 100,000) and probability of death for the relevant age and calendar year. Beginning at age 65 for each birth cohort (and assuming no AD before age 65), we obtained the number of people developing AD at each subsequent age and, therefore, calendar year by multiplying the number of people alive without AD at the beginning of the one-year age interval by the probability of incident AD for each group jointly defined by age, sex and race. We added the new cases to the number with AD carried over from the previous age in the previous calendar year and subtracted them from the disease-free number.
Using 2.13 as the relative risk of dying with AD10 and an iterative algorithm, we divided the total number of deaths in the age interval into those dying with and without AD. We subtracted the deaths from the AD and the AD-free numbers to provide the new numbers of people with and without AD for the next age in the subsequent year. The final calculation in the process (for the age of interest in the year of interest) contains the estimated number of deaths among people with AD for that age and year in the theoretical birth cohort. We divided that number by the total number alive at the beginning of the year to obtain the proportion of the cohort dying with AD for that race, sex and year of age. Because deaths occur throughout the year, we repeated the entire prevalence procedure, subtracting the deaths at each age before rather than after computing the new cases of AD to provide a range for the prevalence proportion and the AD proportion of deaths. We then averaged the proportions calculated by the two procedures for each age-sex-race group.
In the final stage we multiplied the proportion of the cohort dying with AD by the census estimate of number of people in each age-sex-race group and summed across groups to obtain total numbers of people dying with AD.
2.4. Standard Protocol Approvals, Registrations and Patient Consents
The study was approved by the Rush University Medical Center Institutional Review Board.
3. Results
In 2010, an estimated 600,000 deaths occurred among older adults (ages 65 and older) in the US who had AD (Table 1). This estimate encompasses both individuals who were identified during the routine delivery of medical care as having AD and those who were not. The number of deaths with AD increased to 700,000 in 2011, but is expected to increase only modestly for the ensuing three decades, reaching 900,000 in 2030. By 2040, however, the number of deaths is predicted to be more than double (1.3 million) the 2010 count, and by 2050, the number of deaths is predicted to reach 1.6 million.
Table 1.
Predicted number of deaths among older adults (ages 65+) in the US with Alzheimer’s disease dementia (AD; in hundreds of thousands), by year.
| Year | Number* of deaths | |
|---|---|---|
| Among older adults with AD | Among all older adults | |
| 2010 | 6 | 19 |
| 2011 | 7 | 19 |
| 2012 | 7 | 20 |
| 2013 | 7 | 20 |
| 2014 | 7 | 20 |
| 2015 | 7 | 21 |
| 2016 | 7 | 21 |
| 2017 | 7 | 21 |
| 2018 | 7 | 21 |
| 2019 | 7 | 22 |
| 2020 | 7 | 22 |
| 2030 | 9 | 27 |
| 2040 | 13 | 33 |
| 2050 | 16 | 37 |
in hundreds of thousands.
The total number of deaths among older adults in the US also is expected to increase over the same period (Table 1). Yet, after 2020, the fraction of AD deaths among all older adult deaths will begin to surge, such that by 2050, about 43% of all older adult deaths will be deaths among individuals with AD, compared with 32% in 2010 (Figure 1).
Figure 1.
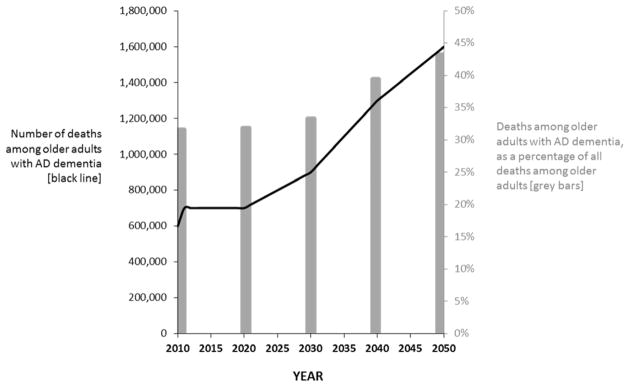
Deaths among older adults (ages 65 and older) in the US who have Alzheimer’s disease dementia (AD), number and percentage of all deaths (2010 to 2050). (Weuve J et al.)
4. Discussion
We estimated that well over half a million deaths per year in the US occur among older adults with AD. About two and a half times as many AD deaths will occur in 2050, short of the emergence of a broadly applied intervention that prevents the condition or delays its onset. Two considerations underlie the substantial numbers of deaths. First, there is no recovery from AD, and the condition persists until death. Second, most deaths in the US occur in older adulthood,4 the age of greatest risk for AD. In particular, the striking ascent in the number of AD deaths after 2020 is due to the aging of the post-World-War-II baby boom generation into the age of greatest AD and mortality risk. For instance, in 2030, this generation will be 66 to 84 years old.
Deaths among individuals with AD make up a considerable proportion of all older adult deaths in the US, a proportion that will increase from 32% in 2010 to 43% in 2050. Underlying this increase is the growing proportion, after 2030, of persons with AD who are 85 years old or older, the age band with the highest number of deaths and mortality rate in the US.4 More than half of AD cases will be 85+ by 2050.10 Thus, the aging baby boom generation not only underlies the surge in the number of AD cases, but also the expected growth in both the number of AD deaths and the percentage of deaths that are people with AD.
Importantly our study does not at all confront the idea of attributing a death to a particular disease as a cause. Our estimates are counts of deaths among people with AD, rather than counts of deaths pathogenically attributed to AD. Thus, our estimate of deaths with AD can be regarded as an upper limit of deaths that could be attributed to AD based on clinical features present during life. Attribution of a death wholly or partially to a particular disease is a complex process. Two facets are especially relevant to AD. Many older adults have multiple co-morbid conditions17–19 so that deciding the degree to which of these co-existing conditions is responsible for death is a matter of judgment that varies substantially.20–23 However, there is also a continuing secular trend in the US for a greater proportion of death certificates to list AD as a cause of death.24–25
Studies that have attempted to confront the complex idea of attribution have, of course, resulted in estimates below this upper limit, but may still understate the burden of AD. Using an algorithm based on death certificate data, the National Center for Health Statistics (NCHS) estimated that 82,616 older adults died because of their AD in 2010, comprising 5% of older adult deaths.4 Comparisons of death certificate data with other supporting data show that presiding physicians severely underreport AD on death certificates.20 This underreporting may be due, in part, to training and awareness of a previously made diagnosis. In a study that relied on health care data, Tinetti and colleagues modeled the risk of death as predicted by diagnoses documented in fee-for-service Medicare claims from 2002 to 2006, and they estimated that 14% of older adult deaths involved AD as an underlying cause.26 Historically, however, many individuals with AD—more than half in some studies—have not been diagnosed.27 To counter the tendency for AD to be underdiagnosed and underdocumented, we conducted systematic diagnostic evaluations in CHAP, irrespective of participants’ memory complaints or their interaction with the health care system
We estimated that about 32% of older adult deaths in 2010 were persons with AD, similar to the 33% of older adult deaths that were persons with AD or other dementias, as calculated from 2009 Medicare claims data and reported by the Alzheimer’s Association.28 The similarity is, at first, surprising. There is some evidence that the accuracy of Medicare claims in representing dementia diagnoses may have improved.29–30 Ironically, however, the number of cases who are missing documentation in Medicare claims (sensitivity, 85%) is more than offset by the number of non-cases that claims data identify as having dementia (specificity, 89%).30
Our study has limitations that warrant mention. We were not able to incorporate information on severity of disease into our estimates, because there is no standard rate of progression of the disease. In addition, these estimates assumed the risks of developing and dying from AD are the same for people of Hispanic origin and the racial group with which they identify. If these assumptions do not hold, the number of deaths among individuals with AD may differ, especially in the future when a larger proportion of the older population will be Hispanic.31
The data that underlay our incidence estimates and the AD mortality hazard ratio (HR) came from a limited geographic area, and our mortality estimation process assumes that these will remain unchanged over time. Yet, our estimated mortality HR is nearly identical to the AD mortality HR estimated over a decade ago in the East Boston cohort of the Established Populations for Epidemiologic Study of the Elderly (EPESE) Project.32 Similar to this cohort, the community-based CHAP study population systematically underwent comprehensive assessments for AD, so that incidence and mortality estimates did not hinge on diagnoses made within the medical care system—a function of access to and quality of care—or the recording of AD diagnoses on death certificates. Moreover, CHAP’s approach to diagnosis included evaluation of people from all strata of cognitive performance, including the best performers, which prevented undercounting new cases and undercounting AD among participants who died.33 Thus, relying on incidence and mortality rates derived from a study that systematically assessed its participants in this way likely improved the accuracy of our death estimates.
Our study indicates that an enormous number of older adults and their families are facing the end-of-life experience associated with AD. This experience is hardly uniform, with meaningful variation in quality of care and considerable costs imposed on both public insurance programs and affected individuals and their loved ones. The prevention of AD could lessen the future impact of AD deaths by reducing the number of people who develop this condition. Closer to the present, however, it may be possible to improve the quality of care provided to individuals with AD—its coordination, the degree to which it is palliative and helpful versus burdensome and inappropriate, and the involvement of loved ones in understanding the course of the disease.34
RESEARCH IN CONTEXT.
Systematic review
We searched United States (US) government statistics and MEDLINE to identify previous estimates of the number of older adult deaths attributed to Alzheimer disease (AD) in the US. We are unaware of any previous reports on how many older adult deaths are persons who have AD.
Interpretation
Individuals with AD comprise a substantial number of deaths in the US, a number expected to rise considerably in coming decades. The estimated number of deaths among persons with AD eclipses estimates that have relied on the idea of attribution. Yet, the former measure reveals substantial burden on the population, indicating that an enormous number of individuals and their families face the end-of-life experience associated with AD.
Future directions
The prevention of AD could lessen its end-of-life burden in the future. Closer to the present, it may be possible to improve the quality of care provided to individuals with AD.
Acknowledgments
This work was funded by National Institutes of Health/National Institute on Aging grant AG011101 and the Alzheimer’s Association. These sponsors did not have a role in the study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
The analyses were performed by Liesi Hebert, Paul Scherr, Ken Tonnissen, and Todd Beck, Rush Institute for Healthy Aging. Dr. Liesi Hebert had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Authors’ contributions
J. Weuve: conception and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, obtaining funding.
L. E. Hebert: conception and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, obtaining funding, supervision.
P. A. Scherr: conception and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis.
D. A. Evans: conception and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, obtaining funding, supervision.
Authors’ potential conflicts of interest
Below are each author’s financial relationships that might lead to a perceived conflict of interest.
J. Weuve is a consultant for the Alzheimer’s Association and the AlzRisk Project (www.alzrisk.org). She is also funded by Alzheimer’s Association grant NIRG-12-242395 and NIH grants R21ES019712 and R21ES020404.
L. E. Hebert is funded by NIH grants NR010211, AG303544, AG011101, AG036650, and AG009966.
P. A. Scherr reports no disclosures.
D. A. Evans is funded by NIH grants AG11101, AG036650, AG09966, AG030146, AG10161, AG021972, ES10902, NR009543, HL084209, and AG12505l.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jones DS, Podolsky SH, Greene JA. The burden of disease and the changing task of medicine. N Engl J Med. 2012;366(25):2333–2338. doi: 10.1056/NEJMp1113569. [DOI] [PubMed] [Google Scholar]
- 2.Hoyert DL. 75 years of mortality in the United States 1935–2010. NCHS Data Brief. 2012;(88):1–8. [PubMed] [Google Scholar]
- 3.Arias E. United States life tables, 2008. Natl Vital Stat Rep. 2012;61(3):1–64. [PubMed] [Google Scholar]
- 4.National Center for Health Statistics. National vital statistics reports. 3. Vol. 61. Hyattsville, MD: National Center for Health Statistics;in press; Detailed Tables for Deaths: Final Data for 2010, National vital statistics reports. [Google Scholar]
- 5.Minino AM, Arias E, Kochanek KD, Murphy SL, Smith BL. Deaths: final data for 2000. Natl Vital Stat Rep. 2002;50(15):1–119. [PubMed] [Google Scholar]
- 6.Howden LM, Meyer JA. Age and sex composition: 2010. Washington, D.C: United States Census Bureau, Economics and Statistics Administration, U.S. Department of Commerce; May, 2011. p. C2010BR-03. [Google Scholar]
- 7.Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309(5):470–477. doi: 10.1001/jama.2012.207624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teno JM, Mitchell SL, Skinner J, et al. Churning: the association between health care transitions and feeding tube insertion for nursing home residents with advanced cognitive impairment. J Palliat Med. 2009;12(4):359–362. doi: 10.1089/jpm.2008.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gozalo P, Teno JM, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011;365(13):1212–1221. doi: 10.1056/NEJMsa1100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013 doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project (CHAP) J Alzheimers Dis. 2003;5(5):349–355. doi: 10.3233/jad-2003-5501. [DOI] [PubMed] [Google Scholar]
- 12.Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60(2):185–189. doi: 10.1001/archneur.60.2.185. [DOI] [PubMed] [Google Scholar]
- 13.Bienias JL, Kott PS, Evans DA. Application of the delete-a-group jackknife variance estimator to analyses of data from a complex longitudinal survey. Proceedings of the Annual Meeting of the American Statistical Association - Section on Survey Research Methods [CD-ROM]; 2003. pp. 539–544. [Google Scholar]
- 14.Bienias JL, Kott PS, Beck TL, Evans DA. Incorporating multiple observations into logistic regression models of incident disease. Proceedings of the Annual Meeting of the American Statistical Association - Section on Survey Research Methods [CD-ROM]; 2005. pp. 2767–2774. [Google Scholar]
- 15.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 16.Little RJA, Rubin DB. Statistical Analysis with Missing Data. 2. New York: Wiley; 2002. [Google Scholar]
- 17.Kuo TC, Zhao Y, Weir S, Kramer MS, Ash AS. Implications of comorbidity on costs for patients with Alzheimer disease. Med Care. 2008;46(8):839–846. doi: 10.1097/MLR.0b013e318178940b. [DOI] [PubMed] [Google Scholar]
- 18.Parekh Ak BMB. The challenge of multiple comorbidity for the us health care system. JAMA. 2010;303(13):1303–1304. doi: 10.1001/jama.2010.381. [DOI] [PubMed] [Google Scholar]
- 19.Wolff Jl SBAG. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Archives of Internal Medicine. 2002;162(20):2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 20.Ives DG, Samuel P, Psaty BM, Kuller LH. Agreement between nosologist and cardiovascular health study review of deaths: implications of coding differences. J Am Geriatr Soc. 2009;57(1):133–139. doi: 10.1111/j.1532-5415.2008.02056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madsen A, Thihalolipavan S, Maduro G, et al. An intervention to improve cause-of-death reporting in New York City hospitals, 2009–2010. Prev Chronic Dis. 2012;9:E157. doi: 10.5888/pcd9.120071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbins JA. Commentary: death certificate reporting needs to be fixed. J Public Health Policy. 2012;33(2):215–217. doi: 10.1057/jphp.2012.5. [DOI] [PubMed] [Google Scholar]
- 23.Smith Sehdev AE, Hutchins GM. Problems with proper completion and accuracy of the cause-of-death statement. Arch Intern Med. 2001;161(2):277–284. doi: 10.1001/archinte.161.2.277. [DOI] [PubMed] [Google Scholar]
- 24.Tejada-Vera B. Mortality from Alzheimer’s disease in the United States: data for 2000 and 2010. NCHS Data Brief. 2013;(116):1–8. [PubMed] [Google Scholar]
- 25.Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9(2):208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Tinetti ME, McAvay GJ, Murphy TE, Gross CP, Lin H, Allore HG. Contribution of individual diseases to death in older adults with multiple diseases. J Am Geriatr Soc. 2012;60(8):1448–1456. doi: 10.1111/j.1532-5415.2012.04077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alzheimer’s Association. 2011 Alzheimer’s disease facts and figures. Alzheimers Dement. 2011;7(2):208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Thies W, Bleiler L. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9(2):208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Newcomer R, Clay T, Luxenberg JS, Miller RH. Misclassification and selection bias when identifying Alzheimer’s disease solely from Medicare claims records. J Am Geriatr Soc. 1999;47(2):215–219. doi: 10.1111/j.1532-5415.1999.tb04580.x. [DOI] [PubMed] [Google Scholar]
- 30.Taylor DH, Jr, Ostbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis. 2009;17(4):807–815. doi: 10.3233/JAD-2009-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vincent GK, Velkoff VA. Current Population Reports. Washington, DC: U.S. Census Bureau; 2010. The Next Four Decades, The Older Population in the United States: 2010 to 2050. [Google Scholar]
- 32.Hebert LE, Scherr PA, McCann JJ, Beckett LA, Evans DA. Is the risk of developing Alzheimer’s disease greater for women than for men? Am J Epidemiol. 2001;153(2):132–136. doi: 10.1093/aje/153.2.132. [DOI] [PubMed] [Google Scholar]
- 33.Brookmeyer R, Evans DA, Hebert L, et al. National estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimers Dement. 2011;7(1):61–73. doi: 10.1016/j.jalz.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529–1538. doi: 10.1056/NEJMoa0902234. [DOI] [PMC free article] [PubMed] [Google Scholar]


