Abstract
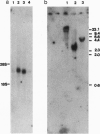
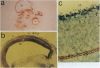
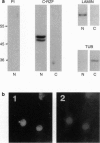
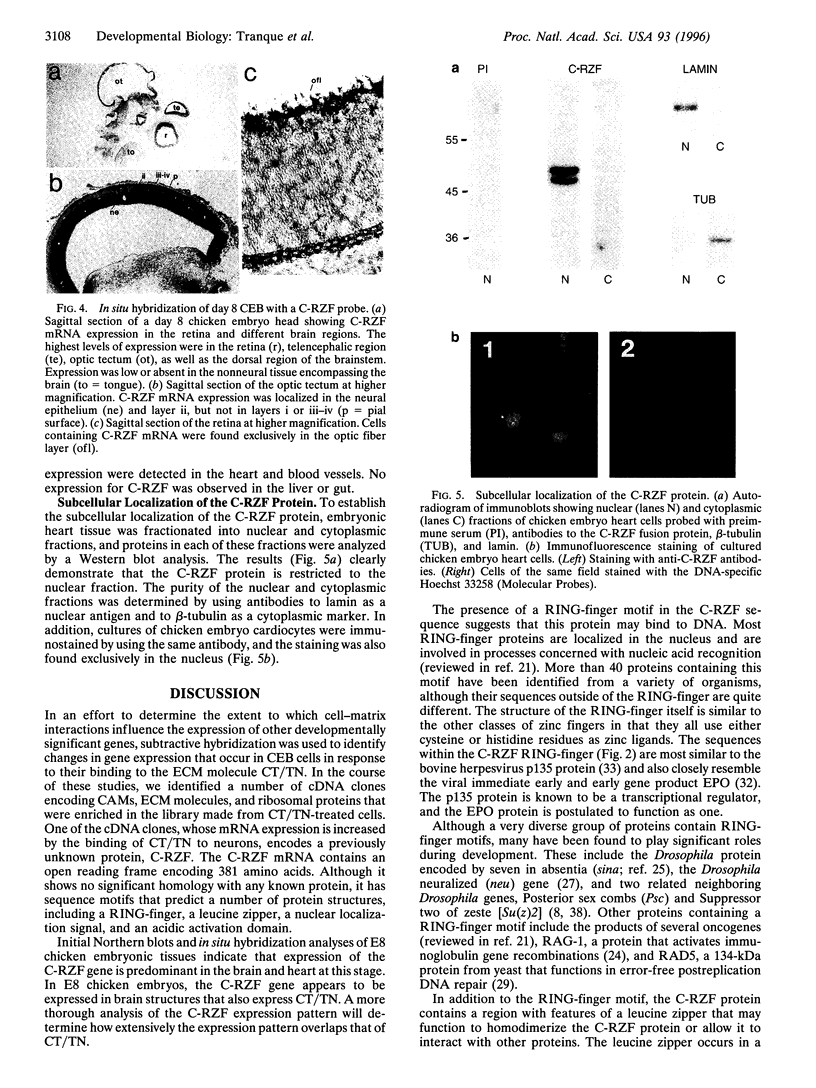
To identify changes in gene expression that occur in chicken embryo brain (CEB) cells as a consequence of their binding to the extracellular matrix molecule cytotactin/tenascin (CT/TN), a subtractive hybridization cloning strategy was employed. One of the cDNA clones identified was predicted to encode 381 amino acids and although it did not resemble any known sequences in the nucleic acid or protein data bases, it did contain the sequence motif for the cysteine-rich C3HC4 type of zinc finger, also known as a RING-finger. This sequence was therefore designated the chicken-RING zinc finger (C-RZF). In addition to the RING-finger, the C-RZF sequence also contained motifs for a leucine zipper, a nuclear localization signal, and a stretch of acidic amino acids similar to the activation domains of some transcription factors. Southern analysis suggested that C-RZF is encoded by a single gene. Northern and in situ hybridization analyses of E8 chicken embryo tissues indicated that expression of the C-RZF gene was restricted primarily to brain and heart. Western analysis of the nuclear and cytoplasmic fractions of chicken embryo heart cells and immunofluorescent staining of chicken embryo cardiocytes with anti-C-RZF antibodies demonstrated that the C-RZF protein was present in the nucleus. The data suggest that we have identified another member of the RING-finger family of proteins whose expression in CEB cells may be affected by CT/TN and whose nuclear localization and sequence motifs predict a DNA-binding function in the nucleus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber T. Structure of the leucine zipper. Curr Opin Genet Dev. 1992 Apr;2(2):205–210. doi: 10.1016/s0959-437x(05)80275-8. [DOI] [PubMed] [Google Scholar]
- Bange F. C., Vogel U., Flohr T., Kiekenbeck M., Denecke B., Böttger E. C. IFP 35 is an interferon-induced leucine zipper protein that undergoes interferon-regulated cellular redistribution. J Biol Chem. 1994 Jan 14;269(2):1091–1098. [PubMed] [Google Scholar]
- Boulikas T. Putative nuclear localization signals (NLS) in protein transcription factors. J Cell Biochem. 1994 May;55(1):32–58. doi: 10.1002/jcb.240550106. [DOI] [PubMed] [Google Scholar]
- Brackenbury R., Greenberg M. E., Edelman G. M. Phenotypic changes and loss of N-CAM-mediated adhesion in transformed embryonic chicken retinal cells. J Cell Biol. 1984 Dec;99(6):1944–1954. doi: 10.1083/jcb.99.6.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk B. P., Martin E. C., Adler P. N. Drosophila genes Posterior Sex Combs and Suppressor two of zeste encode proteins with homology to the murine bmi-1 oncogene. Nature. 1991 Sep 26;353(6342):351–353. doi: 10.1038/353351a0. [DOI] [PubMed] [Google Scholar]
- Byrd D. A., Sweet D. J., Panté N., Konstantinov K. N., Guan T., Saphire A. C., Mitchell P. J., Cooper C. S., Aebi U., Gerace L. Tpr, a large coiled coil protein whose amino terminus is involved in activation of oncogenic kinases, is localized to the cytoplasmic surface of the nuclear pore complex. J Cell Biol. 1994 Dec;127(6 Pt 1):1515–1526. doi: 10.1083/jcb.127.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A., Bouchard T. S., Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990 Apr;110(4):1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheun J. E., Yeh H. H. Differentiation of a stem cell line toward a neuronal phenotype. Int J Dev Neurosci. 1991;9(4):391–404. doi: 10.1016/0736-5748(91)90061-p. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Crossin K. L. Cell adhesion molecules in embryogenesis and disease. Ann N Y Acad Sci. 1991;615:172–186. doi: 10.1111/j.1749-6632.1991.tb37759.x. [DOI] [PubMed] [Google Scholar]
- Crossin K. L. Functional role of cytotactin/tenascin in morphogenesis: a modest proposal. Perspect Dev Neurobiol. 1994;2(1):21–32. [PubMed] [Google Scholar]
- Driscoll D. M., Williams J. G. Two divergently transcribed genes of Dictyostelium discoideum are cyclic AMP-inducible and coregulated during development. Mol Cell Biol. 1987 Dec;7(12):4482–4489. doi: 10.1128/mcb.7.12.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Crossin K. L. Cell adhesion molecules: implications for a molecular histology. Annu Rev Biochem. 1991;60:155–190. doi: 10.1146/annurev.bi.60.070191.001103. [DOI] [PubMed] [Google Scholar]
- Freemont P. S., Hanson I. M., Trowsdale J. A novel cysteine-rich sequence motif. Cell. 1991 Feb 8;64(3):483–484. doi: 10.1016/0092-8674(91)90229-r. [DOI] [PubMed] [Google Scholar]
- Freemont P. S. The RING finger. A novel protein sequence motif related to the zinc finger. Ann N Y Acad Sci. 1993 Jun 11;684:174–192. doi: 10.1111/j.1749-6632.1993.tb32280.x. [DOI] [PubMed] [Google Scholar]
- Gregorio C. C., Weber A., Bondad M., Pennise C. R., Fowler V. M. Requirement of pointed-end capping by tropomodulin to maintain actin filament length in embryonic chick cardiac myocytes. Nature. 1995 Sep 7;377(6544):83–86. doi: 10.1038/377083a0. [DOI] [PubMed] [Google Scholar]
- Hanson I. M., Poustka A., Trowsdale J. New genes in the class II region of the human major histocompatibility complex. Genomics. 1991 Jun;10(2):417–424. doi: 10.1016/0888-7543(91)90327-b. [DOI] [PubMed] [Google Scholar]
- Haupt Y., Alexander W. S., Barri G., Klinken S. P., Adams J. M. Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. Cell. 1991 May 31;65(5):753–763. doi: 10.1016/0092-8674(91)90383-a. [DOI] [PubMed] [Google Scholar]
- Johnson R. E., Henderson S. T., Petes T. D., Prakash S., Bankmann M., Prakash L. Saccharomyces cerevisiae RAD5-encoded DNA repair protein contains DNA helicase and zinc-binding sequence motifs and affects the stability of simple repetitive sequences in the genome. Mol Cell Biol. 1992 Sep;12(9):3807–3818. doi: 10.1128/mcb.12.9.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. L., Boudreau N., Myers C. A., Erickson H. P., Bissell M. J. Tenascin-C inhibits extracellular matrix-dependent gene expression in mammary epithelial cells. Localization of active regions using recombinant tenascin fragments. J Cell Sci. 1995 Feb;108(Pt 2):519–527. doi: 10.1242/jcs.108.2.519. [DOI] [PubMed] [Google Scholar]
- Keane M. M., Rivero-Lezcano O. M., Mitchell J. A., Robbins K. C., Lipkowitz S. Cloning and characterization of cbl-b: a SH3 binding protein with homology to the c-cbl proto-oncogene. Oncogene. 1995 Jun 15;10(12):2367–2377. [PubMed] [Google Scholar]
- Keynes R. J., Cook G. M. Repellent cues in axon guidance. Curr Opin Neurobiol. 1992 Feb;2(1):55–59. doi: 10.1016/0959-4388(92)90162-e. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Shew J. Y., Hong F. D., Sery T. W., Donoso L. A., Young L. J., Bookstein R., Lee E. Y. The retinoblastoma susceptibility gene encodes a nuclear phosphoprotein associated with DNA binding activity. Nature. 1987 Oct 15;329(6140):642–645. doi: 10.1038/329642a0. [DOI] [PubMed] [Google Scholar]
- Lewin B. Commitment and activation at pol II promoters: a tail of protein-protein interactions. Cell. 1990 Jun 29;61(7):1161–1164. doi: 10.1016/0092-8674(90)90675-5. [DOI] [PubMed] [Google Scholar]
- Lovering R., Hanson I. M., Borden K. L., Martin S., O'Reilly N. J., Evan G. I., Rahman D., Pappin D. J., Trowsdale J., Freemont P. S. Identification and preliminary characterization of a protein motif related to the zinc finger. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2112–2116. doi: 10.1073/pnas.90.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro V. P., Krushel L. A., Cunningham B. A., Edelman G. M. Homophilic and heterophilic binding activities of Nr-CAM, a nervous system cell adhesion molecule. J Cell Biol. 1992 Oct;119(1):191–202. doi: 10.1083/jcb.119.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro V. P., Wood I. C., Krushel L., Crossin K. L., Edelman G. M. Cell adhesion alters gene transcription in chicken embryo brain cells and mouse embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2868–2872. doi: 10.1073/pnas.91.7.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patarca R., Freeman G. J., Schwartz J., Singh R. P., Kong Q. T., Murphy E., Anderson Y., Sheng F. Y., Singh P., Johnson K. A. rpt-1, an intracellular protein from helper/inducer T cells that regulates gene expression of interleukin 2 receptor and human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2733–2737. doi: 10.1073/pnas.85.8.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry L. J., Rixon F. J., Everett R. D., Frame M. C., McGeoch D. J. Characterization of the IE110 gene of herpes simplex virus type 1. J Gen Virol. 1986 Nov;67(Pt 11):2365–2380. doi: 10.1099/0022-1317-67-11-2365. [DOI] [PubMed] [Google Scholar]
- Price B. D., Chang Z., Smith R., Bockheim S., Laughon A. The Drosophila neuralized gene encodes a C3HC4 zinc finger. EMBO J. 1993 Jun;12(6):2411–2418. doi: 10.1002/j.1460-2075.1993.tb05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto A. L., Andersson-Fisone C., Crossin K. L. Characterization of multiple adhesive and counteradhesive domains in the extracellular matrix protein cytotactin. J Cell Biol. 1992 Nov;119(3):663–678. doi: 10.1083/jcb.119.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z., Brunovskis P., Rauscher F., 3rd, Lee L., Kung H. J. Transactivation activity of Meq, a Marek's disease herpesvirus bZIP protein persistently expressed in latently infected transformed T cells. J Virol. 1995 Jul;69(7):4037–4044. doi: 10.1128/jvi.69.7.4037-4044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy B. A., Kloc M., Etkin L. The cloning and characterization of a maternally expressed novel zinc finger nuclear phosphoprotein (xnf7) in Xenopus laevis. Dev Biol. 1991 Nov;148(1):107–116. doi: 10.1016/0012-1606(91)90321-s. [DOI] [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989 Dec 22;59(6):1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- Soullam B., Worman H. J. Signals and structural features involved in integral membrane protein targeting to the inner nuclear membrane. J Cell Biol. 1995 Jul;130(1):15–27. doi: 10.1083/jcb.130.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K. Helix-turn-helix, zinc-finger, and leucine-zipper motifs for eukaryotic transcriptional regulatory proteins. Trends Biochem Sci. 1989 Apr;14(4):137–140. doi: 10.1016/0968-0004(89)90145-X. [DOI] [PubMed] [Google Scholar]
- Söderman E., Mattsson J., Svenson M., Borkird C., Engström P. Expression patterns of novel genes encoding homeodomain leucine-zipper proteins in Arabidopsis thaliana. Plant Mol Biol. 1994 Oct;26(1):145–154. doi: 10.1007/BF00039527. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremble P., Chiquet-Ehrismann R., Werb Z. The extracellular matrix ligands fibronectin and tenascin collaborate in regulating collagenase gene expression in fibroblasts. Mol Biol Cell. 1994 Apr;5(4):439–453. doi: 10.1091/mbc.5.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoy M., Leuther K. K., Kodadek T., Johnston S. A. The acidic activation domains of the GCN4 and GAL4 proteins are not alpha helical but form beta sheets. Cell. 1993 Feb 26;72(4):587–594. doi: 10.1016/0092-8674(93)90077-4. [DOI] [PubMed] [Google Scholar]
- Wirth U. V., Fraefel C., Vogt B., Vlcek C., Paces V., Schwyzer M. Immediate-early RNA 2.9 and early RNA 2.6 of bovine herpesvirus 1 are 3' coterminal and encode a putative zinc finger transactivator protein. J Virol. 1992 May;66(5):2763–2772. doi: 10.1128/jvi.66.5.2763-2772.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lohuizen M., Verbeek S., Scheijen B., Wientjens E., van der Gulden H., Berns A. Identification of cooperating oncogenes in E mu-myc transgenic mice by provirus tagging. Cell. 1991 May 31;65(5):737–752. doi: 10.1016/0092-8674(91)90382-9. [DOI] [PubMed] [Google Scholar]