Abstract
The changing of omega-6/omega-3 polyunsaturated fatty acids (PUFA) in the food supply of Western societies occurred over the last 150 years is thought to promote the pathogenesis of many inflammatory-related diseases, including depressive disorders. Several epidemiological studies reported a significant inverse correlation between intake of oily fish and depression or bipolar disorders. Studies conducted specifically on the association between omega-3 intake and depression reported contrasting results, suggesting that the preventive role of omega-3 PUFA may depend also on other factors, such as overall diet quality and the social environment. Accordingly, tertiary prevention with omega-3 PUFA supplement in depressed patients has reached greater effectiveness during the last recent years, although definitive statements on their use in depression therapy cannot be yet freely asserted. Among the biological properties of omega-3 PUFA, their anti-inflammatory effects and their important role on the structural changing of the brain should be taken into account to better understand the possible pathway through which they can be effective both in preventing or treating depression. However, the problem of how to correct the inadequate supply of omega-3 PUFA in the Westernized countries' diet is a priority in order to set food and health policies and also dietary recommendations for individuals and population groups.
1. Introduction
Polyunsaturated fatty acids (PUFA) are fatty acids that contain more two or more carbon-carbon double bonds not saturated with hydrogen atoms at multiple (poly) locations within the molecule. PUFA can be classified into various groups by their chemical structure in omega-3 and omega-6 fatty acids: the omega-3 PUFA (also called ω-3 fatty acids or n-3 fatty acids) refers to a group of PUFA in which the first double bond is 3 carbons from the end (omega) carbon atom of the molecule; the omega-6 (also referred to as ω-6 fatty acids or n-6 fatty acids) are a family of PUFA that have in common a final carbon–carbon double bond in the n-6 position, that is, the sixth bond, counting from the methyl end [1]. Omega-3 PUFA are synthetized by dietary shorter-chained omega-3 fatty acid alpha-linolenic acid (ALA) to form the more important long-chain omega-3 fatty acids: eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Figure 1). Omega-6 PUFA derive from linoleic acid (LA), which can be converted also into the 18-carbon gamma linolenic acid (GLA), and the 20 carbon arachidonic (AA) and dihomo-gamma-linolenic acids (DGLA) (Figure 1). Both LA and ALA are considered essential fatty acids because mammalian cells are unable to synthesize these fatty acids from simpler precursors. Omega-3 PUFA have been long investigated for their anti-inflammatory effects in inflammatory-related diseases [2]. Omega-6 PUFA can be converted into AA and then metabolized into the omega-6 eicosanoids, which has proinflammatory action (Figure 1). On the other hand, omega-3 PUFA increase EPA in the cell membrane. This competes with AA for enzymatic conversion into its own metabolites, the omega-3 derived eicosanoids. These are less active and can partly oppose or antagonize the proinflammatory actions of the omega-6 eicosanoids. Noninflammatory eicosanoid balance is maintained throughout the body by way of a homeostatic balance between omega-3 and omega-6 fatty acids in cell membranes. Eicosanoid balance then exerts a “downstream” balancing influence on cytokines.
Figure 1.
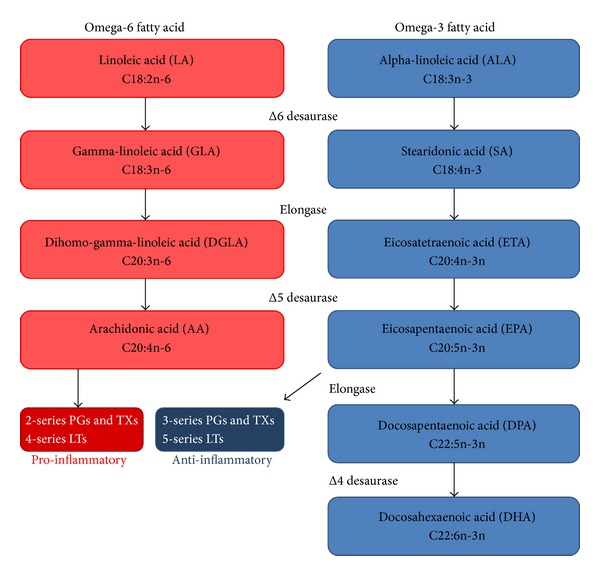
Biosynthesis of the principal polyunsaturated fatty acids and their metabolism.
In the context of the modern human lifestyle and diet, an absolute change of omega-6/omega-3 in the food supply of Western societies has occurred over the last 150 years [4]. Although the eicosanoid metabolites of EPA are crucial to provide anti-inflammatory effects by balancing the potentially proinflammatory eicosanoid metabolites of the omega-6 AA, a ratio of omega-6/omega-3 of 15 : 1 to 16.7 : 1, instead of 1 : 1 as is the case with animal and prehistoric human being has been reported [5]. Thus, the existing balance between omega-6 and omega-3 PUFA for the years during the long evolutionary history of the human being has rapidly changed over a short period of time, not accompanied by corresponding genetic changes. In other words, humans living in modern societies are exposed to a nutritional environment that differs from their genetic constitution. Not by chance, omega-3 PUFA have been considered of particular interest for the treatment of certain forms of chronic diseases [6]. In particular, many epidemiological and experimental studies emphasized their possible role in the prevention or treatment of depressive disorders. Due to evidence from animal and human studies reporting that omega-3 deficiency leads to impaired neuronal function (especially of serotoninergic and dopaminergic neurotransmitters) and altered inflammatory status, the biological plausibility of the effects of the omega-3 PUFA raised several hypotheses although merely speculative [7]. The aim of this study was to review the current knowledge about the association between the omega-3 PUFA and depression, taking into account both the epidemiological and experimental studies. The biological mechanisms of action of omega-3 PUFA in preventing or treating depression have been also reviewed.
2. Epidemiological Aspects Regarding Depressive Disorders and Diet
2.1. Burden of the Disease
Depression is a mental disorder characterized by sadness, loss of interest in activities, and decreased energy. Other symptoms include loss of confidence and self-esteem, inappropriate guilt, thoughts of death and suicide, diminished concentration, and disturbance of sleep and appetite. There are multiple variations of depression that a person can suffer from: (i) depressive episode involves symptoms such as depressed mood, loss of interest and enjoyment, and increased fatigability, categorized as mild, moderate, or severe; (ii) bipolar affective disorders typically consist of both manic and depressive episodes separated by periods of normal mood. Diagnostic criteria for a major depressive episode (DSM-IV) include a depressed mood, a marked reduction of interest or pleasure in virtually all activities, or both, lasting for at least 2 weeks. In addition, 3 or more of the following must be present: gain or loss of weight, increased or decreased sleep, increased or decreased level of psychomotor activity, fatigue, feelings of guilt or worthlessness, diminished ability to concentrate, and recurring thoughts of death or suicide. Particularly when long-lasting and with moderate or severe intensity, depression may become a serious health condition. In about 20% of cases, however, depression follows a chronic course with low rates of remission, especially when adequate treatment is not available. The recurrence rate for those who recover from the first episode is around 35% within 2 years and about 60% at 12 years. The recurrence rate is higher in those who are more than 45 years of age. Depression is associated with significant disability [8] and with excess mortality, particularly increasing the risk of cardiovascular diseases [9]. By 2020, depression is projected to be the second leading cause of disease burden worldwide after heart disease. Depression is associated with dysregulation of circadian rhythms, high incidence of sleep disorders, and anxiety.
Depression is estimated to affect 350 million people. The World Mental Health Survey conducted in 17 countries found that on average about 1 in 20 people reported having an episode of depression in the previous year. Depression is a leading cause of disability worldwide (in terms of total years lost due to disability), especially in high-income countries, ranging from 3% in Japan to 17% in the US (Table 1) [10]. In most countries, the number of people who would suffer from depression during their lives falls within an 8–12% range [11, 12], suggesting significant increased rates of depression in high-prevalence populations (i.e., the US population) than large-sample estimates from the 1980s and 1990s [13]. Furthermore, more recent studies reported that prospectively observed cumulative prevalence of depression resulted nearly twice as high as the lifetime prevalence of major depressive episodes reported by cross-sectional studies during the same time period [14]. Nevertheless, the mental health budgets of the majority of countries constitute less than 1% of their total health expenditures. More than 40% of countries have no mental health policy and over 30% have no mental health programs [15]. Moreover, both direct economic costs of depression in terms of cost of treatment and indirect costs through lost days of work and reduced productivity represent a major issue for public health operators [16].
Table 1.
Age-standardized disability-adjusted life year (DALY) rates by income (Global Burden of Disease: 2004 Update, WHO, Geneva, 2008).
| World | Low-income countries | Middle-income countries | High-income countries | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease or injury | DALYs (millions) | % of total DALYs | Disease or injury | DALYs (millions) | % of total DALYs | Disease or injury | DALYs (millions) | % of total DALYs | Disease or injury | DALYs (millions) | % of total DALYs | |
| 1 | Lower respiratory infections | 94.5 | 6.2 | Lower respiratory infections | 76.9 | 9.3 | Unipolar depressive disorders | 29.0 | 5.1 | Unipolar depressive disorders | 10.0 | 8.2 |
| 2 | Diarrhoeal diseases | 72.8 | 4.8 | Diarrhoeal diseases | 59.2 | 7.2 | Ischaemic heart disease | 28.9 | 5.0 | Ischaemic heart disease | 7.7 | 6.3 |
| 3 | Unipolar depressive disorders | 65.5 | 4.3 | HIV/AIDS | 42.9 | 5.2 | Cerebrovascular disease | 27.5 | 4.8 | Cerebrovascular disease | 4.8 | 3.9 |
| 4 | Ischaemic heart disease | 62.6 | 4.1 | Malaria | 32.8 | 4.0 | Road traffic accidents | 21.4 | 3.7 | Alzheimer and other dementias | 4.4 | 3.6 |
| 5 | HIV/AIDS | 58.5 | 3.8 | Prematurity and low birth weight | 32.1 | 3.9 | Lower respiratory infections | 16.3 | 2.8 | Alcohol use disorders | 4.2 | 3.4 |
| 6 | Cerebrovascular disease | 46.6 | 3.1 | Neonatal infections | 31.4 | 3.8 | COPD | 16.1 | 2.8 | Hearing loss, adult onset | 4.2 | 3.4 |
| 7 | Prematurity and low birth weight | 44.3 | 2.9 | Birth asphyxia and birth trauma | 29.8 | 3.6 | HIV/AIDS | 15.0 | 2.6 | COPD | 3.7 | 3.0 |
| 8 | Birth asphyxia and birth trauma | 41.7 | 2.7 | Unipolar depressive disorders | 26.5 | 3.2 | Alcohol use disorders | 14.9 | 2.6 | Diabetes mellitus | 3.6 | 3.0 |
| 9 | Road traffic accidents | 41.2 | 2.7 | Ischaemic heart disease | 26 | 3.1 | Refractive errors | 13.7 | 2.4 | Trachea, bronchus, lung cancers | 3.6 | 3.0 |
| 10 | Neonatal infections and otherb | 40.4 | 2.7 | Tuberculosis | 22.4 | 2.7 | Diarrhoeal diseases | 13.1 | 2.3 | Road traffic accidents | 3.1 | 2.6 |
COPD: chronic obstructive pulmonary disease.
2.2. Depression and Diet, the Association with Fish Consumption
Mental, physical, and social health represents fundamental components for the general well-being of a person. These factors are closely interwoven and deeply interdependent. For instance, the increased prevalence of depression over last decades in Western countries has been accompanied by parallel increased prevalence of cardiovascular diseases and fundamental changes in dietary habits [16, 17]. Several studies suggest that depression may share common pathophysiologic characteristics with cardiovascular diseases and their risk factors [18], such as the increased production of proinflammatory cytokines [19], endothelial dysfunction [20], and elevations in plasma homocysteine levels [21]. Depressive and cardiovascular disorders share blood flow abnormalities (i.e., in depression, hypoperfusion in the limbic system and prefrontal cortex) [22] and decreased glucose metabolism (i.e., low glucose utilization in a number of brain regions correlating negatively with severity of depression) [23]. Given the increases in prevalence of both depression and cardiovascular diseases, it has been hypothesized that a common underlying environmental influence may account for these changes. A comprehensive causal pathway of the relationship between depression and cardiovascular diseases included behavioral and genetic mechanisms [24]. One factor that could explain the relationship between such diseases and explain this parallel increase is the significant shift over the last century in the dietary intake of long-chain PUFA towards an increase in saturated fat and an increase in the ratio of omega-6 to omega-3 fatty acids [25]. Omega-3 PUFA have been reported to both inhibit endothelial cell proliferation [26] and influence glucose uptake [27, 28] and utilization [29] in the brain cells by reducing the expression of both isoforms of the brain glucose transporter GLUT1 in rats [30].
The fatty acid composition of the modern Western diet has changed dramatically during the last century, being characterized by an excessive amount of omega-6 PUFA and a very high omega-6/omega-3 ratio. This pattern of fatty acids intake is thought to promote the pathogenesis of many inflammatory-related diseases, including cardiovascular disease, cancer, and autoimmune diseases, whereas increased levels of omega-3 PUFA and a low omega-6/omega-3 ratio may exert suppressive effects [31]. The increased intake of saturated fatty acids and n-6 essential fatty acids and the reduced consumption of foods containing omega-3 fatty acid, which may exert anti-inflammatory properties, have been hypothesized to correlate with depressive and cardiovascular diseases, increasing the incidence of both disorders [32].
The main sources of fatty acids may vary greatly among countries, mostly depending on food availability and cultural influences. Per capita consumption of EPA and DHA in the US has been reported to be about 50 mg and 80 mg/day, respectively [33]. Evidence from prospective secondary prevention studies suggests that EPA/DHA supplementation ranging from 50 to 180 mg/day (either as fatty fish or supplements) significantly reduces subsequent cardiac and all-cause mortality. For ALA, total intakes of 150 to 300 mg/day seem to be beneficial. Dietary Guidelines suggest including at least two servings of fish per week (particularly fatty fish). In addition, the data support inclusion of vegetable oils (i.e., soybean, canola, walnut, and flaxseed) and food sources (i.e., walnuts and flaxseeds) high in ALA in a healthy diet for the general population [34]. A joint expert consultation of the United Nations Food and Agriculture Organization (FAO) and the World Health Organization (WHO) recommends an intake of 1-2 servings of fish, where each serving is defined as providing 200 to 500 mg/week DHA and EPA [35]. Further recommendations by the National Health and Medical Research Council (NHMRC) issued Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes, which recommended an intake of combined DHA, EPA, and DPA of 610 mg/day for men and 430 mg/day for women to prevent chronic disease [36].
The first observation of an inverse association between per capita fish consumption and national annual prevalence of major depression across nine countries was reported about 15 years ago [37]. Since then, several epidemiological studies on oily fish consumption and depression reported a significant inverse correlation between intake of oily fish and prevalence [38–43] and incidence [44–46] of depression and bipolar disorder [47], setting a threshold of vulnerability of about 650 mg/day. We performed an analysis using the data by the Food and Agriculture Organization of the United Nations (FAOSTAT) [48] regarding the total and marine fish consumption by country and the last report of the WHO regarding the global burden of disease, including unipolar and bipolar depressive disorders [10] (Figure 2). Matching together these datasets, we found an inverse correlation between the fish consumption and the age-standardized disability-adjusted life year (DALY) rates for both unipolar and bipolar depressive disorders (Figure 3). A specific analysis of the same variables from 2000 to 2007 in the United Kingdom [3] resulted in a significant inverse correlation between fish consumption and mixed anxiety and depressive disorders and in a significant trend of increased prevalence over time of such disorders (Figure 4). As observed in Figure 2, despite the high consumption of fish, increased rates of depression and/or depressive symptoms have been reported in Western countries. Besides in industrialized countries, such as US and Japan, where stressful lifestyles and the condition of the society may counteract the potential beneficial effects of high fish consumption and increase the overall morbidity burden of depression, in the most of other countries fish consumption seems to correlate with the DALY. It has been hypothesized that this finding might be related to the low quality of diet consumed, especially in such countries [49–53]. Regarding the Mediterranean countries, several studies reported a decreased prevalence [54] and incidence [55–57] of depression and/or depressive symptoms in subjects more adherent to the whole Mediterranean dietary pattern, which include a higher consumption of fish. The favorable effects of the Mediterranean diet on mental health may depend on the synergic positive actions of a variety of foods with a high content of PUFA, such as oily fish [58]. Such positive effects on mental health of long-chain fatty acids contained in the Mediterranean diet also translate the numerous evidences of the protection of such dietary pattern against cardiovascular diseases [59]. However, these conclusions are still not definitive, since other components of the Mediterranean dietary pattern, such as B vitamins, or other fish nutrients, such as iodine and selenium, may exert considerable positive effects on the brain and have a protective role against depression undermining the forthcoming evidence regarding omega-3 fatty acids [60].
Figure 2.
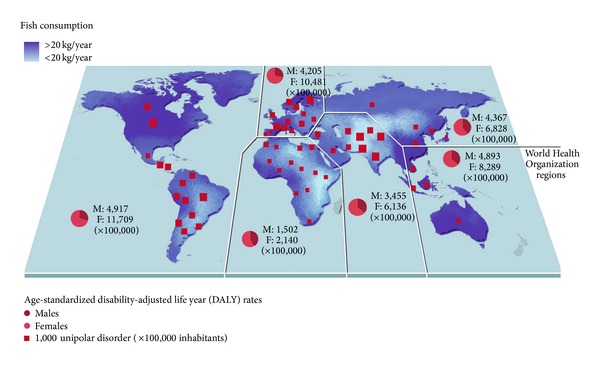
Per capita annual fish consumption and age-standardized disability-adjusted life year for unipolar disorder distribution across countries. DALY rates by gender are also reported per all World Health Organization regions (year 2004). High-income regions reported higher rates of DALY despite their increased consumption of fish, suggesting the role of social environment in the establishment of unipolar depressive disorder. Source: Consumption of Fish and Fishery Products, Fishery and Aquaculture Department 2011, Food and Agriculture Organization of the United Nations (FAOSTAT); the Global Burden of Disease: 2004 Update, World Health Organization, Geneva, 2008.
Figure 3.
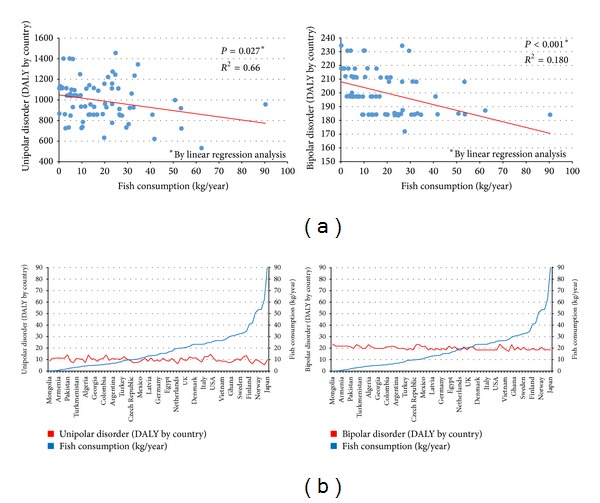
(a) Association between per capita annual fish consumption and age-standardized disability-adjusted life year for unipolar and bipolar disorders by country (year 2004). (b) Countries ordered by increasing fish consumption and relative depressive disorders trends. Both types of graphs demonstrate that DALY rates for unipolar and bipolar disorders are decreased in countries with increased fish consumption. Source: Consumption of Fish and Fishery Products, Fishery and Aquaculture Department 2011, Food and Agriculture Organization of the United Nations (FAOSTAT); the Global Burden of Disease: 2004 Update, World Health Organization, Geneva, 2008.
Figure 4.
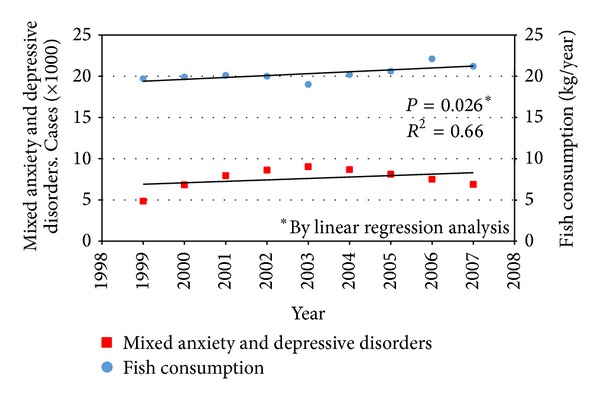
Trend over time (1999–2007) of per capita annual fish consumption and mixed anxiety and depressive disorders in the United Kingdom. Despite a diametric-shaped distribution of cases and relative fish consumption, a significant increasing trend has been found. Source: Consumption of Fish and Fishery Products, Fishery and Aquaculture Department 2011, Food and Agriculture Organization of the United Nations (FAOSTAT); to Walters et al. [3].
3. Evidence of Efficacy of Omega-3 Consumption against Depression
3.1. Epidemiological Studies
Data from cross-sectional studies exploring the association between omega-3 dietary intake and the prevalence of depression are of a various and contrasting nature. Several studies reported inconclusive results, especially in nonclinical populations [61, 62]. An inverse relationship between intake of omega-3 intake and depression was observed in some studies, although after adjusting for other lifestyle confounders the relationship was no longer significant, suggesting that the relation between depressed mood and omega-3 fatty acids intake may reflect a wider association between depressed mood and lifestyle [63, 64]. Conversely, other cross-sectional studies reported a significant association between omega-3 fatty acids intake and depressive symptoms [43, 65, 66].
Similar discrepancies occur also in prospective cohort studies. A population-based study conducted on 29,133 men of 50 to 69 years living in Finland reported no associations between the dietary intake of omega-3 fatty acids or fish consumption and depressed mood, major depressive episodes, or suicide [67]. Other inconclusive results regarding the association between omega-3 fatty acids and depression were reported in two minor population-based surveys (<1000 subjects) conducted in Australia [68] and Greece [69], although in the Greek one the 90th percentile of the GDS score (towards the high end) exhibited significant negative associations with monounsaturated fatty acids (MUFA) and olive oil [69]. Data from the nationwide Health 2000 Survey (n = 5492) and the Fishermen Study on Finnish professional fishermen and their family members (n = 1265) revealed a potential protective effect of general fish intake rather than intake or serum concentrations of omega-3 PUFA, although the associations were strongly influenced by lifestyle factors (i.e., high alcohol intake, occasional smoking, or having intermediate physical activity) [41]. Similarly, in a study conducted on 54,202 Danish women followed for 1 year postpartum, a higher risk of postpartum depression was found for the lowest compared with the highest fish intake group, but no association was observed with respect to omega-3 PUFA intake [70]. Despite such contrasting results, high levels of depressive symptoms during pregnancy were reported in women with low omega-3 fatty acid intake from fish [71] and high omega-6/omega-3 ratio [72], especially when results were adjusted for lifestyle factors (i.e., current smokers and women of single marital status) [61]. Results of a large longitudinal study conducted on 54,632 US women from the Nurses' Health Study who were 50–77 years of age and free from depressive symptoms at baseline did not support a protective effect of long-chain n-3 from fish on depression risk after 10 years of followup but support the hypothesis that higher ALA and lower linoleic acid intakes reduce depression risk [73]. On the contrary, results of a study conducted on a subsample from the French Supplementation with Antioxidant Vitamins and Minerals (SU.VI.MAX) cohort followed for 2 years showed that subjects consuming fatty fish or with an intake of long-chain omega-3 PUFA higher than 0.10% of energy intake had a significantly lesser risk of any depressive episode and of recurrent depressive episodes [46]. A recent update from a cohort retrieved by the same study reported no association between omega-3 PUFA intake and incidence of depressive symptoms; an association was observed in cross-sectional analyses, which may reflect unhealthy dietary patterns among subjects with depressive symptoms [74]. Also a study performed in 7,903 participants to the SUN cohort study followed for 2 years suggested a potential benefit of omega-3 fatty acids intake on mental disorders, although no linear trend was apparent [45].
Some studies also considered suicide as a proxy of severe depression and the relationship of suicide rates to omega-3 PUFA and fish consumption. It has been observed that attempters [75] and suicide [76] ate significantly less fish and lower intake of overall PUFA [77], but other studies did not support a protective role of higher intake of fish, EPA, or DHA against suicide [78]. The uncertainty of the results obtained from both cross-sectional and prospective studies on omega-3 fatty acids consumption and prevalence and incidence of depression may depend on the limitations of the methodology used. Indeed, cross-sectional studies do not allow demonstrating a causal relationship between the factors studied because the temporal variable is lacking. On the other hand, prospective studies may suffer by misclassification of exposure, since omega-3 dietary intake was assumed to be constant over the entire follow-up periods. Finally, omega-3 estimation methods by food frequency questionnaires may lead to recall biases in both types of studies.
3.2. Experimental Studies
Although current evidence increasingly supports an inverse association between omega-3 PUFA and depression, the validity of findings from experimental research is limited by several methodological issues. Previous meta-analytic studies reported a general positive effect of omega-3 PUFA intake on ameliorating symptoms of depression [79, 80]. On the other hand, incongruent results have been reported in other systematic revisions of the literature [81] and in an updated analysis [82]. The reasons for such variability in these findings depend on the significant heterogeneity among studies examined, weakening the results of the analyses. It has been pointed out that publication bias, unstandardized depression assessment, variability of omega-3 regime employed, and duration of the trial may have affected the analysis. The main limitation of the pooled analysis relied on the selection of studies to be included, taking into account that pathophysiological processes of depressive symptoms involved in MDD patients are likely to be very different from those in patients with depression occurring in other clinical conditions (i.e., bipolar disorder, pregnancy, primary diseases other than depression) and in nonhomogenous patients (i.e., community sample of individuals). The substantial inefficacy of omega-3 PUFA in patients with bipolar disorder or perinatal depression may depend on the fact that the specific pathophysiological processes occurring in MDD patients (which omega-3 PUFA are supposed to affect) are lacking. To the same extent, comorbid depression secondary to CVD, Alzheimer, and schizophrenia may strongly depend on the primary disease. Indeed, besides the differences in omega-3 PUFA regime, length of the trial, and overall quality of the study, it seems that type of diagnosis (i.e., MDD versus depressed mood without diagnosis by DSM-IV criteria) and the homogeneity of the population study (i.e., MDD diagnosed patients versus volunteers recruited by shopping malls, radio and television advertising, and newspapers) were the characteristics mostly affecting the efficacy of the treatment with omega-3 PUFA in these pooled analyses [83].
Some meta-analyses focused on the type of fatty acid used, resulting in a positive effect on depressive symptoms of EPA rather than DHA content of the regime [84, 85]. The most recent meta-analysis of clinical trials concluded that supplements containing EPA ≥60% of total EPA + DHA, in a dose range of 200 to 2,200 mg/d of EPA in excess of DHA, were effective against primary depression [86]. It has been also reported that the more severe was the depression, the more likely omega-3 PUFA supplementation would reduce depressive symptoms.
4. Hypothesized Mechanisms of Action
Although epidemiological data and clinical trials suggest that omega-3 PUFA may have preventive and therapeutic effects on depression, the underlying mechanisms are still unclear. The protective role of omega-3 fatty acids against depression has been hypothesized to depend on the physiological mechanisms in which fatty acids take part.
4.1. Neuroendocrine Modulation of Omega-3 PUFA in Depression
The pathophysiology of depression has been dominated by the monoamine hypothesis, suggesting that an imbalance, mainly in serotonergic and noradrenergic neurotransmission, is at the core of the pathophysiology of depression. The current therapeutic strategies against depression include drugs which enhance either serotonergic neurotransmission (i.e., selective serotonin reuptake inhibitors (SSRI)), noradrenergic neurotransmission (i.e., noradrenergic reuptake inhibitors (NARI)), or both (i.e., tricyclic antidepressants and more recently serotonin noradrenaline reuptake inhibitors (SNRI)) [87]. However, in 30% of the cases, there is little or no response to the medication, and almost half of patients treated with current antidepressant drugs do not show significant clinical improvements [87].
An effect of omega-3 intake suggested to positively influence the depressive status is the potential interaction with the serotoninergic and dopaminergic transmission, including metabolism, release, uptake, and receptor function. The highly unsaturated nature of EPA and DHA provides them with the quality of highly influencing membrane order (namely the fluidity) of several types of cells [88]. Omega-3 PUFA also regulate the signal transduction by enhancing G-protein-mediated signal transduction [89, 90], membrane-bound enzymes (Na/K-dependent ATP'ase) [91], and protein kinase C [92]. The membrane changing induced by omega-3 PUFA intake may affect different neurotransmitter system altering the regulation of dopaminergic and serotonergic neurotransmission, which are dysfunctional in depressed patients. Changes in serotonin (5-HT) and dopamine receptor (DR-2) number and function caused by changes in PUFA provide the theoretical rationale connecting fatty acids with the current receptor and neurotransmitter theories of depression. Cerebrospinal fluid (CSF) 5-hydroxyindoleacetic acid (5-HIAA), a metabolite that reflects serotonin turnover, has been reported to be decreased in several psychiatric conditions, including violent suicide attempts during depression [93]. It has been reported that higher concentrations of plasma DHA predict an increase in serotonergic neurotransmission (higher CSF 5-HIAA) in healthy adults [94] and in an experimental animal model of depression [95]. Conversely, omega-3 deficiency results in an increase of serotonin receptor (5HT2) density in the frontal cortex, probably due to an adaptation to reduced serotoninergic function [96, 97]. A preclinical animal experiment reported that erythrocyte DHA was inversely correlated, and AA and the AA/DHA and AA/EPA ratios were positively correlated with plasma IL-6, TNFα, and CRP levels, whereas plasma IL-6 levels were positively correlated with 5-HIAA/5-HT ratios in all brain regions, providing evidence for a functional link between n-3 fatty acid deficiency, elevated peripheral inflammatory signaling, and increased central 5-HT turnover [98].
Regarding the dopamine neurotransmission, in animal experimental models of depression, decreased levels of dopamine turnover in the prefrontal cortex and dopamine levels up to 6-fold higher in the nucleus accumbens have been reported [99, 100]. Similar observations were reported in omega-3 PUFA-deficient rats, in which the expression of the dopamine receptor (D2R) was decreased in the frontal cortex and increased in the nucleus accumbens [96, 101, 102]. Conversely, supplementing the diet of rats with omega 3 PUFA led to a 40% increase in dopamine levels in the frontal cortex as well as an increase in the binding to the dopamine D2 receptor [103].
Beside the well-known deficiency in serotonergic neurotransmission as pathophysiological correlate of major depression, recent evidence points out to an important role of increased glutamate receptor activation as well [104]. Indeed, an increased activity of the glutamatergic system and N-methyl-D-aspartate (NMDA) receptor agonism has been associated with depressed mood, whereas a reduction of the glutamatergic activity may exert antidepressant action. These effects of the glutamatergic system on mood may depend on its direct or indirect influence on the serotonergic and noradrenergic neurotransmission, since NMDA receptor antagonists increase the serotonin levels in the brain [105, 106]. Omega-3 deficiency has been demonstrated to promote age-induced degradation of glutamatergic transmission and its associated astroglial regulation in the hippocampus [107] by slowing astroglial glutamate transport via a specific signal-like effect [108]. Further experimental models confirmed that dietary omega-3 content is relevant for the glutamatergic system development and for behavioral performance in adulthood [109]. At a molecular level, it has been demonstrated that the NMDA receptor can be stimulated by the protein kinase C, whose conformational changes and optimal activation depend on for membrane content of omega-PUFA [110, 111]. The putative correlation among the neurochemical and behavioral alterations caused by dietary omega-3 PUFA and glutamatergic transmission must be further investigated in future research.
Glucocorticoids play a fundamental role in attenuating the inflammation processes following exposure to a variety of stress-related conditions [112]. Glucocorticoids suppress critical inflammatory signaling pathways including nuclear factor-κB (NF-κB) and inhibit stress-related outflow pathways including the corticotropin releasing hormone (CRH), the hypothalamic-pituitary-adrenal (HPA) axis, and the sympathetic nervous system [113]. Failure of glucocorticoids to inhibit inflammatory and neuroendocrine responses to challenge may contribute to disease development, although the etiology of glucocorticoid resistance in both inflammatory and neuropsychiatric disorders is unknown. Depression has been associated with a high level of cortisol in blood due to the hyperactivity of HPA axis, largely due to a hypersecretion of CRH [104]. EPA may regulate the HPA axis dysfunction associated with depression by reducing corticotrophin releasing factor expression and corticosterone secretion [114]. Some animal studies reported that the response to chronic stress can be modulated by the omega-3 fatty acid supply, since a dietary deficiency has been found to be deleterious while enrichment has protected against stress [115]. These effects were associated with the reduction of corticosterone levels promoted by the PUFA supplementation in the stress-induced animals [116, 117]. From a mechanistic point of view, it has been demonstrated that omega-3 PUFA inhibit the P-glycoprotein (P-gp) activity [117], which are transport proteins responsible of the increase in cortisol transport through the blood-brain barrier (BBB) in depressive subjects [118–122]. The normalization in brain penetration of cortisol would normalize the feedback control of the HPA axis. Another study demonstrated a modulatory effect of omega-3 PUFA by increasing the cortisol transport in the BBB models not through the inhibition of P-gp efflux, but thanks to membrane fluidification and some effect on tight junction integrity [123].
4.2. Anti-Inflammatory Effects of Omega-3 PUFA
Recent studies indicate that factors other than monoamine deficiency or hyperactivation of the HPA axis must be considered when examining the pathogenesis of major depression such as an altered activation of immune system and chronic inflammation with a specific impairment in the signaling of neurotrophins, such as transforming growth factor β1 (TGF-β1) [124, 125].
According to recent evidence, chronic stress can elicit a neuroinflammatory response through the activation of microglia in CNS, with ensuing release of inflammatory mediators such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) [126]. The neuroinflammatory response leads to inhibition of neurotrophin signaling and can also elicit both sickness behavior and psychological pain. In addition, chronic stress alters activation of immune system in the periphery, which might account for the state of chronic inflammation observed in depressed patients [127]. Different studies have demonstrated a positive correlation between the severity of the symptoms of depression and the increase in the inflammatory status [127]. Proinflammatory cytokines interfere with many of the pathophysiological mechanisms that characterize the pathogenesis of depression, altering serotonin metabolism, and reducing both synaptic plasticity and hippocampal neurogenesis [127]. On the other hand, reduced levels of anti-inflammatory cytokines, such as interleukin-4 (IL-4), interleukin-10 (IL-10), and TGF-β1, have been found in the plasma of depressed patients [127, 128].
Chronic systemic inflammation also contributes to the progression of neurodegeneration [129]. The key anti-inflammatory effect of omega-3 fatty acids has been long recognized to depend on their action on eicosanoids. Eicosanoids are biologically active lipid mediators produced from PUFA which play a role in inflammation and regulation of immune function [130]. To produce these eicosanoids, AA is released from membrane phospholipids through the action of phospholipase A2 enzymes and then acts as a substrate for cyclooxygenase (COX), lipoxygenase, or cytochrome P450 enzymes. COX enzymes lead to PG and thromboxanes, lipoxygenase enzymes lead to leukotrienes (LT), and cytochrome P450 enzymes lead to hydroxyeicosatetraenoic and epoxyeicosatrienoic acids. Omega-3 EPA and DHA incorporation in cell membrane decreases their AA content and reduces the amount of substrate available to produce inflammatory and immunoregulatory eicosanoids [131]. LTB5, a product of EPA, is a competitive antagonist to LTB4, a highly proinflammatory eicosanoid derived from AA [132]. A series of studies gave important information regarding the omega-3 fatty acids as mediators of inflammatory response in depressive status. Indeed, it has been demonstrated that severity of depression varies with the degree of omega-3 fatty acids in erythrocyte membranes, which are decreased in more severe status, as an indicator of oxidative damage [133–136]. It has been also reported that plasma fatty acids composition and depression are associated with a significant higher ratio of omega-6 to omega-3 PUFA in depressed subjects [137–140]. Many studies also focused on analysis of fasting bloods for detection of plasma fatty acid analysis in risk population. Results from a case-control study conducted on 16 depressed and 22 nondepressed women recruited during the third trimester of pregnancy demonstrated that high DHA, high total n-3, and a low n-6 : n-3 ratio were associated with significantly lower odds of depression [141]. Similar findings were reported in some studies conducted on depressed postmyocardial infarction [142] and acute coronary syndromes patients [143, 144] in which, compared with control group, lower levels of long-chain omega-3 PUFA as measured by a mean AA/EPA ratio were found. Moreover, a low DEA percentage and low omega-3 proportions of lipid profile predicted risk of suicidal behavior among depressed patients over the 2-year period [145]. Other evidences come from a case-control study conducted on 150 subjects reporting an association between fatty acids with serotonergic and immunological markers in depressive patients but not in patients with somatization syndrome suggesting a different biological mechanism of depression and somatoform disorders [146]. This may lead to the speculation of a potential bias in previous studies on depression assessment concerning the indiscriminate merging together of both disorders that could affect the outcome. Similarly, an association between omega-3 fatty acids in adipose tissue and major depression has been shown [147–149], although not univocally reported [150, 151].
Dysregulation of the functional activity of the immune system in depression is a phenomenon that has been widely reviewed [152]. As discussed above, the peripheral immune activation observed in major depression, through the release of proinflammatory cytokines, is responsible for the variety of behavioral, neuroendocrine, and neurochemical alterations that are associated with this psychiatric condition [152]. Depression has been associated with excessive production (during an acute phase response) of proinflammatory cytokines, such as IL-1 beta, IL-12, and interferon-gamma. A recent meta-analysis of experimental studies reported a significantly higher concentration of the proinflammatory cytokines tumor necrosis factor-alpha and IL-6 in depressed subjects compared with control subjects [153]. The actions of omega-3 on cells include the changing of the expression of key cell surface proteins and the modulation of the production of proinflammatory cytokines. Indeed, omega-3 PUFA have been reported to decrease production of TNF, IL-1b, and IL-6 in in vitro studies and decrease production of TNF, IL-1b, IL-6, and various growth factors in healthy human subjects, although not all studies confirm this effect [154]. At the cellular level, they have been demonstrated to decrease activation of NF-κB, a key transcription factor involved in upregulation of inflammatory cytokine [154]. The question arises as to whether the decreased prevalence of depressive symptoms accompanying the higher plasma content of omega-3 PUFA is also associated with improved central inflammation, that is, cytokine activation, in the brain. Recent studies have pointed out the possible role of omega-3 PUFA inducing a central antidepressant-like effect by modulating oxidative reactions and inflammatory cytokine production in microglial and neuronal cells. This determines a reduction of expressions of tumor necrosis factor-α, interleukin-6, nitric oxide synthase, and cyclooxygenase-2, an induction by interferon-γ, and an induction of upregulation of heme oxygenase-1 (HO-1) in BV-2 microglia [155]. However, results of experimental studies on cytokines response after administration of omega-3 fatty acids are not univocal. For example, long-term intake of omega-3 increased plasma serotonin concentration and the hippocampus c-AMP response element binding protein (CREB) and reducing interleukin-6 (IL-6) expression in rats, but clear dose-dependent effects and significant differences in expressions of IL-1β, tumor necrosis factor-α, brain-derived neurotrophic factors, or phosphorylated CREB were not found [156]. Moreover, another experimental study on mice demonstrated that high level of brain DHA was associated with a decrease in depressive-like symptoms throughout aging independently on the cytokines response (in fact, increased interleukin-6 and decreased IL-10 expressions were found in the cortex of aged mice independently of the diets) [157].
Among the anti-inflammatory actions of omega-3, it is noteworthy that they have been recently discovered as a source of docosanoids, metabolites with a novel stereospecificity unlike that of the known eicosanoids [158]. The three known classes, namely, docosatrienes, resolvins, and protectins, are produced mainly from controlled oxidative breakdown of DHA within the membrane and demonstrated anti-inflammatory properties [159]. Novel research on depression focused on the role of resolvins, which are thought to terminate ongoing inflammatory cascades and may be responsible for the potential anti-inflammatory effects of omega-3 PUFA in preventing or ameliorating the depressive status [160]. Resolvins are grouped into E-series and D-series, depending on if derived by EPA or DHA, respectively. Resolvin E1 has been reported to reduce inflammation by suppressing the activation of the transcription factor nuclear factor-κB and subsequent synthesis of inflammatory cytokines and chemokines [160].
As discussed above, major depression is characterized by increased levels of proinflammatory cytokines and reduced levels of anti-inflammatory cytokines such as IL-10 and TGF-β1 [124, 127]. Plasma TGF-β1 levels are reduced in major depressed patients and show a significant negative correlation with the Hamilton Depression Rating Scale [128, 161, 162]. Interestingly, TGF-β1 levels significantly increase after antidepressant treatment [128], and SSRI drugs such as sertraline might exert immunomodulatory effects in vivo through a decrease in the proinflammatory cytokine IL-12 and an increase in the anti-inflammatory cytokines such as IL-4 and TGF-β1 [162]. Similarly, therapeutic concentrations of venlafaxine prevent microglial activation, reduce proinflammatory cytokine secretion, and finally increase the release of TGF-β1 in an astroglia-microglia coculture model [163]. Recent studies suggest that omega-3 fatty acids can increase both in vitro and in vivo the synthesis of TGF-β1 [164, 165] and, in particular, in pregnant women [166], although no studies have been yet conducted in depressed patients. On the basis of this evidence, it might be worth assessing whether TGF-β1 signaling is a common target both for omega-3 fatty acids and antidepressant drugs, and whether omega-3 fatty acids can exert their antidepressant in vivo effects via the rescue of TGF-β1 signaling.
5. Conclusions
The role of omega-3 in preventing psychiatric diseases remains to be clarified. It can be speculated that all types of action can occur simultaneously: on one hand, by maintaining and increasing the brain structures and preserving their function by interacting with phospholipid metabolism and, hence, the modulation of signal transduction; on the other hand, preventing or decreasing the inflammatory status occurring during depression. However, the problem of how to correct the inadequate supply of omega-3 fatty acids in Westernized countries' diet is a priority in order to set food and health policies and dietary recommendations for individuals and population groups. Moreover, accompanying the increased dietary intake of omega-3 fatty acids, an omega-6/omega-3 ratio maintained not above 5 is highly desirable. If omega-3 PUFA will result to be effective for both the prevention and treatment of depression, substantial implications with large-scale impact through dietary interventions could be realized. Although many other factors may also contribute to the rise in depression and for which effective (although not efficient) treatments already exist, dietary recommendations suggesting proper intake of omega-3 PUFA and dietary interventions including omega-3 PUFA supplement can result in substantial benefits for the general population.
Acknowledgments
Giuseppe Grosso and Fabio Galvano equally contributed to the paper. Giuseppe Grosso and Michele Malaguarnera were supported by the International Ph.D. Program in Neuropharmacology, University of Catania Medical School, Catania, Italy.
Disclosure
Filippo Drago and Filippo Caraci are co-last authors.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomedicine and Pharmacotherapy. 2002;56(8):365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 2.Din JN, Newby DE, Flapan AD. Omega 3 fatty acids and cardiovascular disease—fishing for a natural treatment. British Medical Journal. 2004;328(7430):30–35. doi: 10.1136/bmj.328.7430.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walters K, Rait G, Griffin M, Buszewicz M, Nazareth I. Recent trends in the incidence of anxiety diagnoses and symptoms in primary care. PLoS ONE. 2012;7(8) doi: 10.1371/journal.pone.0041670.e41670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simopoulos AP. Executive summary of the international conference on genetic variation and nutrition. World Review of Nutrition and Dietetics. 1990;63:1–13. doi: 10.1159/000418492. [DOI] [PubMed] [Google Scholar]
- 5.Simopoulos AP, Ordovas J. Nutrigenetics and Nutrigenomics. Vol. 93. Washington, DC, USA: Karger; 2004. [Google Scholar]
- 6.Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomedicine and Pharmacotherapy. 2006;60(9):502–507. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 7.Sinclair AJ, Begg D, Mathai M, Weisinger RS. Omega 3 fatty acids and the brain: review of studies in depression. Asia Pacific Journal of Clinical Nutrition. 2007;16(supplement 1):391–397. [PubMed] [Google Scholar]
- 8.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: global burden of disease study. The Lancet. 1997;349(9063):1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 9.Rivelli S, Jiang W. Depression and ischemic heart disease: what have we learned from clinical trials? Current Opinion in Cardiology. 2007;22(4):286–291. doi: 10.1097/HCO.0b013e3281ead011. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. The Global Burden of Disease: 2004 Update. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 11.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Journal of the American Medical Association. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 12.Andrade L, Caraveo-Anduaga JJ, Berglund P, et al. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. International Journal of Methods in Psychiatric Research. 2003;12(1):3–21. doi: 10.1002/mpr.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Archives of General Psychiatry. 2005;62(10):1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- 14.Patten SB. Accumulation of major depressive episodes over time in a prospective study indicates that retrospectively assessed lifetime prevalence estimates are too low. BMC Psychiatry. 2009;9, article 19 doi: 10.1186/1471-244X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. A Public Health Approach to Mental Health. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 16.Sobocki P, Jönsson B, Angst J, Rehnberg C. Cost of depression in Europe. Journal of Mental Health Policy and Economics. 2006;9(2):87–98. [PubMed] [Google Scholar]
- 17.Tiihonen J, Lonnqvist J, Wahlbeck K, et al. No mental health without physical health. The Lancet. 2011;377(9766):p. 611. doi: 10.1016/S0140-6736(11)60211-0. [DOI] [PubMed] [Google Scholar]
- 18.Prince M, Patel V, Saxena S, et al. No health without mental health. The Lancet. 2007;370(9590):859–877. doi: 10.1016/S0140-6736(07)61238-0. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez-Villegas A, Martinez-Gonzalez MA. Diet, a new target to prevent depression? BMC Medicine. 2013;11, article 3 [Google Scholar]
- 20.Machado-Vieira R, Mallinger AG. Abnormal function of monoamine oxidase-A in comorbid major depressive disorder and cardiovascular disease: pathophysiological and therapeutic implications (review) Molecular Medicine Reports. 2012;6(5):915–922. doi: 10.3892/mmr.2012.1062. [DOI] [PubMed] [Google Scholar]
- 21.Do DP, Beam Dowd J, Ranjit N, House JS, Kaplan GA. Hopelessness, depression, and early markers of endothelial dysfunction in U.S. adults. Psychosomatic Medicine. 2010;72(7):613–619. doi: 10.1097/PSY.0b013e3181e2cca5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Severus WE, Littman AB, Stoll AL. Omega-3 fatty acids, homocysteine, and the increased risk of cardiovascular mortality in major depressive disorder. Harvard Review of Psychiatry. 2001;9(6):280–293. doi: 10.1080/10673220127910. [DOI] [PubMed] [Google Scholar]
- 23.Ito H, Kawashima R, Awata S, et al. Hypoperfusion in the limbic system and prefrontal cortex in depression: SPECT with anatomic standardization technique. Journal of Nuclear Medicine. 1996;37(3):410–414. [PubMed] [Google Scholar]
- 24.Kimbrell TA, Ketter TA, George MS, et al. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biological Psychiatry. 2002;51(3):237–252. doi: 10.1016/s0006-3223(01)01216-1. [DOI] [PubMed] [Google Scholar]
- 25.Stapelberg NJ, Neumann DL, Shum DHK, McConnell H, Hamilton-Craig I. A topographical map of the causal network of mechanisms underlying the relationship between major depressive disorder and coronary heart disease. Australian and New Zealand Journal of Psychiatry. 2011;45(5):351–369. doi: 10.3109/00048674.2011.570427. [DOI] [PubMed] [Google Scholar]
- 26.Puri BK. Cardiovascular disease and depression: the PUFA connection. International Journal of Clinical Practice. 2008;62(3):355–357. doi: 10.1111/j.1742-1241.2007.01649.x. [DOI] [PubMed] [Google Scholar]
- 27.Cui PH, Petrovic N, Murray M. The ω-3 epoxide of eicosapentaenoic acid inhibits endothelial cell proliferation by p38 MAP kinase activation and cyclin D1/CDK4 down-regulation. British Journal of Pharmacology. 2011;162(5):1143–1155. doi: 10.1111/j.1476-5381.2010.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pifferi F, Jouin M, Alessandri JM, et al. n-3 Fatty acids modulate brain glucose transport in endothelial cells of the blood-brain barrier. Prostaglandins Leukotrienes and Essential Fatty Acids. 2007;77(5-6):279–286. doi: 10.1016/j.plefa.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Pifferi F, Jouin M, Alessandri JM, et al. N-3 long-chain fatty acids and regulation of glucose transport in two models of rat brain endothelial cells. Neurochemistry International. 2010;56(5):703–710. doi: 10.1016/j.neuint.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Da Silva AX, Lavialle F, Gendrot G, Guesnet P, Alessandri J-M, Lavialle M. Glucose transport and utilization are altered in the brain of rats deficient in n-3 polyunsaturated fatty acids. Journal of Neurochemistry. 2002;81(6):1328–1337. doi: 10.1046/j.1471-4159.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 31.Pifferi F, Roux F, Langelier B, et al. (n-3) polyunsaturated fatty acid deficiency reduces the expression of both isoforms of the brain glucose transporter GLUT1 in rats. Journal of Nutrition. 2005;135(9):2241–2246. doi: 10.1093/jn/135.9.2241. [DOI] [PubMed] [Google Scholar]
- 32.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Experimental Biology and Medicine. 2008;233(6):674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 33.Hibbeln JR, Salem N., Jr. Dietary polyunsaturated fatty acids and depression: when cholesterol does not satisfy. The American Journal of Clinical Nutrition. 1995;62(1):1–9. doi: 10.1093/ajcn/62.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. The American Journal of Clinical Nutrition. 2011;93(5):950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. Report of the Joint WHO/FAO Expert Consultation. Geneva, Switzerland: World Health Organization; 2003. Diet, nutrition and the prevention of chronic diseases. [Google Scholar]
- 37.National Health Medical Research Council. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes. NHMRC; 2006. [Google Scholar]
- 38.Tanskanen A, Hibbeln JR, Tuomilehto J, et al. Fish consumption and depressive symptoms in the general population in Finland. Psychiatric Services. 2001;52(4):529–531. doi: 10.1176/appi.ps.52.4.529. [DOI] [PubMed] [Google Scholar]
- 39.Bountziouka V, Polychronopoulos E, Zeimbekis A, et al. Long-term fish intake is associated with less severe depressive symptoms among elderly men and women: the MEDIS (MEDiterranean ISlands Elderly) epidemiological study. Journal of Aging and Health. 2009;21(6):864–880. doi: 10.1177/0898264309340693. [DOI] [PubMed] [Google Scholar]
- 40.Timonen M, Horrobin D, Jokelainen J, Laitinen J, Herva A, Räsänen P. Fish consumption and depression: the Northern Finland 1966 birth cohort study. Journal of Affective Disorders. 2004;82(3):447–452. doi: 10.1016/j.jad.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Suominen-Taipale AL, Partonen T, Turunen AW, Männistö S, Jula A, Verkasalo PK. Fish consumption and omega-3 polyunsaturated fatty acids in relation to depressive episodes: a cross-sectional analysis. PLoS ONE. 2010;5(5) doi: 10.1371/journal.pone.0010530.e10530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Appleton KM, Woodside JV, Yarnell JWG, et al. Depressed mood and dietary fish intake: direct relationship or indirect relationship as a result of diet and lifestyle? Journal of Affective Disorders. 2007;104(1–3):217–223. doi: 10.1016/j.jad.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Murakami K, Miyake Y, Sasaki S, Tanaka K, Arakawa M. Fish and n-3 polyunsaturated fatty acid intake and depressive symptoms: Ryukyus child health study. Pediatrics. 2010;126(3):e623–e630. doi: 10.1542/peds.2009-3277. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Dai Q, Ekperi LI, Dehal A, Zhang J. Fish consumption and severely depressed mood, findings from the first national nutrition follow-up study. Psychiatry Research. 2011;190(1):103–109. doi: 10.1016/j.psychres.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez-Villegas A, Henríquez P, Figueiras A, Ortuño F, Lahortiga F, Martínez-González MA. Long chain omega-3 fatty acids intake, fish consumption and mental disorders in the SUN cohort study. European Journal of Nutrition. 2007;46(6):337–346. doi: 10.1007/s00394-007-0671-x. [DOI] [PubMed] [Google Scholar]
- 46.Astorg P, Couthouis A, Bertrais S, et al. Association of fish and long-chain n-3 polyunsaturated fatty acid intakes with the occurrence of depressive episodes in middle-aged French men and women. Prostaglandins Leukotrienes and Essential Fatty Acids. 2008;78(3):171–182. doi: 10.1016/j.plefa.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. The American Journal of Psychiatry. 2003;160(12):2222–2227. doi: 10.1176/appi.ajp.160.12.2222. [DOI] [PubMed] [Google Scholar]
- 48.Food and Agriculture Organization of the United Nations. Consumption of Fish and Fishery Products, 2001, http://www.fao.org/fishery/statistics/global-consumption/en.
- 49.Akbaraly TN, Sabia S, Shipley MJ, Batty GD, Kivimaki M. Adherence to healthy dietary guidelines and future depressive symptoms: evidence for sex differentials in the Whitehall II study. The American Journal of Clinical Nutrition. 2013;97(2):419–427. doi: 10.3945/ajcn.112.041582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akbaraly TN, Brunner EJ, Ferrie JE, Marmot MG, Kivimaki M, Singh-Manoux A. Dietary pattern and depressive symptoms in middle age. British Journal of Psychiatry. 2009;195(5):408–413. doi: 10.1192/bjp.bp.108.058925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacka FN, Kremer PJ, Berk M, et al. A prospective study of diet quality and mental health in adolescents. PLoS ONE. 2011;6(9) doi: 10.1371/journal.pone.0024805.e24805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacka FN, Mykletun A, Berk M, Bjelland I, Tell GS. The association between habitual diet quality and the common mental disorders in community-dwelling adults: the hordaland health study. Psychosomatic Medicine. 2011;73(6):483–490. doi: 10.1097/PSY.0b013e318222831a. [DOI] [PubMed] [Google Scholar]
- 53.Jacka FN, Pasco JA, Mykletun A, et al. Association of western and traditional diets with depression and anxiety in women. The American Journal of Psychiatry. 2010;167(3):305–311. doi: 10.1176/appi.ajp.2009.09060881. [DOI] [PubMed] [Google Scholar]
- 54.Skarupski KA, Tangney CC, Li H, Evans DA, Morris MC. Mediterranean diet and depressive symptoms among older adults over time. The Journal of Nutrition Health and Aging. 2013;17(5):441–445. doi: 10.1007/s12603-012-0437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sánchez-Villegas A, Delgado-Rodríguez M, Alonso A, et al. Association of the Mediterranean dietary pattern with the incidence of depression: The Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Archives of General Psychiatry. 2009;66(10):1090–1098. doi: 10.1001/archgenpsychiatry.2009.129. [DOI] [PubMed] [Google Scholar]
- 56.Rienks J, Dobson AJ, Mishra GD. Mediterranean dietary pattern and prevalence and incidence of depressive symptoms in mid-aged women: results from a large community-based prospective study. European Journal of Clinical Nutrition. 2013;67(1):75–82. doi: 10.1038/ejcn.2012.193. [DOI] [PubMed] [Google Scholar]
- 57.Hodge A, Almeida OP, English DR, Giles GG, Flicker L. Patterns of dietary intake and psychological distress in older Australians: benefits not just from a Mediterranean diet. International Psychogeriatrics. 2013;25(3):456–466. doi: 10.1017/S1041610212001986. [DOI] [PubMed] [Google Scholar]
- 58.Luciano M, Mottus R, Starr JM, et al. Depressive symptoms and diet: their effects on prospective inflammation levels in the elderly. Brain, Behavior, and Immunity. 2012;26(5):717–720. doi: 10.1016/j.bbi.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Antonogeorgos G, Panagiotakos DB, Pitsavos C, et al. Understanding the role of depression and anxiety on cardiovascular disease risk, using structural equation modeling, the mediating effect of the Mediterranean diet and physical activity: the ATTICA study. Annals of Epidemiology. 2012;22(9):630–637. doi: 10.1016/j.annepidem.2012.06.103. [DOI] [PubMed] [Google Scholar]
- 60.Sánchez-Villegas A, Henríquez P, Bes-Rastrollo M, Doreste J. Mediterranean diet and depression. Public Health Nutrition. 2006;9(8):1104–1109. doi: 10.1017/S1368980007668578. [DOI] [PubMed] [Google Scholar]
- 61.Sontrop J, Avison WR, Evers SE, Speechley KN, Campbell MK. Depressive symptoms during pregnancy in relation to fish consumption and intake of n-3 polyunsaturated fatty acids. Paediatric and Perinatal Epidemiology. 2008;22(4):389–399. doi: 10.1111/j.1365-3016.2008.00941.x. [DOI] [PubMed] [Google Scholar]
- 62.Murakami K, Mizoue T, Sasaki S, et al. Dietary intake of folate, other B vitamins, and ω-3 polyunsaturated fatty acids in relation to depressive symptoms in Japanese adults. Nutrition. 2008;24(2):140–147. doi: 10.1016/j.nut.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 63.Oddy WH, Hickling S, Smith MA, et al. Dietary intake of omega-3 fatty acids and risk of depressive symptoms in adolescents. Depression and Anxiety. 2011;28(7):582–588. doi: 10.1002/da.20822. [DOI] [PubMed] [Google Scholar]
- 64.Appleton KM, Peters TJ, Hayward RC, et al. Depressed mood and n-3 polyunsaturated fatty acid intake from fish: non-linear or confounded association? Social Psychiatry and Psychiatric Epidemiology. 2007;42(2):100–104. doi: 10.1007/s00127-006-0142-3. [DOI] [PubMed] [Google Scholar]
- 65.Panagiotakos DB, Mamplekou E, Pitsavos C, et al. Fatty acids intake and depressive symptomatology in a greek sample: an epidemiological analysis. Journal of the American College of Nutrition. 2010;29(6):586–594. doi: 10.1080/07315724.2010.10719897. [DOI] [PubMed] [Google Scholar]
- 66.Colangelo LA, He K, Whooley MA, Daviglus ML, Liu K. Higher dietary intake of long-chain ω-3 polyunsaturated fatty acids is inversely associated with depressive symptoms in women. Nutrition. 2009;25(10):1011–1019. doi: 10.1016/j.nut.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hakkarainen R, Partonen T, Haukka J, Virtamo J, Albanes D, Lönnqvist J. Is low dietary intake of omega-3 fatty acids associated with depression? The American Journal of Psychiatry. 2004;161(3):567–569. doi: 10.1176/appi.ajp.161.3.567. [DOI] [PubMed] [Google Scholar]
- 68.Jacka FN, Pasco JA, Hensry MJ, Kotowicz MA, Nicholson GC, Berk M. Dietary omega-3 fatty acids and depression in a community sample. Nutritional Neuroscience. 2004;7(2):101–106. doi: 10.1080/10284150410001710438. [DOI] [PubMed] [Google Scholar]
- 69.Kyrozis A, Psaltopoulou T, Stathopoulos P, Trichopoulos D, Vassilopoulos D, Trichopoulou A. Dietary lipids and geriatric depression scale score among elders: the EPIC-Greece cohort. Journal of Psychiatric Research. 2009;43(8):763–769. doi: 10.1016/j.jpsychires.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Strøm M, Mortensen EL, Halldorsson TI, Thorsdottir I, Olsen SF. Fish and long-chain n-3 polyunsaturated fatty acid intakes during pregnancy and risk of postpartum depression: a prospective study based on a large national birth cohort. The American Journal of Clinical Nutrition. 2009;90(1):149–155. doi: 10.3945/ajcn.2009.27552. [DOI] [PubMed] [Google Scholar]
- 71.Golding J, Steer C, Emmett P, Davis JM, Hibbeln JR. High levels of depressive symptoms in pregnancy with low omega-3 fatty acid intake from fish. Epidemiology. 2009;20(4):598–603. doi: 10.1097/EDE.0b013e31819d6a57. [DOI] [PubMed] [Google Scholar]
- 72.Da Rocha CMM, Kac G. High dietary ratio of omega-6 to omega-3 polyunsaturated acids during pregnancy and prevalence of post-partum depression. Maternal and Child Nutrition. 2012;8(1):36–48. doi: 10.1111/j.1740-8709.2010.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lucas M, Mirzaei F, O’Reilly EJ, et al. Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: a 10-y prospective follow-up study. The American Journal of Clinical Nutrition. 2011;93(6):1337–1343. doi: 10.3945/ajcn.111.011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kesse-Guyot E, Touvier M, Andreeva VA, et al. Cross-sectional but not longitudinal association between n-3 fatty acid intake and depressive symptoms: results from the SU.VI.MAX 2 study. The American Journal of Epidemiology. 2012;175(10):979–987. doi: 10.1093/aje/kwr472. [DOI] [PubMed] [Google Scholar]
- 75.Li Y, Zhang J, McKeown RE. Cross-sectional assessment of diet quality in individuals with a lifetime history of attempted suicide. Psychiatry Research. 2009;165(1-2):111–119. doi: 10.1016/j.psychres.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 76.Nanri A, Mizoue T, Poudel-Tandukar K, et al. Dietary patterns and suicide in Japanese adults: the Japan Public Health Center-based Prospective Study. The British Journal of Psychiatry. 2013;203(6):422–427. doi: 10.1192/bjp.bp.112.114793. [DOI] [PubMed] [Google Scholar]
- 77.Zhang J, Li Y, Torres ME. How does a suicide attempter eat differently from others? Comparison of macronutrient intakes. Nutrition. 2005;21(6):711–717. doi: 10.1016/j.nut.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 78.Poudel-Tandukar K, Nanri A, Iwasaki M, et al. Long chain n-3 fatty acids intake, fish consumption and suicide in a cohort of Japanese men and women—the Japan public health center-based (JPHC) prospective study. Journal of Affective Disorders. 2011;129(1–3):282–288. doi: 10.1016/j.jad.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 79.Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. Journal of Clinical Psychiatry. 2006;67(12):1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 80.Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. Journal of Clinical Psychiatry. 2007;68(7):1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- 81.Appleton KM, Hayward RC, Gunnell D, et al. Effects of n-3 long-chain polyunsaturated fatty acids on depressed mood: systematic review of published trials. The American Journal of Clinical Nutrition. 2006;84(6):1308–1316. doi: 10.1093/ajcn/84.6.1308. [DOI] [PubMed] [Google Scholar]
- 82.Rogers PJ, Appleton KM, Kessler D, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. British Journal of Nutrition. 2008;99(2):421–431. doi: 10.1017/S0007114507801097. [DOI] [PubMed] [Google Scholar]
- 83.Lin PY, Mischoulon D, Freeman MP, et al. Are omega-3 fatty acids antidepressants or just mood-improving agents? The effect depends upon diagnosis, supplement preparation, and severity of depression. Molecular Psychiatry. 2012;17(12):1161–1163. doi: 10.1038/mp.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids in Health and Disease. 2007;6, article 21 doi: 10.1186/1476-511X-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. Journal of the American College of Nutrition. 2009;28(5):525–542. doi: 10.1080/07315724.2009.10719785. [DOI] [PubMed] [Google Scholar]
- 86.Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of Eicosapentaenoic Acid (EPA) in clinical trials in depression. Journal of Clinical Psychiatry. 2011;72(12):1577–1584. doi: 10.4088/JCP.10m06634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Massart R, Mongeau R, Lanfumey L. Beyond the monoaminergic hypothesis: neuroplasticity and epigenetic changes in a transgenic mouse model of depression. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2012;367(1601):2485–2494. doi: 10.1098/rstb.2012.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Calder PC, Yaqoob P, Harvey DJ, Watts A, Newsholme EA. Incorporation of fatty acids by concanavalin A-stimulated lymphocytes and the effect on fatty acid composition and membrane fluidity. Biochemical Journal. 1994;300(2):509–518. doi: 10.1042/bj3000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee CR, Hamm MW. Effect of dietary fat and cholesterol supplements on glucagon receptor binding and adenylate cyclase activity of rat liver plasma membrane. Journal of Nutrition. 1989;119(4):539–546. doi: 10.1093/jn/119.4.539. [DOI] [PubMed] [Google Scholar]
- 90.Ahmad SN, Alma BS, Alam SQ. Dietary omega-3 fatty acids increase guanine nucleotide binding proteins and adenylate cyclase activity in rat salivary glands. The FASEB Journal. 1989;3, article A948 [Google Scholar]
- 91.Bowen RA, Clandinin MT. Dietary low linolenic acid compared with docosahexaenoic acid alter synaptic plasma membrane phospholipid fatty acid composition and sodium-potassium ATPase kinetics in developing rats. Journal of Neurochemistry. 2002;83(4):764–774. doi: 10.1046/j.1471-4159.2002.01156.x. [DOI] [PubMed] [Google Scholar]
- 92.Vaidyanathan VV, Rao KV, Sastry PS. Regulation of diacylglycerol kinase in rat brain membranes by docosahexaenoic acid. Neuroscience Letters. 1994;179(1-2):171–174. doi: 10.1016/0304-3940(94)90961-x. [DOI] [PubMed] [Google Scholar]
- 93.Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biological Psychiatry. 1997;41(2):162–171. doi: 10.1016/s0006-3223(96)00217-x. [DOI] [PubMed] [Google Scholar]
- 94.Hibbeln JR, Linnoila M, Umhau JC, Rawlings R, George DT, Salem N., Jr. Essential fatty acids predict metabolites of serotonin and dopamine in cerebrospinal fluid among healthy control subjects, and early- and late- onset alcoholics. Biological Psychiatry. 1998;44(4):235–242. doi: 10.1016/s0006-3223(98)00141-3. [DOI] [PubMed] [Google Scholar]
- 95.Vines A, Delattre AM, Lima MMS, et al. The role of 5-HT1A receptors in fish oil-mediated increased BDNF expression in the rat hippocampus and cortex: a possible antidepressant mechanism. Neuropharmacology. 2012;62(1):184–191. doi: 10.1016/j.neuropharm.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 96.Delion S, Chalon S, Guilloteau D, Besnard J-C, Durand G. α-Linolenic acid dietary deficiency alters age-related changes of dopaminergic and serotoninergic neurotransmission in the rat frontal cortex. Journal of Neurochemistry. 1996;66(4):1582–1591. doi: 10.1046/j.1471-4159.1996.66041582.x. [DOI] [PubMed] [Google Scholar]
- 97.McNamara RK, Able J, Liu Y, et al. Omega-3 fatty acid deficiency during perinatal development increases serotonin turnover in the prefrontal cortex and decreases midbrain tryptophan hydroxylase-2 expression in adult female rats: dissociation from estrogenic effects. Journal of Psychiatric Research. 2009;43(6):656–663. doi: 10.1016/j.jpsychires.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McNamara RK, Jandacek R, Rider T, Tso P, Cole-Strauss A, Lipton JW. Omega-3 fatty acid deficiency increases constitutive pro-inflammatory cytokine production in rats: relationship with central serotonin turnover. Prostaglandins Leukotrienes and Essential Fatty Acids. 2010;83(4–6):185–191. doi: 10.1016/j.plefa.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heidbreder CA, Weiss IC, Domeney AM, et al. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100(4):749–768. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 100.Zangen A, Overstreet DH, Yadid G. Increased catecholamine levels in specific brain regions of a rat model of depression: normalization by chronic antidepressant treatment. Brain Research. 1999;824(2):243–250. doi: 10.1016/s0006-8993(99)01214-7. [DOI] [PubMed] [Google Scholar]
- 101.Zimmer L, Vancassel S, Cantagrel S, et al. The dopamine mesocorticolimbic pathway is affected by deficiency in n-3 polyunsaturated fatty acids. The American Journal of Clinical Nutrition. 2002;75(4):662–667. doi: 10.1093/ajcn/75.4.662. [DOI] [PubMed] [Google Scholar]
- 102.Zimmer L, Delion-Vancassel S, Durand G, et al. Modification of dopamine neurotransmission in the nucleus accumbens of rats deficient in n-3 polyunsaturated fatty acids. Journal of Lipid Research. 2000;41(1):32–40. [PubMed] [Google Scholar]
- 103.Chalon S, Delion-Vancassel S, Belzung C, et al. Dietary fish oil affects monoaminergic neurotransmission and behavior in rats. Journal of Nutrition. 1998;128(12):2512–2519. doi: 10.1093/jn/128.12.2512. [DOI] [PubMed] [Google Scholar]
- 104.Müller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Molecular Psychiatry. 2007;12(11):988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- 105.Yan Q-S, Reith MEA, Jobe PC, Dailey JW. Dizocilpine (MK-801) increases not only dopamine but also serotonin and norepinephrine transmissions in the nucleus accumbens as measured by microdialysis in freely moving rats. Brain Research. 1997;765(1):149–158. doi: 10.1016/s0006-8993(97)00568-4. [DOI] [PubMed] [Google Scholar]
- 106.Martin P, Carlsson ML, Hjorth S. Systemic PCP treatment elevates brain extracellular 5-HT: a microdialysis study in awake rats. NeuroReport. 1998;9(13):2985–2988. doi: 10.1097/00001756-199809140-00012. [DOI] [PubMed] [Google Scholar]
- 107.Latour A, Grintal B, Champeil-Potokar G, et al. Omega-3 fatty acids deficiency aggravates glutamatergic synapse and astroglial aging in the rat hippocampal CA1. Aging Cell. 2013;12(1):76–84. doi: 10.1111/acel.12026. [DOI] [PubMed] [Google Scholar]
- 108.Grintal B, Champeil-Potokar G, Lavialle M, Vancassel S, Breton S, Denis I. Inhibition of astroglial glutamate transport by polyunsaturated fatty acids: evidence for a signalling role of docosahexaenoic acid. Neurochemistry International. 2009;54(8):535–543. doi: 10.1016/j.neuint.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 109.Moreira JD, Knorr L, Ganzella M, et al. Omega-3 fatty acids deprivation affects ontogeny of glutamatergic synapses in rats: relevance for behavior alterations. Neurochemistry International. 2010;56(6-7):753–759. doi: 10.1016/j.neuint.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 110.Speizer LA, Watson MJ, Brunton LL. Differential effects of omega-3 fish oils on protein kinase activities in vitro. The American Journal of Physiology—Endocrinology and Metabolism. 1991;261(1):E109–E114. doi: 10.1152/ajpendo.1991.261.1.E109. [DOI] [PubMed] [Google Scholar]
- 111.Holian O, Nelson R. Action of long-chain fatty acids on protein kinase C activity: comparison of omega-6 and omega-3 fatty acids. Anticancer Research. 1992;12(3):975–980. [PubMed] [Google Scholar]
- 112.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. The American Journal of Psychiatry. 2003;160(9):1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 113.Pace TWW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain, Behavior, and Immunity. 2007;21(1):9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schiepers OJG, Wichers MC, Maes M. Cytokines and major depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29(2):201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 115.Hennebelle M, Balasse L, Latour A, et al. Influence of omega-3 fatty acid status on the way rats adapt to chronic restraint stress. PLoS ONE. 2012;7(7) doi: 10.1371/journal.pone.0042142.e42142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ferraz AC, Delattre AM, Almendra RG, et al. Chronic ω-3 fatty acids supplementation promotes beneficial effects on anxiety, cognitive and depressive-like behaviors in rats subjected to a restraint stress protocol. Behavioural Brain Research. 2011;219(1):116–122. doi: 10.1016/j.bbr.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 117.Song C, Li X, Leonard BE, Horrobin DF. Effects of dietary n-3 or n-6 fatty acids on interleukin-1β-induced anxiety, stress, and inflammatory responses in rats. Journal of Lipid Research. 2003;44(10):1984–1991. doi: 10.1194/jlr.M300217-JLR200. [DOI] [PubMed] [Google Scholar]
- 118.Murck H, Song C, Horrobin DF, Uhr M. Ethyl-eicosapentaenoate and dexamethasone resistance in therapy-refractory depression. International Journal of Neuropsychopharmacology. 2004;7(3):341–349. doi: 10.1017/S1461145704004249. [DOI] [PubMed] [Google Scholar]
- 119.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23(5):477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 120.Karssen AM, Meijer OC, van der Sandt ICJ, et al. Multidrug resistance P-glycoprotein hampers the access of cortisol but not of corticosterone to mouse and human brain. Endocrinology. 2001;142(6):2686–2694. doi: 10.1210/endo.142.6.8213. [DOI] [PubMed] [Google Scholar]
- 121.Pariante CM, Makoff A, Lovestone S, et al. Antidepressants enhance glucocorticoid receptor function in vitro by modulating the membrane steroid transporters. British Journal of Pharmacology. 2001;134(6):1335–1343. doi: 10.1038/sj.bjp.0704368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biological Psychiatry. 2001;49(5):391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 123.Mason BL, Pariante CM. The effects of antidepressants on the hypothalamic-pituitary-adrenal axis. Drug News and Perspectives. 2006;19(10):603–608. doi: 10.1358/dnp.2006.19.10.1068007. [DOI] [PubMed] [Google Scholar]
- 124.Navarro C, González-Álvarez I, González-Álvarez M, et al. Influence of polyunsaturated fatty acids on Cortisol transport through MDCK and MDCK-MDR1 cells as blood-brain barrier in vitro model. European Journal of Pharmaceutical Sciences. 2011;42(3):290–299. doi: 10.1016/j.ejps.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 125.Caraci F, Copani A, Nicoletti F, Drago F. Depression and Alzheimer’s disease: neurobiological links and common pharmacological targets. European Journal of Pharmacology. 2010;626(1):64–71. doi: 10.1016/j.ejphar.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 126.Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. The American Journal of Psychiatry. 2010;167(11):1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wager-Smith K, Markou A. Depression: a repair response to stress-induced neuronal microdamage that can grade into a chronic neuroinflammatory condition? Neuroscience and Biobehavioral Reviews. 2011;35(3):742–764. doi: 10.1016/j.neubiorev.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maes M, Yirmyia R, Noraberg J, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metabolic Brain Disease. 2009;24(1):27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- 129.Myint A-M, Leonard BE, Steinbusch HWM, Kim Y-K. Th1, Th2, and Th3 cytokine alterations in major depression. Journal of Affective Disorders. 2005;88(2):167–173. doi: 10.1016/j.jad.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 130.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nature Reviews Immunology. 2007;7(2):161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 131.Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. Journal of Clinical Investigation. 2001;108(1):15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kidd PM. Omega-3 DHA and EPA for cognition, behavior, and mood: clinical findings and structural-functional synergies with cell membrane phospholipids. Alternative Medicine Review. 2007;12(3):207–227. [PubMed] [Google Scholar]
- 133.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. The American Journal of Clinical Nutrition. 2006;83(supplement 6):S1505–S1519. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 134.Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biological Psychiatry. 1998;43(5):315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- 135.Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. Journal of Affective Disorders. 1998;48(2-3):149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 136.Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Meltzer HY. Lowered ω3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Research. 1999;85(3):275–291. doi: 10.1016/s0165-1781(99)00014-1. [DOI] [PubMed] [Google Scholar]
- 137.Adams PB, Lawson S, Sanigorski A, Sinclair AJ. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31(supplement 3):S157–S161. doi: 10.1007/BF02637069. [DOI] [PubMed] [Google Scholar]
- 138.Maes M, Smith R, Christophe A, Cosyns P, Desnyder R, Meltzer H. Fatty acid composition in major depression: decreased omega 3 fractions in cholesteryl esters and increased C20: 4 omega 6/C20:5 omega 3 ratio in cholesteryl esters and phospholipids. Journal of Affective Disorders. 1996;38(1):35–46. doi: 10.1016/0165-0327(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 139.Tiemeier H, van Tuijl HR, Hofman A, Kiliaan AJ, Breteler MMB. Plasma fatty acid composition and depression are associated in the elderly: The Rotterdam Study. The American Journal of Clinical Nutrition. 2003;78(1):40–46. doi: 10.1093/ajcn/78.1.40. [DOI] [PubMed] [Google Scholar]
- 140.Kiecolt-Glaser JK, Belury MA, Porter K, Beversdorf DQ, Lemeshow S, Glaser R. Depressive symptoms, omega-6:omega-3 fatty acids, and inflammation in older adults. Psychosomatic Medicine. 2007;69(3):217–224. doi: 10.1097/PSY.0b013e3180313a45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rizzo AM, Corsetto PA, Montorfano G, et al. Comparison between the AA/EPA ratio in depressed and non depressed elderly females: omega-3 fatty acid supplementation correlates with improved symptoms but does not change immunological parameters. Nutrition Journal. 2012;11, article 82 doi: 10.1186/1475-2891-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rees AM, Austin MP, Owen C, Parker G. Omega-3 deficiency associated with perinatal depression: case control study. Psychiatry Research. 2009;166(2-3):254–259. doi: 10.1016/j.psychres.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 143.Schins A, Crijns HJ, Brummer R-JM, et al. Altered omega-3 polyunsaturated fatty acid status in depressed post-myocardial infarction patients. Acta Psychiatrica Scandinavica. 2007;115(1):35–40. doi: 10.1111/j.1600-0447.2006.00830.x. [DOI] [PubMed] [Google Scholar]
- 144.Frasure-Smith N, Lespérance F, Julien P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biological Psychiatry. 2004;55(9):891–896. doi: 10.1016/j.biopsych.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 145.Parker GB, Heruc GA, Hilton TM, et al. Low levels of docosahexaenoic acid identified in acute coronary syndrome patients with depression. Psychiatry Research. 2006;141(3):279–286. doi: 10.1016/j.psychres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 146.Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. The American Journal of Psychiatry. 2006;163(6):1100–1102. doi: 10.1176/ajp.2006.163.6.1100. [DOI] [PubMed] [Google Scholar]
- 147.Riemer S, Maes M, Christophe A, Rief W. Lowered ω-3 PUFAs are related to major depression, but not to somatization syndrome. Journal of Affective Disorders. 2010;123(1–3):173–180. doi: 10.1016/j.jad.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 148.Mamalakis G, Tornaritis M, Kafatos A. Depression and adipose essential polyunsaturated fatty acids. Prostaglandins Leukotrienes and Essential Fatty Acids. 2002;67(5):311–318. doi: 10.1054/plef.2002.0435. [DOI] [PubMed] [Google Scholar]
- 149.Mamalakis G, Kalogeropoulos N, Andrikopoulos N, et al. Depression and long chain n-3 fatty acids in adipose tissue in adults from Crete. European Journal of Clinical Nutrition. 2006;60(7):882–888. doi: 10.1038/sj.ejcn.1602394. [DOI] [PubMed] [Google Scholar]
- 150.Mamalakis G, Jansen E, Cremers H, Kiriakakis M, Tsibinos G, Kafatos A. Depression and adipose and serum cholesteryl ester polyunsaturated fatty acids in the survivors of the seven countries study population of Crete. European Journal of Clinical Nutrition. 2006;60(8):1016–1023. doi: 10.1038/sj.ejcn.1602413. [DOI] [PubMed] [Google Scholar]
- 151.Mamalakis G, Kiriakakis M, Tsibinos G, et al. Lack of an association of depression with n-3 polyunsaturated fatty acids in adipose tissue and serum phospholipids in healthy adults. Pharmacology Biochemistry and Behavior. 2008;89(1):6–10. doi: 10.1016/j.pbb.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 152.Mamalakis G, Kiriakakis M, Tsibinos G, Kafatos A. Depression and adipose polyunsaturated fatty acids in an adolescent group. Prostaglandins Leukotrienes and Essential Fatty Acids. 2004;71(5):289–294. doi: 10.1016/j.plefa.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 153.Wichers M, Maes M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. International Journal of Neuropsychopharmacology. 2002;5(4):375–388. doi: 10.1017/S1461145702003103. [DOI] [PubMed] [Google Scholar]
- 154.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 155.Calder PC. N-3 fatty acids, inflammation and immunity: new mechanisms to explain old actions. Proceedings of the Nutrition Society. 2013;72(3):326–336. doi: 10.1017/S0029665113001031. [DOI] [PubMed] [Google Scholar]
- 156.Lu DY, Tsao YY, Leung YM, Su KP. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: implications of antidepressant effects for omega-3 fatty acids. Neuropsychopharmacology. 2010;35(11):2238–2248. doi: 10.1038/npp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Park Y, Moon HJ, Kim SH. N-3 polyunsaturated fatty acid consumption produces neurobiological effects associated with prevention of depression in rats after the forced swimming test. Journal of Nutritional Biochemistry. 2011 doi: 10.1016/j.jnutbio.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 158.Moranis A, Delpech JC, de Smedt-Peyrusse V, et al. Long term adequate n-3 polyunsaturated fatty acid diet protects from depressive-like behavior but not from working memory disruption and brain cytokine expression in aged mice. Brain, Behavior, and Immunity. 2012;26(5):721–731. doi: 10.1016/j.bbi.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 159.Serhan CN. Novel eicosanoid and docosanoid mediators: resolvins, docosatrienes, and neuroprotectins. Current Opinion in Clinical Nutrition and Metabolic Care. 2005;8(2):115–121. doi: 10.1097/00075197-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 160.Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat. Journal of Neurochemistry. 2007;101(3):577–599. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- 161.Prescott SM, Stenson WF. Fish oil fix. Nature Medicine. 2005;11(6):596–598. doi: 10.1038/nm0605-596. [DOI] [PubMed] [Google Scholar]
- 162.Kim Y, Lee C. The gene encoding transforming growth factor β1 confers risk of ischemic stroke and vascular dementia. Stroke. 2006;37(11):2843–2845. doi: 10.1161/01.STR.0000244782.76917.87. [DOI] [PubMed] [Google Scholar]
- 163.Sutcigil L, Oktenli C, Musabak U, et al. Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clinical and Developmental Immunology. 2007;2007 doi: 10.1155/2007/76396.76396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vollmar P, Haghikia A, Dermietzel R, Faustmann PM. Venlafaxine exhibits an anti-inflammatory effect in an inflammatory co-culture model. International Journal of Neuropsychopharmacology. 2008;11(1):111–117. doi: 10.1017/S1461145707007729. [DOI] [PubMed] [Google Scholar]
- 165.Hida M, Fujita H, Ishikura K, Omori S, Hoshiya M, Awazu M. Eicosapentaenoic acid inhibits PDGF-induced mitogenesis and cyclin D1 expression via TGF-β in mesangial cells. Journal of Cellular Physiology. 2003;196(2):293–300. doi: 10.1002/jcp.10298. [DOI] [PubMed] [Google Scholar]
- 166.Thienprasert A, Samuhaseneetoo S, Popplestone K, West AL, Miles EA, Calder PC. Fish oil n-3 polyunsaturated fatty acids selectively affect plasma cytokines and decrease illness in Thai schoolchildren: a randomized, double-blind, placebo-controlled intervention trial. Journal of Pediatrics. 2009;154(3):391–395. doi: 10.1016/j.jpeds.2008.09.014. [DOI] [PubMed] [Google Scholar]


