Abstract
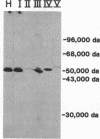
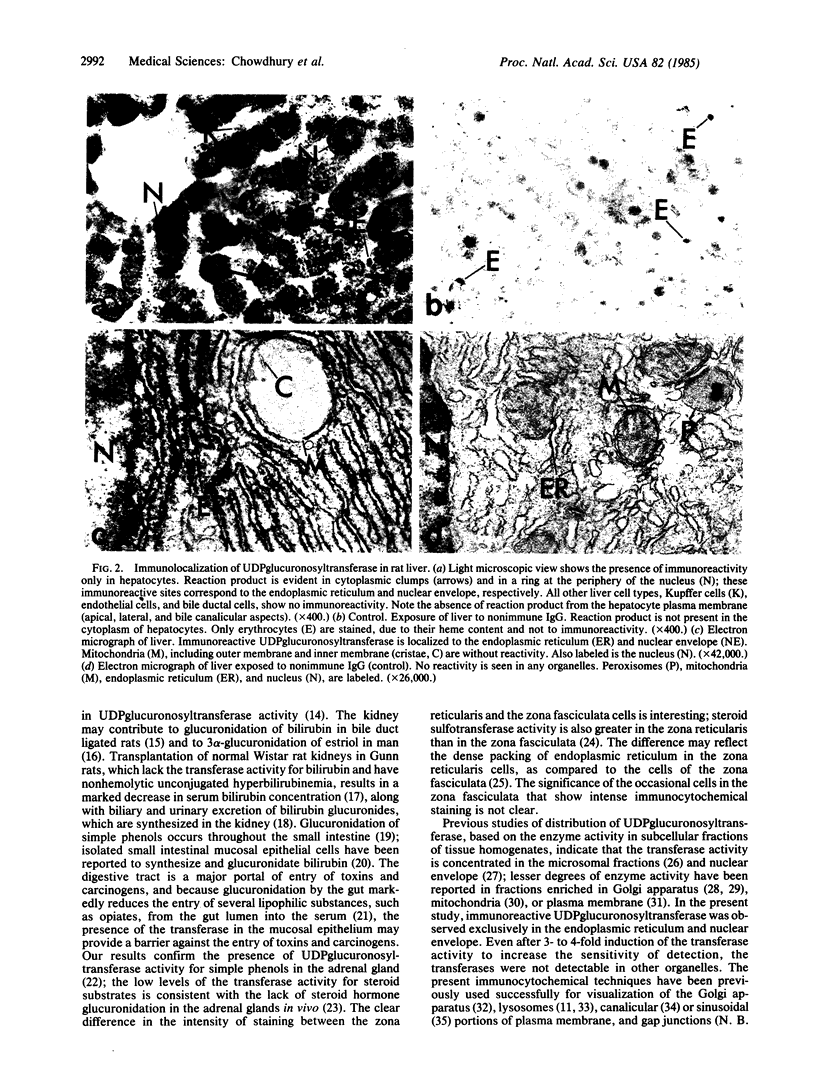
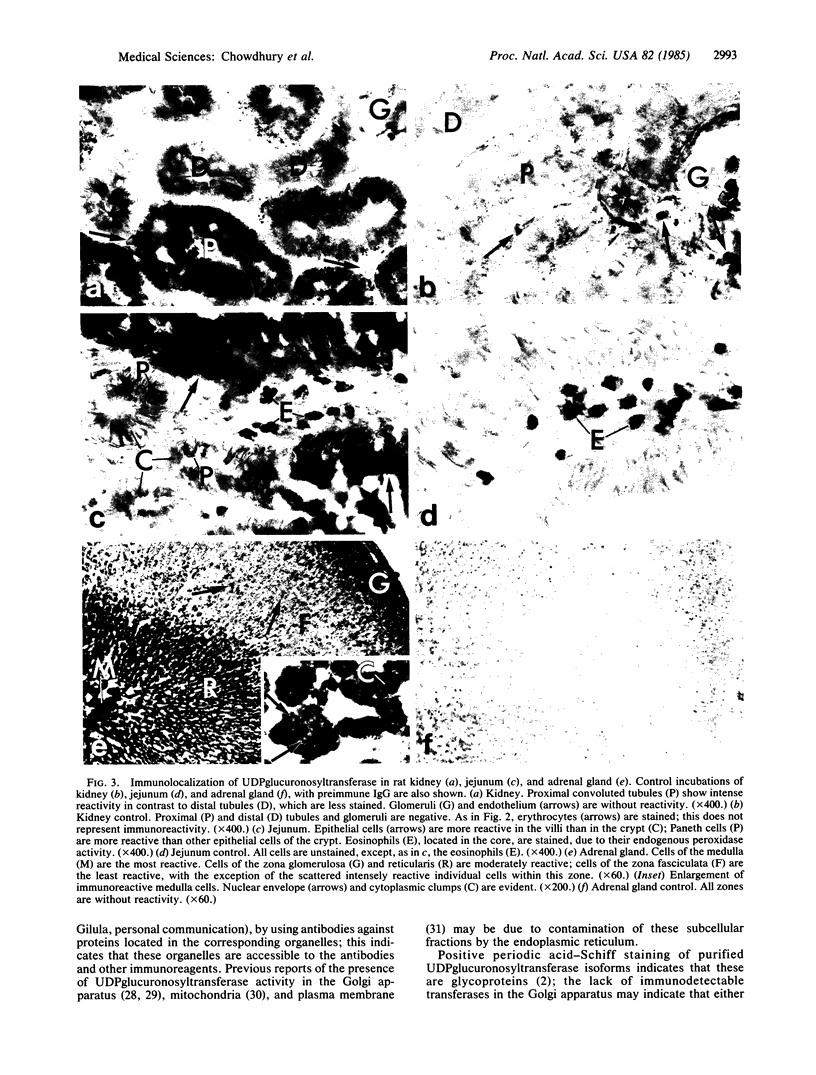
UDPglucuronosyltransferase [UDPglucuronate beta-D-glucuronosyltransferase (acceptor-unspecific), EC 2.4.1.17] is a group of enzymes with distinct but partially overlapping substrate specificity. A rabbit antiserum raised against one purified rat liver UDPglycuronosyltransferase isoform was specific for UDPglucuronosyltransferase and recognized all transferase isoforms by immunodiffusion or immunotransblot analysis. The transferase activity toward all substrates was immunoabsorbed from solubilized rat liver microsomes by IgG purified from the antiserum. The purified IgG was used for immunocytochemical localization of UDP-glucuronosyltransferase in rat liver, jejunum, kidney, and adrenal gland. In the liver, UDPglucuronosyltransferase was present exclusively in hepatocytes and was uniformly distributed within all zones of the hepatic lobule. In the jejunum, the transferase was present exclusively in the epithelial cells and showed a progressive increase in concentration from the crypt to the villar tip. In the kidney, the greatest concentration of the transferase was observed in the epithelial cells of the proximal convoluted tubule. Adrenal medullary cells showed intense immunocytochemical staining; the zona glomerulosa and the zona reticularis of the adrenal cortex were more intensely stained than the zona fasciculata. By light microscopy, UDPglucuronosyltransferase was found in the endoplasmic reticulum and nuclear envelope of all the four organs; this was confirmed in the hepatocyte by electron microscopy. The transferase was not observed in mitochondria, Golgi apparatus, lysosomes, peroxisomes, and plasma membrane, even after 3- to 4-fold induction of various substrate-specific UDPglucuronosyltransferase activities.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson P. H., Lee J. T. Co-translational excision of alpha-glucose and alpha-mannose in nascent vesicular stomatitis virus G protein. J Cell Biol. 1984 Jun;98(6):2245–2249. doi: 10.1083/jcb.98.6.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufay H., Amar-Costesec A., Feytmans E., Thinès-Sempoux D., Wibo M., Robbi M., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. I. Biochemical methods. J Cell Biol. 1974 Apr;61(1):188–200. doi: 10.1083/jcb.61.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cameron E. H., Jones T., Jones D., Anderson A. B., Griffiths K. Further studies on the relationship between C19- and C21-steroid synthesis in the human adrenal gland. J Endocrinol. 1969 Oct;45(2):215–230. doi: 10.1677/joe.0.0450215. [DOI] [PubMed] [Google Scholar]
- Chowdhury J. R., Chowdhury N. R., Moscioni A. D., Tukey R., Tephly T., Arias I. M. Differential regulation by triiodothyronine of substrate-specific uridinediphosphoglucuronate glucuronosyl transferases in rat liver. Biochim Biophys Acta. 1983 Nov 22;761(1):58–65. doi: 10.1016/0304-4165(83)90362-8. [DOI] [PubMed] [Google Scholar]
- Chowdhury J. R., Chowdhury N. R., Wu G., Shouval R., Arias I. M. Bilirubin mono- and diglucuronide formation by human liver in vitro: assay by high-pressure liquid chromatography. Hepatology. 1981 Nov-Dec;1(6):622–627. doi: 10.1002/hep.1840010610. [DOI] [PubMed] [Google Scholar]
- Chowdhury J. R., Jansen P. L., Fischberg E. B., Daniller A., Arias I. M. Hepatic conversion of bilirubin monoglucuronide to diglucuronide in uridine diphosphate-glucuronyl transferase-deficient man and rat by bilirubin glucuronoside glucuronosyltransferase. J Clin Invest. 1978 Jul;62(1):191–196. doi: 10.1172/JCI109105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahl W. E., Shen A. L., Jefcoate C. R. UDP-glucuronosyl transferase and the conjugation of benzo(a)pyrene metabolites to DNA. Biochem Biophys Res Commun. 1978 Dec 14;85(3):891–899. doi: 10.1016/0006-291x(78)90627-7. [DOI] [PubMed] [Google Scholar]
- Falany C. N., Chowdhury J. R., Chowdhury N. R., Tephly T. R. Steroid 3- and 17-OH UDP-glucuronosyltransferase activities in rat and rabbit liver microsomes. Drug Metab Dispos. 1983 Sep-Oct;11(5):426–432. [PubMed] [Google Scholar]
- Field P. R., Shanker S., Murphy A. M. The use of protein A-sepharose affinity chromatography for separation and detection of specific IgM antibody in acquired rubella infection: a comparison with absorption by staphylococci containing protein A and density gradient ultracentrifugation. J Immunol Methods. 1980;32(1):59–70. doi: 10.1016/0022-1759(80)90117-9. [DOI] [PubMed] [Google Scholar]
- Foliot A., Christoforov B., Petite J. P., Etienne J. P., Housset E., Dubois M. Bilirubin UDP-glucuronyltransferase activity of wistar rat kidney. Am J Physiol. 1975 Aug;229(2):340–343. doi: 10.1152/ajplegacy.1975.229.2.340. [DOI] [PubMed] [Google Scholar]
- Hartiala K. Metabolism of hormones, drugs and other substances by the gut. Physiol Rev. 1973 Apr;53(2):496–534. doi: 10.1152/physrev.1973.53.2.496. [DOI] [PubMed] [Google Scholar]
- Hartmann F., Bissell D. M. Metabolism of heme and bilirubin in rat and human small intestinal mucosa. J Clin Invest. 1982 Jul;70(1):23–29. doi: 10.1172/JCI110598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S. C., Ziurys J. C., Gollan J. L. Subcellular distribution and regulation of hepatic bilirubin UDP-glucuronyltransferase. J Biol Chem. 1984 Apr 10;259(7):4527–4533. [PubMed] [Google Scholar]
- Jansen P. L., Chowdhury J. R., Fischberg E. B., Arias I. M. Enzymatic conversion of bilirubin monoglucuronide to diglucuronide by rat liver plasma membranes. J Biol Chem. 1977 Apr 25;252(8):2710–2716. [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadlubar F. F., Miller J. A., Miller E. C. Hepatic microsomal N-glucuronidation and nucleic acid binding of N-hydroxy arylamines in relation to urinary bladder carcinogenesis. Cancer Res. 1977 Mar;37(3):805–814. [PubMed] [Google Scholar]
- Magdalou J., Antoine B., Ratanasavanh D., Siest G. Phenobarbital induction of cytochrome P-450 and UDP-glucuronosyltransferase in rabbit liver plasma membranes. Enzyme. 1982;28(1):41–47. doi: 10.1159/000459083. [DOI] [PubMed] [Google Scholar]
- Mellor J. D., Hobkirk R. In vitro synthesis of estrogen glucuronides and sulfates by human renal tissue. Can J Biochem. 1975 Jul;53(7):779–783. doi: 10.1139/o75-105. [DOI] [PubMed] [Google Scholar]
- Novikoff P. M., La Russo N. F., Novikoff A. B., Stockert R. J., Yam A., Le Sage G. D. Immunocytochemical localization of lysosomal beta-galactosidase in rat liver. J Cell Biol. 1983 Nov;97(5 Pt 1):1559–1565. doi: 10.1083/jcb.97.5.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff P. M., Tulsiani D. R., Touster O., Yam A., Novikoff A. B. Immunocytochemical localization of alpha-D-mannosidase II in the Golgi apparatus of rat liver. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4364–4368. doi: 10.1073/pnas.80.14.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyquist S. E., Morré D. J. Distribution of UDP-glucuronyl transferase among cell fractions of rat liver. J Cell Physiol. 1971 Aug;78(1):9–12. doi: 10.1002/jcp.1040780103. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance M. J., Shillingford J. S. The role of the gut in the metabolism of strong analgesics. Biochem Pharmacol. 1976 Mar 15;25(6):735–741. doi: 10.1016/0006-2952(76)90255-0. [DOI] [PubMed] [Google Scholar]
- Saez J. M., Morera A. M., Dazord A., Bertrand J. Adrenal and testicular contribution to plasma oestrogens. J Endocrinol. 1972 Oct;55(1):41–49. doi: 10.1677/joe.0.0550041. [DOI] [PubMed] [Google Scholar]
- Schumacher R., Rao G. S., Rao M. L., Breuer H. Steroidglucuronyltransferases. 3. Oestradiol-17 3-glucuronyltransferase of the mitochondria of pig intestine. Hoppe Seylers Z Physiol Chem. 1972 Nov;353(11):1784–1788. doi: 10.1515/bchm2.1972.353.2.1784. [DOI] [PubMed] [Google Scholar]
- Spater H. W., Quintana N., Becker F. F., Novikoff A. B. Immunocytochemical localization of gamma-glutamyltransferase in induced hyperplastic nodules of rat liver. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4742–4746. doi: 10.1073/pnas.80.15.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey R. H., Tephly T. R. Purification of properties of rabbit liver estrone and p-nitrophenol UDP-glucuronyltransferases. Arch Biochem Biophys. 1981 Jul;209(2):565–578. doi: 10.1016/0003-9861(81)90314-3. [DOI] [PubMed] [Google Scholar]