Abstract
Prostate cancer (PCa) is the most common malignancy in elderly men. The progressive ageing of the world male population will further increase the need for tailored assessment and treatment of PCa patients. The determinant role of androgens and sexual hormones for PCa growth and progression has been established. However, several trials on androgens and PCa are recently focused on urinary continence, quality of life, and sexual function, suggesting a new point of view on the whole endocrinological aspect of PCa. During aging, metabolic syndrome, including diabetes, hypertension, dyslipidemia, and central obesity, can be associated with a chronic, low-grade inflammation of the prostate and with changes in the sex steroid pathways. These factors may affect both the carcinogenesis processes and treatment outcomes of PCa. Any treatment for PCa can have a long-lasting negative impact on quality of life and sexual health, which should be assessed by validated self-reported questionnaires. In particular, sexual health, urinary continence, and bowel function can be worsened after prostatectomy, radiotherapy, or hormone treatment, mostly in the elderly population. In the present review we summarized the current knowledge on the role of hormones, metabolic features, and primary treatments for PCa on the quality of life and sexual health of elderly Pca survivors.
1. Introduction
Prostate cancer (PCa) is the most common malignancy in elderly men. Age is a relevant risk factor, with a proven histological PCa being found in 60% of men by the age of 70 years and 80% by the age of 80 [1]. In fact, PCa is considered a chronic disease, needing a long period for initiation, development, and progression, through the development of early and later precancerous modifications, such as high-grade prostate intraepithelial neoplasia (HG-PIN), leading to the development of a clinically relevant cancer [2, 3]. Therefore, PCa is frequent in old men, likely becoming the prevalent cancer because of the ageing of population [4].
Although androgen receptor (AR) pathway is crucial for prostate cancer growth and progression, evidence supporting a favorable risk-benefit ratio of androgen deprivation therapy (ADT) is currently limited to high-risk PCa or metastatic disease [5, 6]. Furthermore, hypogonadism is common in elderly men and men who have PCa: the symptoms of hypogonadism, such as depression, erectile dysfunction (ED), and lower urinary tract symptoms, can impair a man's quality of life (QoL) [7]. Therefore, androgens and AR play a critical role in management of elderly men with PCa.
The current literature suggests an association between metabolic syndrome (MetS) and PCa, although the evidence for a causal relationship remains unknown [8]. In particular, a recent review pointed out that men with MetS seem to have more likely high-grade and advanced PCa: moreover, they resulted in greater risk of progression and cancer specific death, even if the overall analyses did not reveal any association between MetS and the risk to develop the disease [8]. Therefore, MetS should be assessed as a new domain in basic and clinical research in elderly men with PCa.
The primary goal of any definitive treatment of PCa is the improvement of survival and QoL: although surgery, radiotherapy, and hormone therapy can lead to long-term survival, these treatments can cause lasting side effects [9]. Therefore, patients survival has to be considered in treatment decision making, but patients' quality of life must also be considered before and after any treatment [10]. Moreover, an accurate assessment of QoL in PCa patients must be performed with validated, self-reported, and disease specific instruments [11]. Therefore, there is a need for a tailored approach in the management of PCa in the elderly men, to avoid unnecessary intervention with permanent adverse event [12].
The aim of present review is to summarize the current knowledge on the role of androgens pathways, metabolic factors, and primary treatments on the overall QoL and sexual health of elderly PCa survivors.
2. Endocrinological Aspects of Prostate Cancer
2.1. The Role of Androgens and of Androgen Receptor (AR) in Carcinogenesis and Progression of Prostate Cancer
Prostate volume and function are age- and androgen-dependent [13] and in hypogonadal subjects therapy with testosterone restores the volume of the prostate to that of eugonadal men [14]. Androgens and AR play a fundamental role in the development of PCa which is androgen-dependent for its growth, as demonstrated in the pioneering work of Huggins and Hodges [15] who showed that castration causes complete regression of the disease.
How actions of AR become tumorigenic and lead to uncontrolled growth remains poorly understood. In a high percentage of PCa, fusions between the androgen-dependent gene TMPRSS2 and ETS transcription factors (such as ERG) occur through chromosomal translocations [16], leading to elevated expression of these oncogenic factors under androgen control. However, whether TMPRSS2:ETS fusions are sufficient to promote PCa is discussed [17, 18] and the initial enthusiasm about such chromosomal aberrations has been dampened by the controversial results of clinical studies investigating their role in PCa progression [19].
Androgen deprivation therapy (ADT) represents a valuable treatment of metastatic PCa. However, ADT provides palliation but not cure and most PCa regrow as castration-resistant PCa (CRPCa) able to survive and grow in a milieu virtually deprived of androgens. The detailed mechanisms of why ADT ultimately fails and a more aggressive cancer recurs remain unclear (Figure 1). In the past decade, based on in vitro or in vivo evidence, several hypotheses involving the AR have been generated to explain development of CRPCa, such as AR mutations (found in about 20% of metastatic specimens) or amplifications that confer the ability to bind other steroids and even antiandrogens (acting as agonists), changes in AR-coregulators interactions, and activation of AR by growth factors or other signal pathways (reviewed in [20]). In addition, recent work has highlighted the role of intraprostatic androgen synthesis as the driving force of recurrent disease (see below for further details).
Figure 1.
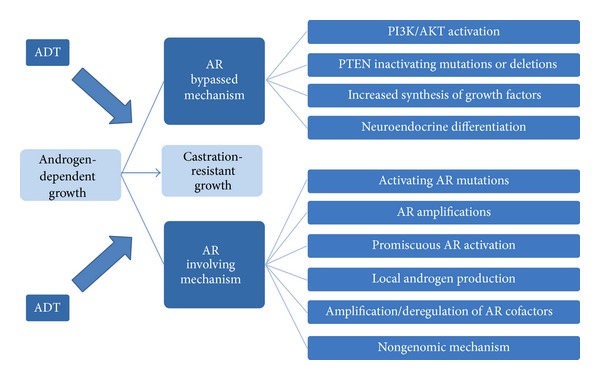
Schematic representation of the main pathways involved in development of castration-resistant prostate cancer (CRPC). ADT: androgen-deprivation therapy, AR: androgen receptor. Modified from 43.
Interestingly, low expression of mutated AR may drive in vitro growth of CRPCa cell lines also by nongenomic (rapid signalling) mechanisms [21]. However, more studies are needed in order to better understand the role, if any, of nongenomic AR signalling in PCa growth and progression. These AR-involving hypotheses do not completely explain why patients receiving ADT tend to have an earlier development of more aggressive cancer. Alternative pathways of growth and invasion may develop in PCa cells (Figure 1) bypassing the necessity of androgens: among these, PTEN inactivating mutation has been found in a high proportion of PCa [22] leading to suppression of apoptotic pathways and consequent uncontrolled growth. Neuroendocrine differentiation also plays an important role in development of CRPCa [23]. In summary, development of CRPCa is a very complex event, potentially involving both androgen-regulated and androgen-alternative pathways (Figure 1). Such a complexity makes the development of therapeutic strategies very difficult, and, as today, CRPCa is basically incurable.
Currently, research is mainly directed to understand the role of these multiple pathways and their interregulation with the aim of identifying potential therapeutical targets. One hot topic of research is aimed at understating the role of AR. In a recent survey of the literature concerning the relationship between AR expression in PCa specimen and disease prognosis, we have highlighted the conflicting results reported so far [24]. These studies evidenced both the highly variable expression of AR among different cancers and a different relation with prognosis. Most studies did not find any association between AR expression and prognosis, including a large one by Minner et al. [25], whereas some studies found an association between high AR expression and better or worse prognosis. Although such contrasting findings may depend on several factors [24], one possible explanation may be related to a different role of AR depending on its location (stroma or epithelium) in the tumor. Recently, a mouse cancer model lacking the AR only in the prostatic epithelium and/or stroma has been generated (ARKO-TRAMP) [26, 27]. These mice paradoxically develop poorly differentiated PCa and, most importantly, restoration of AR function in epithelial basal cells leads to tumor suppression. Conversely, restoration of AR in stromal cells stimulates cancer progression, supporting a differential role of AR in PCa depending on its location. Studies in mice models suggest that stromal AR may promote prostate tumorigenesis via induction of proinflammatory cytokines/chemokines expression [28].
These results substantiate in vitro studies showing that enforced expression of AR in AR-negative PCa cells decreases the metastatic/invasive potential of the cells [26, 29–35]. In a recent paper evaluating the role of androgen signalling in epithelial-mesenchimal transition, Zhu and Kyprianou [36] demonstrated that overexpression of AR in PCa cell lines suppresses androgen-induced epithelial-mesenchimal transition, suggesting that downregulation of AR occurring in androgen-deprived condition [37] may facilitate mesenchimal transition and promote metastasis [36]. There is also evidence that inducing AR expression in PCa cells by targeting methylation of promoter increases differentiation of carcinoma cells and suppresses self-renewal/proliferation of stem cells and tumorigenesis [38].
Clinical data supporting a differentiating role of AR in PCa and, as such, limiting invasiveness have been also published. Following androgen ablation metastatic PCa is promoted in vitro [39] and there is clinical evidence that intermittent ADT benefits patients in PCa progression [40]. In addition, patients with CRPCa displaying amplification of AR gene survive longer than patients without amplification [41]. Results of long-term survival in the PCa prevention trial with the 5 alpha-reductase inhibitor finasteride demonstrated that, although the risk of developing PC is decreased, the Gleason score of developing cancers is significantly higher in the finasteride group and, overall, no difference in life expectancy between the treated and placebo group was observed [42]. Finally, there is evidence in the literature that, in some instances, CRPCa may benefit from androgen-replacement therapies [43–45]. Overall, these studies suggest that AR may have both negative and positive roles in PCa progression by regulating cell growth and invasion ability [46, 47].
In such a complex scenario, it is clear that more studies are needed to define the role of AR in the different PCa compartments. In addition, investigations should be aimed at evaluating the different AR variants present in the tumors. Indeed a recent study [48], performed in a small series of CRPCa bone metastases (n = 30), demonstrated that expression of AR variants lacking the ligand binding domain was associated with poor prognosis and shorter survival rates. If these results will be confirmed in a larger series of subjects, they can open new therapeutic perspectives to target other portions of AR. Of interest, an AR antagonist, EPI-001, able to bind the N-terminal domain of AR, has been recently developed [49, 50]. This antagonist has been found to reduce the growth of CRPCa xenografts [51].
2.2. Prostate Cancer in Hypogonadal Men
Although a causative role for circulating androgens on PCa has been envisaged since the Huggins and Hodges studies, data clearly showed that such a link is at best unproved. In a meta-analysis of 18 prospective studies, including almost 4000 men with incident PCa and 6500 control subjects, no associations were found between the risk of PCa and serum concentrations of Testosterone (T), calculated FT, DHT, and other androgens [52]. Furthermore, some authors have documented that low serum T is associated with more aggressive, ADT-resistant tumors suggesting that low levels of androgens create a selective pressure for PCa cells leading to androgen-independence ([53, 54]; for review see [55, 56]).
In line with these data, the pooled odds ratio for Testosterone Replacement Therapy (TRT) derived from 19 randomized clinical trials was 1.09 (0.48–2.49, 95% CI) for PC and 1.19 (0.67–2.09, 95% CI) for PSA > 4 ng/dL or 1.5% increase during study (for review see [55, 56]). Based on critical analysis of clinical trials and on the aforementioned experimental data on PCa cell lines, so far 11 investigators evaluated the effect of TRT even in PCa patients, with the aim of inducing differentiation in the tumor [55, 56]. Overall these studies included 279 subjects previously treated with radical prostatectomy or radiotherapy. In the vast majority of patients no association with progression or clinical recurrence was reported. Despite this evidence, it should be recognized that the number of reported cases is still small and heterogeneous. In the absence of randomized controlled trials (RCT), the concept of using TRT for PCa survivors is debatable. Accordingly, current recommendations suggest limiting TRT to symptomatic hypogonadal men successfully treated for PCa, after a prudent interval, although the length of that interval is not specified [57].
2.3. Intraprostatic Synthesis of Steroids: Role in PCa Progression
As mentioned above, intraprostatic androgen synthesis may support PCa cell growth even in the virtual absence of androgens contributing to development of CRPCa [13]. There is evidence that androgen levels may remain elevated in the prostate during ADT [58, 59]. Moreover, androgens have been found in locally recurrent CRPCa [60] and in distant metastasis [61]. These studies suggest that the PCa may acquire the capability to synthesize androgens although a direct proof can be only obtained by demonstrating the occurrence of steroidogenic machinery in the cells. Results of the latter experiments are contrasting [62, 63]. In particular, in a recent study [62], expression of the steroidogenic enzymes CYP17A1 and HSD3B1, essential for androgen synthesis, has been detected at low levels only in 19 of the 88 tumor samples leading to the conclusion that intratumoral steroid biosynthesis has a limited contribution. However, the elevated expression of 5 alpha-reductase found in CRPCa samples [63] suggests that de novo steroidogenesis may occur bypassing the requirement of T by 5 alpha-reduction of adrenal precursor steroids [13]. Targeting androgen synthesis in CRPC with abiraterone acetate, a potent inhibitor of CYP17, resulted to be safe and well tolerated, leading to a reduction of the risk of death and increased median survival of some months compared to placebo [64]. At present ongoing investigations are evaluating the efficiency of abiraterone acetate (in combination with other treatments) in the early stages of PC. Efforts are currently directed to understand the mechanisms of resistance to abiraterone acetate and how to prevent it.
2.4. Modifications of Sex Hormone after Radical Treatment for Prostate Cancer
Several studies have analyzed the modifications in the levels of T and gonadotropins following radical treatment, producing controversial results [65–68]. In particular, in 55 males treated with radical prostatectomy (RP), a remarkable increase in T, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) has been reported 1 year after RP [69]. These data were confirmed by Olsson et al. [66]. In a group of 49 men, LH and FSH were increased by 71 and 63%, respectively, 12 months after RP without any evident changes in T, suggesting that the hypothalamic pituitary axis was inhibited in patients with PCa and that this inhibition has been removed following RP [69].
Recently, we enrolled 100 men affected by PCa in a single center prospective study, with the aim to evaluate the changes in the serum levels of T, LH, and FSH within the first 3 months after RP for clinically localized PCa and to analyze the correlation between LH and T at various follow-up times [67]. As expected, we found a remarkable positive correlation between T and LH before surgery (r = 0.370; P < 0.0001), but not 1 month after RP (r = 0.109; P = 0.303). Three months after prostatectomy, the correlation between T and LH was restored (r = 0.273; P = 0.054). Therefore, our data demonstrated that RP can induce an early significant decline in the T levels and a compensatory increase in LH and FSH levels. These data have a critical relevance, suggesting that hormone modifications could have an important role in the loss and the subsequent recovery of both urinary continence and potency [70]. Three months after RP, the full recovery of T levels, with persistent high levels of gonadotropins, seems to delineate the features of compensated hypergonadotropic hypogonadism.
To confirm these data and to analyze the influence of T on sexual activity and urinary continence in men with PCa, we consecutively enrolled 257 patients treated with RP in our center [71]. As expected both age and BMI have a negative impact on preoperative T levels. Moreover, in men with normal T, urinary continence was significantly correlated with sexual function and sexual bother (r = 0.2544: P = 0.01 and r = 0.2512: P = 0.01), whereas this correlation was lost in hypogonadal men.
3. Metabolic Syndrome and Prostate Cancer in Elderly Men
Metabolic syndrome describes the combination or clustering of several metabolic abnormalities including central obesity, dyslipidemia, hypertension, insulin resistance with compensatory hyperinsulinemia, and glucose intolerance [72–74]. Recently, epidemiological, histopathological, molecular pathological, and clinical studies have provided emerging evidence of a possible role of MetS and its components in PCa development and progression [74, 75].
Although the only well-established risk factors associated with PCa are age, race, and family history, the large geographical variations in PCa risk suggest that lifestyle and environmental factors may also contribute to its etiology. The possibility to prevent and treat MetS and its components led to novel therapeutic approaches that have been proposed as a new frontier in the prevention and treatment of PCa [74, 76, 77].
3.1. Definition, Epidemiology, and Pathophysiology
MetS is a constellation of physiological and biochemical abnormalities characterized by diabetes or high fasting glucose, central obesity, abnormal cholesterol and triglyceride levels, and hypertension [78]. Currently, the two most widely used definitions are those proposed by the National Cholesterol Educational Program Adult Treatment Panel III (NCEP:ATP III) and by the International Diabetes Federation (IDF) focusing on abdominal obesity measured by waist circumference. In contrast, the World Health Organization (WHO) and the European Group for the study on Insulin Resistance (EGIR) definitions are principally focused on IR [73, 74].
Prevalence of MetS increases linearly from the age of 20 until age of 50, when it plateaus and affects more than 40% of the population in the United States and nearly 30% in Europe [79, 80]. Similar to western countries, the prevalence of MetS is rapidly increasing in developing countries, ranging from 9.8% in males from urban north India, to 16.3% in Morocco, to 25.4% in urban Brazil, to 33.5% in South Africa, and to 33.7% in Iran [74, 78, 79]. People with MetS are estimated to have twice the risk of developing cardiovascular disease compared to healthy individuals and a fivefold increased risk of type-2 diabetes. MetS has been recently linked to a number of urological diseases including PCa [72, 75]. Although IR and obesity are considered at the core of the pathophysiology of MetS, a number of other factors can also be involved in its pathogenesis and potential interactions [73, 74].
In most cases MetS develops as a result of poor eating habits and/or sedentary lifestyles which are associated with IR and obesity. IR occurs when there is a decrease in the responsiveness of peripheral tissues (skeletal muscle, fat, and liver) to the effect of insulin with a concomitant hyperinsulinemia [80]. Hyperinsulinemia is also responsible for stimulating Insulin Growth Factor-1 (IGF) production in the liver. IGF-1 is a potent mitogenic factor and apoptosis inhibitor which has been linked with PCa risk [81].
Central obesity is also considered an early step in the development and progression of MetS. Visceral adipose tissue secretes various bioactive substances known as adipocytokines which can induce IR and have proinflammatory and proatherogenic effects (Figure 2). Cytokines including resistin, leptin, tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), C-reactive protein (PCR), fibrinogen, and plasminogen activator inhibitor (PAI-1) are normally increased in obese patients and in patients with DMT2. On the contrary, adiponectin, is lower in individuals with visceral fat accumulation. Adiponectin stimulates glucose metabolism and fatty acid oxidation in the muscle, enhances insulin sensitivity in the liver, increases free fatty acid oxidation, reduces hepatic glucose output, and inhibits monocyte adhesion and macrophage transformation to foam cells within the vascular wall [80, 82]. Visceral adiposity may also contribute to hypogonadism, frequently associated with MetS in men, through increased aromatase activity. Its increased activity in obese patients raises estradiol levels, which results in feedback inhibition at the level of the hypothalamus/pituitary to lower T leading to hypogonadrotropic hypogonadism. Elevated estrogen levels lead to a further increase in visceral adipose deposition creating a self-sustained loop [74, 80, 82].
Figure 2.
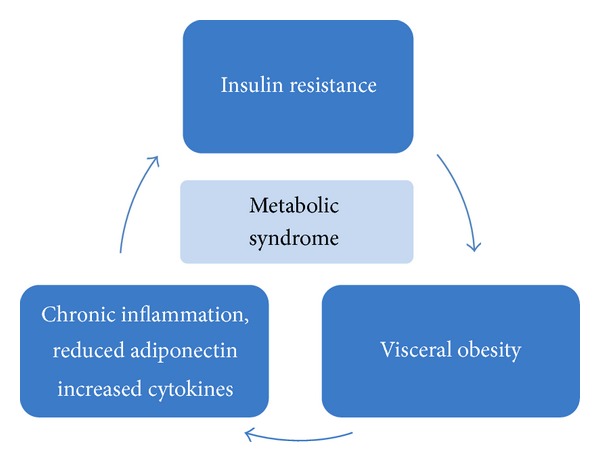
The pathophysiological loop of metabolic syndrome.
MetS has also been associated with a state of chronic, low-grade inflammation. Several studies showed that patients with MetS were more likely than those without to have elevated levels of a marker of inflammation such as C-reactive protein (CRP) as well as proinflammatory cytokines such as TNF-α, IL-8, IL-6, and IL-1β [83, 84].
3.2. Relationship between MetS and PCa
MetS has frequently been associated in human and animal models with carcinogenesis (see Table 1) [85]. It has recently been suggested that evaluating MetS as a single condition may be an inappropriate approach to investigating PCa risk. Specifically, combining all the multiple components of the syndrome into a single variable may confound or obscure the independent effects and interactions of these metabolic components on PCa risk [86]. Each of the primary components of the MetS have been individually observed to be directly associated with PCa risk. DMT2 has been associated with a reduction in PCa risk, probably in relation to the changing action of insulin over the course of diabetes progression [86, 87]. The presence of hypertension may increase PCa risk, in part through increased sympathetic nervous system activity, which can result in androgen-mediated stimulation of PCa cell growth [86]. Men with lower plasma cholesterol were less likely to develop high-grade PCa than men with higher concentrations; this effect might be mediated by several pathways including androgen metabolism and intracellular cholesterol-mediated signaling [88, 89]. Most recent large studies suggest that obesity is associated with a decreased risk of low-grade disease, but an increased risk of high-grade and advanced PCa [74].
Table 1.
Relevant clinical studies of the relationship between MetS and prostate cancer.
| Authors, year | Study design | Country | Population | Time | Age years (range or mean ± SD) | Cohort size | Exposure assessment: MetS criteria | Number of cases | Results (outcome: PCa) | Level of evidence |
|---|---|---|---|---|---|---|---|---|---|---|
| Laukkanen et al., 2004 [91] | Longitudinal cohort study | Finland | Kuopio communities | 1984–2001 | 42–62 | 1880 (White) | WHO | 56 | Risk increase (RR: 1.94, 95% CI: 1.06–3.53) | 2b |
| Håheim et al., 2006 [92] | Longitudinal cohort study | Norway | Oslo study | 1972–1998 | 40–49 | 15 933 (White) | Upper quartile levels ATP III criteria | 507 | Risk increase (RR: 1.56; 95 %CI: 1.21–2) | 2b |
| Martin et al., 2009 [93] | Longitudinal cohort study | Norway | Nord-Trondelag Health study (HUNT 2) | 1996–2005 | 48 ± 16.4 | 29 364 (White) | NCEP: ATP III | 687 | No association (HR: 0.91, 95% CI, 0.877–1.09) | 2b |
| Beebe-Dimmer et al., 2009 [94] | Case-control study | USA | Gene Environment and Prostate Cancer study (GECAP) | 2001–2004 | 62 ± 10.4 | 881 (56% White; 44% African-American) | NCEP: ATP III | 637 | Risk increase in African-American population (OR: 1.71, 95% CI; 0.97–3.01) | 3 |
| Tande et al., 2006 [95] | Longitudinal cohort study | USA | Atherosclerosis Risk in Communities (ARIC); | 1987–2000 | 45–64 | 6429 (49% White; 61% African-American) | NCEP: ATP III | 385 | Risk reduction (RR: 0.77; 95% CI, 0.51–1.05) | 2b |
| Kheterpal et al., 2012 [96] | Longitudinal cohort study | USA | Robotic radical prostatectomy | 2005–2008 | 45–65 | 2756 | BMI ≥30 and ≥2 of the following: hypertension, diabetes or elevated blood glucose, and dyslipidemia | 357 | Greater pathology Gleason grade (≥7: 78% versus 64%, P < 0.001) and pathologic stage (≥T3 disease: 43% versus 32%, P < 0.001) | 3 |
| C. De Nunzio et al., 2011 [78] | Cohort study | Italy | Prostate biopsy cohort study | 2009-2010 | 47–83 | 195 (White) | NCEP: ATP III | 102 | No association (OR: 0.97, 95% CI: 0.48–1.95); Increased risk for Gleason score ≥7 in pts with PCA (OR: 3.82, 95% CI: 1.33–10.9) | 3 |
Moreover, obesity was associated with an increased risk of intraoperative and perioperative complications and with a worse functional outcome, in men treated with RP [90]. In particular, obese men are at threefold greater risk of intraoperative complications and blood transfusions than not-obese men (adjusted odds ratio (OR) = 3.116, P < 0.001, and OR = 2.763, P < 0.050, resp.). Furthermore, the risk of needing at least two pads per day is two and a half times greater in men with a waist circumference of at least 102 cm than in those with a WC below 102 cm (adjusted OR = 2.435, P = 0.007).
In conclusion, further basic and clinical studies are needed to evaluate this association by investigating all these metabolic conditions as a whole and to better evaluate the role the MetS and its mediators with the development and progression of PCa.
4. Measurement of Quality of Life and Sexual Health in Men with Prostate Cancer
Any treatment of PCa can affect urinary and sexual activity, psychosocial function, and overall wellbeing. Several validated questionnaires have been used to asses QoL after RP. An effective evaluation should consider at least 3 categories of QoL: (1) the organ specific function (urinary and sexual); (2) the physical status and the mental health in the patients with any type of cancer; (3) the general health status. The following validated questionnaires are the most accurate to assess the overall health and quality of life for men with PCa.
4.1. UCLA-PCI
The University of California-Los Angeles Prostate Cancer Index (UCLA-PCI) is very accurate to evaluate all aspects related to QoL before and after any treatment for PCA. This questionnaire investigates urinary, bowel and sexual function (UF, BF, and SF), and bowel and sexual bother (UB, BB, and SB) and has been designed either for urologists or radiotherapists [97, 98]. The majority of questions were assigned a score from 0 to 100 (0 = worse health; 100 = better health).
4.2. European Organisation for Research and Treatment of Cancer (Cancer Generic): EORTC QLQ-C-30
This validated questionnaire, designed to evaluate the QoL in men affected by or treated for any cancer, is a 30-item questionnaire composed of multi-item scales and single items that reflect the multidimensionality of the quality-of-life construct [99]. It incorporates five functional scales (physical, role, cognitive, emotional, and social), three symptom scales (fatigue, pain, and nausea/vomiting), and a global health and QoL scale.
4.3. Short Form-12 (SF-12)
The SF-12 is the short version of the SF-36 questionnaire [100]. Through 12 questions, it allows investigating, instead of the 8 original scales, only two indices: the Physical Component Summary (PCS) and the Mental Component Summary (MCS). The strengths of this form are the brevity and relative ease of use from both patients and physicians. For every question there are from 3 to 5 options.
4.4. International Index of Erectile Function (IIEF)
The IIEF questionnaire is a validated multidimensional self-administered questionnaire used to assess the erectile function and the response to treatment in clinical trials. A score of 0–5 is awarded to each question that evaluates 4 domains of sexual health: sexual desire, erectile function, orgasmic function, and intercourse satisfaction [101]. Recently, a short form (IIEF-5), based on 5 questions instead of the original 15 questions, has been used.
5. Impact of Primary Treatment for Pca on QoL and Sexual Health
Currently, more than half of all PCa are clinically localized at the diagnosis, with a 5-year biochemical disease-free survival above 85% [102]. Treatment options for clinically localized PCa include watchful waiting, radical prostatectomy, radical external beam radiation, and hormone treatment. Nevertheless, while active surveillance may have a minimal impact on either QoL or sexual health, more invasive therapies can lead to clinically significant and lasting side effects [103].
In particular, erectile dysfunction and urinary incontinence after RP, bowel, urinary, and sexual complications after radiotherapy or sexual and continence and mood bothersome after hormone treatment can have a negative impact on all the aspects of QoL, including vitality, social and physical, and emotional limitations.
5.1. Primary Treatment in the Elderly Men
The appropriate management of the older men can be a challenge: elderly men are more likely to be diagnosed with higher-grade cancer and the presence of comorbidity and functional decline can impact on treatment tolerance and side effects [104, 105]. Therefore, RT and/or ADT are more commonly used because they are less invasive and do not carry the risk of surgery and anesthesia.
PC is often diagnosed as a result of routine screening in asymptomatic patients, so the development of late treatment sequelae may be particularly alarming [106], also considering the improvement of life expectancy in elderly population (over 17 years at 65 years old) [107].
QoL is a key criterion in the choice of treatment, particularly for early PC or elderly patients, but it is difficult to assess despite the availability of validated questionnaires [108–112]. Late QoL impact is well documented in the literature, but most of the longest QoL studies did not include pretreatment evaluation [113, 114] or collected it retrospectively [115–117].
Generally, recent cohort prospective studies of patients show a different pattern of late sequelae: an increased prevalence of urinary incontinence after prostatectomy, of urinary irritative obstructive symptoms after brachytherapy, of bowel side effects after EBRT, whereas sexual dysfunction as common late event after all these treatment including ADT [110, 111, 118–120].
In particular, erectile dysfunction (ED) is a critical point related to QoL in men treated for PCa and it is strongly associated with depression and significant distress [119, 121, 122]. ED in these patients is the result of several factors: anatomic changes after surgery or radiotherapy, not only hormonal therapy but also psychological and social factors and finally specific comorbidities that often occur in elderly men (metabolic syndrome, diabetes, obesity, osteoporosis, reduced muscle mass, and strength) [123–126].
5.2. Radical Prostatectomy
The major concerns for patients undergoing RP are postprostatectomy incontinence (PPI) and ED. Both QoL and sexual health after RP are strongly dependent on patient age, aging, tumor characteristics, and disease progression [112]. While slight urinary of sexual dysfunction can lead to important bother in younger men, remarkable symptoms can generate minimal bother in the elderly. QoL and sexual health can progressively change owing to anatomical modifications, treatment for PCa, or the natural aging [127]. Therefore, age at time of treatment and perspectives in both sexual and general health have the same clinical relevance of tumor features [128]. Finally, tumor progression or recurrence after RP can generate anxiety and fear that can further worsen QoL and sexual health [129]. In a retrospective, cross-sectional study, enrolling 595 men with PCa treated with radical treatment as primary therapy, we demonstrated that pretreatment tumor characteristics (clinical stage, bioptical Gleason score, and total PSA), treatment timing (age at time of treatment, follow-up duration after treatment, and age at time of follow-up), and posttreatment outcomes (biochemical recurrence and hormonal status) had a remarkable impact on QoL.
One of the leading determinants of QoL and sexual health after RP is the surgical approach: a more conservative procedure, sparing neurovascular bundles, bladder neck, and proximal urethra can strongly increase the chance of better functional outcomes [130, 131]. The decision for the surgical approach is usually a compromise between patient's desire to preserve sexual activity and the eligibility to a conservative surgery based on tumor characteristics (PSA, Clinical stage, bioptical Gleason Score). In a prospective survey on 2,408 PCa patients treated with RP, we demonstrated that at least 737 men (30.6%) were interested in preservation of sexual activity, but not eligible for a nerve-sparing procedure, based on their high-risk PCa features [132]. For 372 (50.5%) of these patients a nerve sparing approach (monolateral or bilateral) was chosen: in these highly selected cases, surgeons' strategy was performed in accordance with patients' desire, without compromising surgical margin status.
The complete recovery of urinary continence after RP is mandatory to preserve general health and to maximize the outcomes of sexual rehabilitation: after catheter removal, most patients reported some level of urinary incontinence [132]. In a multicenter prospective study, we enrolled 1972 men with full continence preoperatively and complete postoperative data: 1 month after RP, 644 (32.7%) were fully continent, 810 (41.1%) were using 0-1 pad/day, and 518 (26.3%) >1 pad/day. Age and nerve sparing were not significant predictors of continence recovery after RP, while preoperative erectile function allowed predicting PPI: the integrity of pelvic vasculature and nerves prior to RP was determinant to avoid of early PPI.
ED and urinary continence can improve even beyond more than 1 year postoperatively, with an average time to sexual and urinary recovery of >6 months [133]. Moreover, Phosphodiesterase type 5 inhibitors (PDE5-I), either in nightly or on-demand dosing, are the gold standard to recover sexual function after nerve-sparing prostatectomy [134]. In a multicenter RCT we have randomized men treated with nerve sparing prostatectomy for localized PCa into 3 groups: (1) PDE5-Is on demand; (2) PDE5-Is once a day; (3) placebo [135]. PDE5-IS improved continence recovery compared with placebo (Improvement of Urinary Function at 3, 6, and 9 months after PDE5-Is once a day versus placebo: P = 0.042, P = 0.044, and P = 0.039, resp.): the positive effect of PDE5-I on continence recovery, even in the absence of the prostatic gland, suggested a direct activity of PD5-Is on lower urinary tract by a pathway not including prostate. Therefore, the long-term use of PDE5-Is after RP can strongly influence the general QoL, the urinary function, and the sexual health.
During the natural aging process in disease-free survivors after RP, both urinary and sexual symptoms and bother can be strongly modified, due to hormonal modification and both vascular and nerve impairment. Moreover, after long-term disease-free follow-up, several men reconsider their QoL status [136]. In two tertiary referral center for PCa we recruited 367 men treated with RP (for clinically localized PCa), without biochemical failure (PSA ≤ 0.2 ng/mL) at the follow-up ≥5 years, with the aim to evaluate long-term general QoL and sexual health in elderly PCa survivors [70]. Older men presented worse urinary continence regardless of age at time of surgery or follow-up duration. Moreover, after more than 8 years after nerve sparing RP without hormone treatment, patients reported substantial sexual dysfunction, but, interestingly, they were minimally sexually bothered (see Figure 3). Our data confirmed that slight urinary incontinence is poorly tolerated even after several years of complete cancer control, while sexual dysfunction is better tolerated, in the daily life of long-term disease-free survivors, perhaps because patients consider ED as a part of their natural aging.
Figure 3.
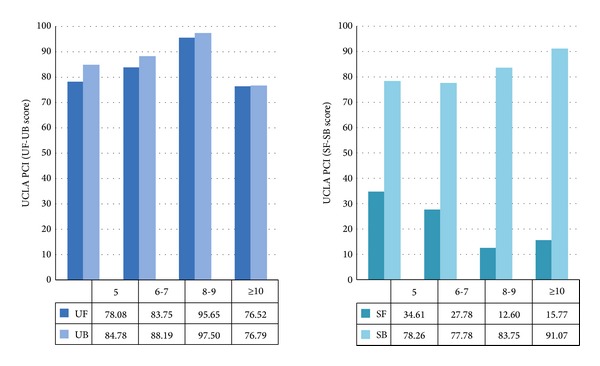
Comparison of function and bother in long-term disease-free survivors after nerve sparing RP without hormone treatment: UF: urinary function; UB: urinary bother; SF: sexual function; SB: sexual bother; RP: radical prostatectomy (adapted from [70]).
5.3. Radiotherapy
Current indications for external-beam radiotherapy (EBRT) in PC include primary treatment (in localized intracapsular tumors or combined with ADT in locally advanced and high-risk PC), adjuvant treatment in patient with adverse pathologic features, (extracapsular extension, positive surgical margins), and salvage radiotherapy (after radical prostatectomy) [137].
However, over recent years, radiotherapy (RT) has seen major advances such as the introduction of intensity-modulated radiation therapy (IMRT) and image-guided RT (IGRT) [138, 139]. The higher radiation doses that can be delivered to the prostate by these new techniques, whilst sparing surrounding organs, have improved progression-free survival and reduced acute and late toxicities [140, 141]. Several studies investigated this aspect of QoL [119] with a short (1–3 years) or intermediate (4-5 years) follow-up, while longer-term outcomes remain largely unknown. Regarding age of patients, some studies have found equal rates of both acute and late side effects in all age groups [142, 143] while others have found older age to be associated with faster onset and more frequent side effects [71]. A prospective trial evaluating patients more than one year following EBRT treatments with final dose of 70–72 Gy found that older age and diabetes were predictive of both preexisting ED and post-EBRT acquired ED [144].
Sanda et al. concluded a substantial decline from baseline of sexual function at 2 years after surgery, but only a moderate decline after EBRT or brachytherapy. Recovery of sexual function was worse in patients treated with androgen suppression combined with radiotherapy, in older patients, obese patients, and patients with a larger prostate size and a high pretreatment PSA. The patient's QoL concerning sexual function was also significantly related to satisfaction in the partner [111].
Pardo et al., in a Spanish study, had similar results after a follow-up of 3 years in patients treated by surgery or EBRT or brachytherapy [145], as well as Rice et al. in a USA study: the authors concluded that EBRT had no significant impact on sexual function at 12 months and may be offered to older patients with minimal QoL impact [146]. As reported in the studies of Potosky et al. [115], the Prostate Cancer Outcomes Study (PCOS), and the one of Miller et al. [113], the patients treated by surgery had an improvement in their sexual function at 2 years after diagnosis, whereas the patients treated by EBRT had slight declines: a possible explanation of this result is the older age of the group of patients treated by radiotherapy. Instead in the Litwin's et al. report long-term sexual function scores were better among surgical patients, but return to baseline was more rapid in patients treated by EBRT [147].
In their report of a long-term follow-up, Resnick et al. [148] described that, although patients undergoing RP were more likely to have ED at 2–5 years, at 15 years the prevalence of ED is very common, affecting 87% of men treated by surgery and 93.9% of men treated by EBRT: it is matter of debate if this decline is due to late sequelae of oncologic treatments, to normal aging process, or to a combination of these factors. Van der Wielen et al. studied the correlation between ED and dose to penile bulb in patients treated with doses of 68–78 Gy: but there was no relation found [149]. Mangar et al. demonstrated that a dose received by 90% of the penile bulb (D90) >50 Gy was significantly associated with ED (P = 0.006) [150], results comparable to the outcomes of the study of Wernicke et al. [151].
The Radiation Therapy Oncology Group 9406 trial examined 158 men with a regular erectile function at baseline and found a greater risk of impotence with a penile mean dose >52.5 Gy (P = 0.039) [152]. So these data are suggestive for a correlation between dose to penile bulb and ED, but more prospective studies are indispensable for predicting preservation of sexual function.
Regarding alternative fractionations, the low α/β ratio of PC causes the high sensitivity of these cells to higher doses for fraction than other tumors [153, 154], even if it is well known that hypofractionated treatments can result in increased rate of late toxicity. In a Canadian prospective study, at 39 months of follow-up, moderate and severe distress related to urinary and bowel symptoms was minimal (3% and 5% of patients, resp.), and the rate of sexual dysfunction was in line with the studies with conventional fractionations [155].
Finally, the few studies available concerning QoL after dose-escalated radiotherapy (thanks to advances in radiotherapy techniques) suggest an increased radiation dose does not result in decline of QoL [156–159].
5.4. Androgen Deprivation Therapy (ADT)
Approximately 50% of men with PCa receive ADT at some time after diagnosis, and most will take it for at least 2 to 3 years [160, 161]. Currently, luteinizing hormone-releasing hormone (LHRH) agonists are the most frequently used agents for ADT. However, other agents including high-dose estrogen, high-dose ketoconazole, abiraterone, and LHRH antagonists can also be used to achieve a castrate level of T. Single-agent antiandrogen therapy is also used as a form of ADT, but it is more likely to reach lower serum T levels [162].
ADT presents several symptoms of “castration syndrome” as side effects, based on low serum T concentration. The symptoms include loss of libido and sexual interest, erectile dysfunction, general fatigue, decreased intellectual ability, depression, loss of muscle strength, increased abdominal fat mass, and loss of vigor [163]. Several cross-sectional studies have described the effect of ADT on self-reported physical function; these studies consistently found that ADT treated men reported decreased physical function in comparison to nontreated [164, 165].
Regarding the side effects of hormonal therapy, in a Canadian retrospective study of Joly et al. [166, 167] patients treated with ADT for at least 3 months for localized PCa both as adjuvant therapy and as biochemical relapse were enrolled. Tests were administered to assess the: patients had significantly poorer scores than controls, especially for urinary disorders and sexuality (P < 0.01). The urinary and sexual symptoms may be primarily due to the prior local treatment. ADT contributed to deterioration in sexual functions, but this study was not designed to address this question; of the patients, 90% reported sexual problem, in agreement with results of other studies [168]. However elderly patients often report that urinary symptoms have a greater impact than sexual functions on global QoL [166, 169].
In an Australian longitudinal study the authors investigated the change to QoL and T level in men starting an intermittent maximal androgen blockade program. Two hundred and fifty men were recruited in this multicentre study: T suppression leads to a significant reduction in global QoL and deterioration in most function as sexual function. Complete loss of libido increased from 37.2% before treatment to 72.2% after hormonal deprivation. Complete sexual inactivity increased from 54.3% to 86.9%. Following treatment cessation, T recovery was gradual and median time to eugonadal levels of the hormone was 9.3 months with an improvement in emotional function, sexual function, fatigue, sleep, and hot flushes [170].
In the American study of Lubeck, QoL of 1178 newly diagnosed patients was examined (mean age at diagnosis was 73 years) which were enrolled in the Cancer of the Prostate Strategic Urologic Research Endeavor Database. General and disease specific QoL outcomes were measured with tests at study entry and quarterly thereafter. Patients were randomized in 3 groups: ADT, surveillance, radical prostatectomy, or EBRT. Men receiving ADT reported poorer urinary and sexual function and a higher rate of urinary and sexual symptoms than patients selecting surveillance. ADT and surveillance QoL scores remained low in the year after treatment, whereas men treated by RP showed improvement in these scales [167].
In the Australian phase 3 trial of Denham, all patients were given six months of leuprorelin, and radiotherapy to the prostate and seminal vesicles after 5 months from randomisation. After leuprorelin, patients were given either no further treatment or an additional 12 months of leuprorelin. In addition to androgen suppression, men who were randomly allocated to the two bisphosphonate groups were given zoledronic acid for 18 months. In this study, 18 months of androgen suppression worsened the adverse changes in the “patients-reported-outcome” score caused by 6 months of androgen suppression and radiotherapy. However, these increases were restricted to only sexual activity, hormone treatment related symptoms, fatigue, and financial problems at 18 months after randomization. The increases were also restricted in time [171].
Mature survival data from men with previously untreated, locally-advanced disease reveal that bicalutamide monotherapy provides survival benefits that do not differ significantly from castration, while offering important advantages with respect to the maintenance of physical capacity and sexual interest [172]. Also the study of Stav confirms that sexual interest appears to be better preserved with bicalutamide than with castration [173].
In the two largest phase III studies comparing bicalutamide 150 mg/die monotherapy with castration (orchiectomy or the LH-RH agonist goserelin acetate) in 1453 patients, the combined analysis at 12 months showed that bicalutamide was associated with a significant advantage for sexual interest compared with castration (P = 0.029), although a decrease was recorded in both groups [174].
6. Conclusions
In conclusion, the present review underlines the double role of androgens and the androgen receptor in the development and proliferation of PCa as well as in maintaining a correct functional state of the prostate of elderly men. Evidence in the literature suggests that maintaining a correct function of the androgen receptor may limit PCa progression by keeping a more differentiate state of the cells. Although more RCT are needed to better define the risk/benefit of androgen therapy in elderly men previously cured for PCa, current evidence indicates that treatment with androgens of hypogonadal men with previous PCa may be safe and may ameliorate both sexual health and QoL.
There are several lines of evidence regarding the emerging role of MetS and its components in PCa development and progression. Moreover, MetS can be associated with a state of chronic, low-grade inflammation, in particular in elderly men. We have summarized the evidence about the involvement of MetS in the pathogenesis of PCa, particularly of high-grade disease and we suggested that MetS should be assessed as a new domain in basic and clinical research in elderly men with PCa. In particular, all the components of MetS should be adequately assessed either before or after any treatment of PCa.
It is mandatory to use validated questionnaire to provide adequate details to patients not only regarding urinary and bowel symptoms, but also regarding their sexual function in order to avoid anxiety in patients and their families, to provide adequate medical and psychological counseling, and to analyze the progressive modifications during the follow-up of PCa survivors.
The adverse effects of surgery, radiotherapy, or androgen deprivation may be more pronounced in the elderly population, especially those with lower functional status and increased comorbidities, in particular regarding their sexual health. Therefore it is important to consider the specific benefits and risks for each treatment modality as they apply to the elderly because of the greater risk in both short- and long-term postoperative complications and mortality following any radical treatment.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Sakr WA, Haas GP, Cassin BF, Pontes JE, Crissman JD. The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. Journal of Urology. 1993;150(2):379–385. doi: 10.1016/s0022-5347(17)35487-3. [DOI] [PubMed] [Google Scholar]
- 2.Guzzo TJ, Kutikov A, Canter DJ, et al. The clinical and pathological history of prostate cancer progression in men with a prior history of high grade prostatic intraepithelial neoplasia. The Canadian Journal of Urology. 2008;15(4):4174–4179. [PubMed] [Google Scholar]
- 3.Alva A, Hussain M. The changing natural history of metastatic prostate cancer. Cancer Journal. 2013;19(1):19–24. doi: 10.1097/PPO.0b013e318281197e. [DOI] [PubMed] [Google Scholar]
- 4.Boyle P, Maisonneuve P, Napalkov P. Incidence of prostate cancer will double by the year 2030: the argument for. European Urology. 1996;29(2):3–9. doi: 10.1159/000473828. [DOI] [PubMed] [Google Scholar]
- 5.Grossmann M, Cheung AS, Zajac JD. Androgens and prostate cancer, pathogenesis and deprivation therapy. Best Practice and Research. Clinical Endocrinology and Metabolism. 2013;27(4):603–616. doi: 10.1016/j.beem.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Hellstrom WJG, Paduch D, Donatucci CF. Importance of hypogonadism and testosterone replacement therapy in current urologic practice: a review. International Urology and Nephrology. 2012;44(1):61–70. doi: 10.1007/s11255-010-9879-4. [DOI] [PubMed] [Google Scholar]
- 7.Hwang TIS, Lo HC, Tsai TF, Chiou HY. Association among hypogonadism, quality of life and erectile dysfunction in middle-aged and aged male in Taiwan. International Journal of Impotence Research. 2007;19(1):69–75. doi: 10.1038/sj.ijir.3901480. [DOI] [PubMed] [Google Scholar]
- 8.Xiang YZ, Xiong H, Cui ZL, et al. The association between metabolic syndrome and the risk of prostate cancer, high-grade prostate cancer, advanced prostate cancer, prostate cancer-specific mortality and biochemical recurrence. Journal of Experimental and Clinical Cancer Research. 2013;32, article 9 doi: 10.1186/1756-9966-32-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boorjian SA, Eastham JA, Graefen M, et al. A critical analysis of the long-term impact of radical prostatectomy on cancer control and function outcomes. European Urology. 2012;61(4):664–675. doi: 10.1016/j.eururo.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 10.Kyrdalen AE, Dahl AA, Hernes E, Småstuen MC, Fosså SD. A national study of adverse effects and global quality of life among candidates for curative treatment for prostate cancer. BJU International. 2013;111(2):221–232. doi: 10.1111/j.1464-410X.2012.11198.x. [DOI] [PubMed] [Google Scholar]
- 11.Bergman J, Saigal CS, Kwan L, Litwin MS. Responsiveness of the University of California-Los Angeles Prostate Cancer Index. Urology. 2010;75(6):1418–1423. doi: 10.1016/j.urology.2009.04.070. [DOI] [PubMed] [Google Scholar]
- 12.Heinzer H, Steuber T. Prostate cancer in the elderly. Urologic Oncology. 2009;27(6):668–672. doi: 10.1016/j.urolonc.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Shafi AA, Yen AE, Weigel NL. Androgen receptors in hormone-dependent and castration-resistant prostate cancer. Pharmacology and Therapeutics. 2013;140(3):223–238. doi: 10.1016/j.pharmthera.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Behre HM, Bohmeyer J, Nieschlag E. Prostate volume in testosterone-treated and untreated hypogonadal men in comparison to age-matched normal controls. Clinical Endocrinology. 1994;40(3):341–349. doi: 10.1111/j.1365-2265.1994.tb03929.x. [DOI] [PubMed] [Google Scholar]
- 15.Huggins C, Hodges CV. Studies on prostatic cancer. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Research. 1941;1:293–297. [Google Scholar]
- 16.Hessels D, Schalken JA. Recurrent gene in prostate cancer: their clinical implications and uses. Current Urology Reports. 2013;14:214–222. doi: 10.1007/s11934-013-0321-1. [DOI] [PubMed] [Google Scholar]
- 17.Carver BS, Tran J, Chen Z, et al. ETS rearrangements and prostate cancer initiation. Nature. 2009;457(7231):p. E1. doi: 10.1038/nature07738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zong Y, Xin L, Goldstein AS, Lawson DA, Teitell MA, Witte ON. ETS family transcription factors collaborate with alternative signaling pathways to induce carcinoma from adult murine prostate cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(30):12465–12470. doi: 10.1073/pnas.0905931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pettersson A, Graff RE, Bauer SR, et al. The TMPRSS2:ERG rearrangement, ERG expression, and prostate cancer outcomes: a cohort study and meta-analysis. Cancer Epidemiology, Biomarkers and Prevention. 2012;21(9):1497–1509. doi: 10.1158/1055-9965.EPI-12-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McPhaul MJ. Mechanisms of prostate cancer progression to androgen independence. Best Practice and Research. Clinical Endocrinology and Metabolism. 2008;22(2):373–388. doi: 10.1016/j.beem.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Jagla M, Fève M, Kessler P, et al. A splicing variant of the androgen receptor detected in a metastatic prostate cancer exhibits exclusively cytoplasmic actions. Endocrinology. 2007;148(9):4334–4343. doi: 10.1210/en.2007-0446. [DOI] [PubMed] [Google Scholar]
- 22.Choucair K, Ejdelman J, Brimo F, et al. PTEN genomic deletion predicts prostate cancer recurrence and is associated with low AR expression and transcriptional activity. BMC Cancer. 2012;12:543–552. doi: 10.1186/1471-2407-12-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sagnak L, Topaloglu H, Ozok U, Ersoy H. Prognostic significance of neuroendocrine differentiation in prostate adenocarcinoma. Clinical Genitourinary Cancer. 2011;9(2):73–80. doi: 10.1016/j.clgc.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Tamburrino L, Salvianti F, Marchiani S, et al. Androgen receptor (AR) expression in prostate cancer and progression of the tumor: lessons from cell lines, animal models and human specimens. Steroids. 2012;77(10):996–1001. doi: 10.1016/j.steroids.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Minner S, Enodien M, Sirma H, et al. ERG status is unrelated to PSA recurrence in radically operated prostate cancer in the absence of antihormonal therapy. Clinical Cancer Research. 2011;17(18):5878–5888. doi: 10.1158/1078-0432.CCR-11-1251. [DOI] [PubMed] [Google Scholar]
- 26.Niu Y, Altuwaijri S, Yeh S, et al. Targeting the stromal androgen receptor in primary prostate tumors at earlier stages. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(34):12188–12193. doi: 10.1073/pnas.0804701105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niu Y, Altuwaijri S, Lai KP, et al. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(34):12182–12187. doi: 10.1073/pnas.0804700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mimeault M, Batra SK. Development of animal models underlining mechanistic connections between prostate inflammation and cancer. World Journal of Clinical Oncology. 2013;4(1):4–13. doi: 10.5306/wjco.v4.i1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonaccorsi L, Carloni V, Muratori M, et al. Androgen receptor expression in prostate carcinoma cells suppresses α6β4 integrin-mediated invasive phenotype. Endocrinology. 2000;141(9):3172–3182. doi: 10.1210/endo.141.9.7640. [DOI] [PubMed] [Google Scholar]
- 30.Bonaccorsi L, Carloni V, Muratori M, et al. EGF receptor (EGFR) signaling promoting invasion is disrupted in androgen-sensitive prostate cancer cells by an interaction between EGFR and androgen receptor (AR) International Journal of Cancer. 2004;112(1):78–86. doi: 10.1002/ijc.20362. [DOI] [PubMed] [Google Scholar]
- 31.Bonaccorsi L, Nosi D, Muratori M, Formigli L, Forti G, Baldi E. Altered endocytosis of epidermal growth factor receptor in androgen receptor positve prostate cancer cell lines. Journal of Molecular Endocrinology. 2007;38(1-2):51–66. doi: 10.1677/jme.1.02155. [DOI] [PubMed] [Google Scholar]
- 32.Cinar B, Koeneman KS, Edlund M, Prins GS, Zhau HE, Chung LWK. Androgen receptor mediates the reduced tumor growth, enhanced androgen responsiveness, and selected target gene transactivation in a human prostate cancer cell line. Cancer Research. 2001;61(19):7310–7317. [PubMed] [Google Scholar]
- 33.Davis R, Jia D, Cinar B, et al. Functional androgen receptor confers sensitization of androgen-independent prostate cancer cells to anticancer therapy via caspase activation. Biochemical and Biophysical Research Communications. 2003;309(4):937–945. doi: 10.1016/j.bbrc.2003.08.096. [DOI] [PubMed] [Google Scholar]
- 34.Guerini V, Sau D, Scaccianoce E, et al. The androgen derivative 5α-androstane-3β,17β-diol inhibits prostate cancer cell migration through activation of the estrogen receptor β subtype. Cancer Research. 2005;65(12):5445–5453. doi: 10.1158/0008-5472.CAN-04-1941. [DOI] [PubMed] [Google Scholar]
- 35.Niu Y, Chang TM, Yeh S, Ma WL, Wang YZ, Chang C. Differential androgen receptor signals in different cells explain why androgen-deprivation therapy of prostate cancer fails. Oncogene. 2010;29(25):3593–3604. doi: 10.1038/onc.2010.121. [DOI] [PubMed] [Google Scholar]
- 36.Zhu M-L, Kyprianou N. Role of androgens and the androgen receptor in epithelial-mesenchymal transition and invasion of prostate cancer cells. The FASEB Journal. 2010;24(3):769–777. doi: 10.1096/fj.09-136994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchiani S, Tamburrino L, Nesi G, et al. Androgen-responsive and -unresponsive prostate cancer cell lines respond differently to stimuli inducing neuroendocrine differentiation. International Journal of Andrology. 2010;33(6):784–793. doi: 10.1111/j.1365-2605.2009.01030.x. [DOI] [PubMed] [Google Scholar]
- 38.Tian J, Lee SO, Liang L, et al. Targeting the unique methylation pattern of androgen receptor (AR) promoter in prostate stem/progenitor cells with 5-aza-2′-deoxycytidine (5-AZA) leads to suppressed prostate tumorigenesis. Journal of Biological Chemistry. 2012;287(47):39954–39966. doi: 10.1074/jbc.M112.395574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleeberger W, Bova GS, Nielsen ME, et al. Roles for the stem cell-associated intermediate filament nestin in prostate cancer migration and metastasis. Cancer Research. 2007;67(19):9199–9206. doi: 10.1158/0008-5472.CAN-07-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boccon-Gibod L, Hammerer P, Madersbacher S, Mottet N, Prayer-Galetti T, Tunn U. The role of intermittent androgen deprivation in prostate cancer. BJU International. 2007;100(4):738–743. doi: 10.1111/j.1464-410X.2007.07053.x. [DOI] [PubMed] [Google Scholar]
- 41.Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. The New England Journal of Medicine. 2004;351(15):1488–1490. doi: 10.1056/NEJMp048178. [DOI] [PubMed] [Google Scholar]
- 42.Thompson IM, Jr., Goodman PJ, Tangen CM, et al. Long-term survival of participants in the prostate cancer prevention trial. The New England Journal of Medicine. 2013;369(7):603–610. doi: 10.1056/NEJMoa1215932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathew P. Prolonged control of progressive castration-resistant metastatic prostate cancer with testosterone replacement therapy: the case for a prospective trial. Annals of Oncology. 2008;19(2):395–396. doi: 10.1093/annonc/mdm568. [DOI] [PubMed] [Google Scholar]
- 44.Morris MJ, Huang D, Kelly WK, et al. Phase 1 trial of high-dose exogenous testosterone in patients with castration-resistant metastatic prostate cancer. European Urology. 2009;56(2):237–244. doi: 10.1016/j.eururo.2009.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chuu CP, Kokontis JM, Hiipakka RA, et al. Androgens as therapy for androgen receptor-positive castration-resistant prostate cancer. Journal of Biomedical Science. 2011;18(1, article 63) doi: 10.1186/1423-0127-18-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baldi E, Bonaccorsi L, Forti G. Androgen receptor: good guy or bad guy in prostate cancer invasion? Endocrinology. 2003;144(5):1653–1655. doi: 10.1210/en.2003-0234. [DOI] [PubMed] [Google Scholar]
- 47.Wen S, Niu Y, Lee SO, Chang C. Androgen receptor (AR) positive vs negative roles in prostate cancer cell deaths including apoptosis, anoikis, entosis, necrosis and autophagic cell death. Cancer Treatment Reviews. 2014;40(1):31–40. doi: 10.1016/j.ctrv.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hörnberg E, Ylitalo EB, Crnalic S, et al. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS ONE. 2011;6(4) doi: 10.1371/journal.pone.0019059.e19059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersen RJ, Mawji NR, Wang J, et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17(6):535–546. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 50.Sadar MD. Advances in small molecule inhibitors of androgen receptor for the treatment of advanced prostate cancer. World Journal of Urology. 2012;30(3):311–318. doi: 10.1007/s00345-011-0745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Myung JK, Banuelos CA, Fernandez JG, et al. An androgen receptor N-terminal domain antagonist for treating prostate cancer. Journal of Clinical Investigation. 2013;123(7):2948–2960. doi: 10.1172/JCI66398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. Journal of the National Cancer Institute. 2008;100(3):170–183. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Severi G, Morris HA, MacInnis RJ, et al. Circulating steroid hormones and the risk of prostate cancer. Cancer Epidemiology Biomarkers and Prevention. 2006;15(1):86–91. doi: 10.1158/1055-9965.EPI-05-0633. [DOI] [PubMed] [Google Scholar]
- 54.Sofikerim M, Eskicorapci S, Ozruç Ö, Özen H. Hormonal predictors of prostate cancer. Urologia Internationalis. 2007;79(1):13–18. doi: 10.1159/000102906. [DOI] [PubMed] [Google Scholar]
- 55.Corona G, Baldi E, Maggi M. Androgen regulation of prostate cancer: where are we now? Journal of Endocrinological Investigation. 2011;34(3):232–243. doi: 10.1007/BF03347072. [DOI] [PubMed] [Google Scholar]
- 56.Corona G, Gacci M, Baldi E, Mancina R, Forti G, Maggi M. Androgen deprivation therapy in prostate cancer: focusing on sexual side effects. Journal of Sexual Medicine. 2012;9(3):887–902. doi: 10.1111/j.1743-6109.2011.02590.x. [DOI] [PubMed] [Google Scholar]
- 57.Buvat J, Maggi M, Guay A, Torres LO. Testosterone deficiency in men: systematic review and standard operating procedures for diagnosis and treatment. Journal of Sexual Medicine. 2013;10:245–284. doi: 10.1111/j.1743-6109.2012.02783.x. [DOI] [PubMed] [Google Scholar]
- 58.Nishiyama T, Hashimoto Y, Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clinical Cancer Research. 2004;10(21):7121–7126. doi: 10.1158/1078-0432.CCR-04-0913. [DOI] [PubMed] [Google Scholar]
- 59.Page ST, Lin DW, Mostaghel EA, et al. Persistent intraprostatic androgen concentrations after medical castration in healthy men. Journal of Clinical Endocrinology and Metabolism. 2006;91(10):3850–3856. doi: 10.1210/jc.2006-0968. [DOI] [PubMed] [Google Scholar]
- 60.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clinical Cancer Research. 2005;11(13):4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 61.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Research. 2008;68(11):4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hofland J, van Weerden WM, Dits NFJ, et al. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Research. 2010;70(3):1256–1264. doi: 10.1158/0008-5472.CAN-09-2092. [DOI] [PubMed] [Google Scholar]
- 63.Chang KH, Li R, Papari-Zareei M, et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(33):13728–13733. doi: 10.1073/pnas.1107898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. The Lancet Oncology. 2012;13(10):983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 65.Miller LR, Partin AW, Chan DW, et al. Influence of radical prostatectomy on serum hormone levels. Journal of Urology. 1998;160(2):449–453. [PubMed] [Google Scholar]
- 66.Madersbacher S, Schatzl G, Bieglmayer C, et al. Impact of radical prostatectomy and TURP on the hypothalamic-pituitary-gonadal hormone axis. Urology. 2002;60(5):869–874. doi: 10.1016/s0090-4295(02)01893-9. [DOI] [PubMed] [Google Scholar]
- 67.Lackner JE, Maerk I, Koller A, et al. Serum inhibin not a cause of low testosterone levels in hypogonadal prostate cancer? Urology. 2008;72(5):1121–1124. doi: 10.1016/j.urology.2008.01.066. [DOI] [PubMed] [Google Scholar]
- 68.Olsson M, Ekström L, Schulze J, et al. Radical prostatectomy: influence on serum and urinary androgen levels. Prostate. 2010;70(2):200–205. doi: 10.1002/pros.21053. [DOI] [PubMed] [Google Scholar]
- 69.Miller LR, Partin AW, Chan DW, et al. Influence of radical prostatectomy on serum hormone levels. Journal of Urology. 1998;160(2):449–453. [PubMed] [Google Scholar]
- 70.Gacci M, Simonato A, Masieri L, et al. Urinary and sexual outcomes in long-term (5+ years) prostate cancer disease free survivors after radical prostatectomy. Health and Quality of Life Outcomes. 2009;7, article 94 doi: 10.1186/1477-7525-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gacci M, Corona G, Apolone A, et al. Influence of serum testosterone on urinary continence and sexual activity in patients undergoing radical prostatectomy for clinically localized prostate cancer. Prostate Cancer and Prostatic Diseases. 2010;13(2):168–172. doi: 10.1038/pcan.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kirby MG, Wagg A, Cardozo L, et al. Overactive bladder: is there a link to the metabolic syndrome in men? Neurourology and Urodynamics. 2010;29(8):1360–1364. doi: 10.1002/nau.20892. [DOI] [PubMed] [Google Scholar]
- 73.Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Medicine. 2011;9, article 48 doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Nunzio C, Aronson W, Freedland SJ, Giovannucci E, Parsons JK. The correlation between metabolic syndrome and prostatic diseases. European Urology. 2012;61(3):560–570. doi: 10.1016/j.eururo.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 75.Gorbachinsky I, Akpinar H, Assimos DG. Metabolic syndrome and urologic diseases. Reviews in Urology. 2010;12(4):e157–e180. [PMC free article] [PubMed] [Google Scholar]
- 76.Hsing AW, Sakoda LC, Chua S., Jr. Obesity, metabolic syndrome, and prostate cancer. The American Jurnal of Clinical Nutrition. 2007;86(3):s843–857. doi: 10.1093/ajcn/86.3.843S. [DOI] [PubMed] [Google Scholar]
- 77.Buschemeyer WC, III, Freedland SJ. Obesity and prostate cancer: epidemiology and clinical implications. European Urology. 2007;52(2):331–343. doi: 10.1016/j.eururo.2007.04.069. [DOI] [PubMed] [Google Scholar]
- 78.De Nunzio C, Kramer G, Marberger M, et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. European Urology. 2011;60(1):106–117. doi: 10.1016/j.eururo.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 79.Gupta A, Gupta V. Metabolic syndrome: what are the risks for humans? Bioscience Tends. 2010;4(5):204–212. [PubMed] [Google Scholar]
- 80.Duvnjak L, Duvnjak M. The metabolic syndrome - an ongoing story. Journal of Physiology and Pharmacology. 2009;60:19–24. [PubMed] [Google Scholar]
- 81.Ngo TH, Barnard RJ, Leung PS, Cohen P, Aronson WJ. Insulin-like growth factor I (IGF-I) and IGF binding protein-1 modulate prostate cancer cell growth and apoptosis: possible mediators for the effects of diet and exercise on cancer cell survival. Endocrinology. 2003;144(6):2319–2324. doi: 10.1210/en.2003-221028. [DOI] [PubMed] [Google Scholar]
- 82.Huang PL. A comprehensive definition for metabolic syndrome. Disease Models and Mechanisms. 2009;2(5-6):231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Penna G, Mondaini N, Amuchastegui S, et al. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. European Urology. 2007;51(2):524–533. doi: 10.1016/j.eururo.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 84.Devaraj S, Singh U, Jialal I. Human C-reactive protein and the metabolic syndrome. Current Opinion in Lipidology. 2009;20(3):182–189. doi: 10.1097/MOL.0b013e32832ac03e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jaggers JR, Sui X, Hooker SP, et al. Metabolic syndrome and risk of cancer mortality in men. European Journal of Cancer. 2009;45(10):1831–1838. doi: 10.1016/j.ejca.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wallner LP, Morgenstern H, McGree ME, et al. The effects of metabolic conditions on prostate cancer incidence over 15 years of follow-up: results from the Olmsted County Study. BJU International. 2011;107(6):929–935. doi: 10.1111/j.1464-410X.2010.09703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Diabetes mellitus and risk of prostate cancer. Cancer Causes and Control. 1998;9(1):3–9. doi: 10.1023/a:1008822917449. [DOI] [PubMed] [Google Scholar]
- 88.Hamilton RJ, Freedland SJ. Review of recent evidence in support of a role for statins in the prevention of prostate cancer. Current Opinion in Urology. 2008;18(3):333–339. doi: 10.1097/MOU.0b013e3282f9b3cc. [DOI] [PubMed] [Google Scholar]
- 89.Mondul AM, Clipp SL, Helzlsouer KJ, Platz EA. Association between plasma total cholesterol concentration and incident prostate cancer in the CLUE II cohort. Cancer Causes and Control. 2010;21(1):61–68. doi: 10.1007/s10552-009-9434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gacci M, Sebastianelli A, Salvi M, et al. Role of abdominal obesity for functional outcomes and complications in men treated with radical prostatectomy for prostate cancer: results of the Multicenter Italian Report on Radical Prostatectomy (MIRROR) study. Scandinavian Journal of Urology. 2013 doi: 10.3109/21681805.2013.803151. [DOI] [PubMed] [Google Scholar]
- 91.Laukkanen C, Laukkanen AJ, Laaksonen DE, et al. Metabolic syndrome and the risk of prostate cancer in finnish men: a population-based study. Cancer Epidemiology Biomarkers and Prevention. 2004;13(10):1646–1650. [PubMed] [Google Scholar]
- 92.Håheim LL, Wisløff TF, Holme I, Nafstad P. Metabolic syndrome predicts prostate cancer in a cohort of middle-aged Norwegian men followed for 27 years. The American Journal of Epidemiology. 2006;164(8):769–774. doi: 10.1093/aje/kwj284. [DOI] [PubMed] [Google Scholar]
- 93.Martin RM, Vatten L, Gunnell D, Romundstad P, Nilsen TIL. Components of the metabolic syndrome and risk of prostate cancer: the HUNT 2 cohort, Norway. Cancer Causes and Control. 2009;20(7):1181–1192. doi: 10.1007/s10552-009-9319-x. [DOI] [PubMed] [Google Scholar]
- 94.Beebe-Dimmer JL, Nock NL, Neslund-Dudas C, et al. Racial differences in risk of prostate cancer associated with metabolic syndrome. Urology. 2009;74(1):185–190. doi: 10.1016/j.urology.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tande AJ, Platz EA, Folsom AR. The metabolic syndrome is associated with reduced risk of prostate cancer. The American Journal of Epidemiology. 2006;164(11):1094–1102. doi: 10.1093/aje/kwj320. [DOI] [PubMed] [Google Scholar]
- 96.Kheterpal E, Sammon JD, Diaz M, et al. Effect of metabolic syndrome on pathologic features of prostate cancer. Urologic Oncology. 31(7):1054–1059. doi: 10.1016/j.urolonc.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 97.Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, Brook RH. The UCLA prostate cancer index: development, reliability, and validity of a health-related quality of life measure. Medical Care. 1998;36(7):1002–1012. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 98.Gacci M, Livi L, Paiar F, et al. Quality of life after radical treatment of prostate cancer: validation of the Italian version of the University of California-Los Angeles Prostate Cancer Index. Urology. 2005;66(2):338–343. doi: 10.1016/j.urology.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 99.Hjermstad MJ, Fossa SD, Bjordal K, Kaasa S. Test/retest study of the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire. Journal of Clinical Oncology. 1995;13(5):1249–1254. doi: 10.1200/JCO.1995.13.5.1249. [DOI] [PubMed] [Google Scholar]
- 100.Ware JE, Jr., Kosinski M, Keller SD. A 12-Item short-form health survey: construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 101.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 102.Mitchell RE, Shah JB, Olsson CA, Benson MC, McKiernan JM. Does year of radical prostatectomy independently predict outcome in prostate cancer? Urology. 2006;67(2):368–372. doi: 10.1016/j.urology.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 103.Daubenmier JJ, Weidner G, Marlin R, et al. Lifestyle and health-related quality of life of men with prostate cancer managed with active surveillance. Urology. 2006;67(1):125–130. doi: 10.1016/j.urology.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 104.Hoffman KE. Management of older men with clinically localized prostate cancer: the significance of advanced age and comorbidity. Seminars in Radiation Oncology. 2012;22(4):284–294. doi: 10.1016/j.semradonc.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 105.Bian SX, Hoffman KE. Management of prostate cancer in elderly men. Seminars in Radiation Oncology. 2013;23(3):198–205. doi: 10.1016/j.semradonc.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 106.Cameron S, Springer C, Fox-Wasylyshyn S, El-Masri MM. A descriptive study of functions, symptoms, and perceived health state after radiotherapy for prostate cancer. European Journal of Oncology Nursing. 2012;16(3):310–314. doi: 10.1016/j.ejon.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 107.National Center for Health Statistics. Health, United States, 2010: with Special Feature on Death and Dying. Hyattsville, Md, USA: National Center for Health Statistics; 2011. [PubMed] [Google Scholar]
- 108.Goineau A, Marchand V, Rigaud J, et al. Prospective evaluation of quality of life 54 months after high-dose intensity-modulated radiotherapy for localized prostate cancer. Radiation Oncology. 2013;8, article 53 doi: 10.1186/1748-717X-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Niezgoda HE, Pater JL. A validation study of the domains of the core EORTC quality of life questionnaire. Quality of Life Research. 1993;2(5):319–325. doi: 10.1007/BF00449426. [DOI] [PubMed] [Google Scholar]
- 110.Ferrer M, Suárez JF, Guedea F, et al. Health-related quality of life 2 years after treatment with radical prostatectomy, prostate brachytherapy, or external beam radiotherapy in patients with clinically localized prostate cancer. International Journal of Radiation Oncology Biology Physics. 2008;72(2):421–432. doi: 10.1016/j.ijrobp.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 111.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. The New England Journal of Medicine. 2008;358(12):1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 112.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the Expanded Prostate Cancer Index Composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56(6):899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 113.Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. Journal of Clinical Oncology. 2005;23(12):2772–2780. doi: 10.1200/JCO.2005.07.116. [DOI] [PubMed] [Google Scholar]
- 114.Frank SJ, Pisters LL, Davis J, Lee AK, Bassett R, Kuban DA. An assessment of quality of life following radical prostatectomy, high dose external beam radiation therapy and brachytherapy iodine implantation as monotherapies for localized prostate cancer. Journal of Urology. 2007;177(6):2151–2156. doi: 10.1016/j.juro.2007.01.134. [DOI] [PubMed] [Google Scholar]
- 115.Potosky AL, Davis WW, Hoffman RM, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. Journal of the National Cancer Institute. 2004;96(18):1358–1367. doi: 10.1093/jnci/djh259. [DOI] [PubMed] [Google Scholar]
- 116.Hoffman RM, Gilliland FD, Penson DF, Stone SN, Hunt WC, Potesky AL. Cross-sectional and longitudinal comparisons of health-related quality of life between patients with prostate carcinoma and matched controls. Cancer. 2004;101(9):2011–2019. doi: 10.1002/cncr.20608. [DOI] [PubMed] [Google Scholar]
- 117.Smith DP, King MT, Egger S, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ. 2009;339:b4817–b4828. doi: 10.1136/bmj.b4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Korfage IJ, Essink-Bot ML, Borsboom GJJM, et al. Five-year follow-up of health-related quality of life after primary treatment of localized prostate cancer. International Journal of Cancer. 2005;116(2):291–296. doi: 10.1002/ijc.21043. [DOI] [PubMed] [Google Scholar]
- 119.Hoppe BS. Erectile function, incontinence, and other quality of life outcomes following proton therapy for prostate cancer in men 60 years old and younger. Cancer. 2012;118(18):4619–4626. doi: 10.1002/cncr.27398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bian SX. Management of prostate cancer in elderly men. Seminars in Radiation Oncology. 2013;23(3):198–205. doi: 10.1016/j.semradonc.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 121.Nelson CJ, Mulhall JP, Roth AJ. The association between erectile dysfunction and depressive symptoms in men treated for prostate cancer. Journal of Sexual Medicine. 2011;8(2):560–566. doi: 10.1111/j.1743-6109.2010.02127.x. [DOI] [PubMed] [Google Scholar]
- 122.Helgason ÁR, Adolfsson J, Dickman P, Fredrikson M, Arver S, Steineck G. Waning sexual function - the most important disease-specific distress for patients with prostate cancer. British Journal of Cancer. 1996;73(11):1417–1421. doi: 10.1038/bjc.1996.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lunenfeld B, Arver S, Moncada I, Rees DA, Schulte HM. How to help the aging male? Current approaches to hypogonadism in primary care. The Aging Male. 2012;15(4):187–197. doi: 10.3109/13685538.2012.729110. [DOI] [PubMed] [Google Scholar]
- 124.Incrocci L, Jensen PT. Pelvic radiotherapy and sexual function in men and women. The Journal of Sexual Medicine. 2013;10(supplement 1):53–64. doi: 10.1111/jsm.12010. [DOI] [PubMed] [Google Scholar]
- 125.Alemozaffar M, Regan MM, Cooperberg MR, et al. Prediction of erectile function following treatment for prostate cancer. Journal of the American Medical Association. 2011;306(11):1205–1214. doi: 10.1001/jama.2011.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Karakiewicz PI, Bhojani N, Neugut A, et al. The effect of comorbidity and socioeconomic status on sexual and urinary function and on general health-related quality of life in men treated with radical prostatectomy for localized prostate cancer. Journal of Sexual Medicine. 2008;5(4):919–927. doi: 10.1111/j.1743-6109.2007.00741.x. [DOI] [PubMed] [Google Scholar]
- 127.Lubeck DP, Litwin MS, Henning JM, Stoddard ML, Flanders SC, Carroll PR. Changes in health-related quality of life in the first year after treatment for prostate cancer: results from caPSURE. Urology. 1999;53(1):180–186. doi: 10.1016/s0090-4295(98)00408-7. [DOI] [PubMed] [Google Scholar]
- 128.Rabbani F, Stapleton AMF, Kattan MW, Wheeler TM, Scardino PT. Factors predicting recovery of erections after radical prostatectomy. Journal of Urology. 2000;164(6):1929–1934. [PubMed] [Google Scholar]
- 129.Knight SJ, Latini DM. Sexual side effects and prostate cancer treatment decisions. Patient information needs and preferences. Cancer Journal. 2009;15(1):41–44. doi: 10.1097/PPO.0b013e31819764cc. [DOI] [PubMed] [Google Scholar]
- 130.Carter S, Le JD, Hu JC. Anatomic and technical considerations for optimizing recovery of sexual function during robotic-assisted radical prostatectomy. Current Opinion in Urology. 2013;23(1):88–94. doi: 10.1097/MOU.0b013e32835b6602. [DOI] [PubMed] [Google Scholar]
- 131.Vora AA, Dajani D, Lynch JH, Kowalczyk KJ. Anatomic and technical considerations for optimizing recovery of urinary function during robotic-assisted radical prostatectomy. Current Opinion in Urology. 2013;23(1):78–87. doi: 10.1097/MOU.0b013e32835b0ae5. [DOI] [PubMed] [Google Scholar]
- 132.Bianco FJ, Jr., Scardino PT, Eastham JA. Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function (“trifecta”) Urology. 2005;66(5):83–94. doi: 10.1016/j.urology.2005.06.116. [DOI] [PubMed] [Google Scholar]
- 133.Litwin MS, Melmed GY, Nakazon T. Life after radical prostatectomy: a longitudinal study. Journal of Urology. 2001;166(2):587–592. [PubMed] [Google Scholar]
- 134.Briganti A, Salonia A, Gallina A, et al. Management of erectile dysfunction after radical prostatectomy in 2007. World Journal of Urology. 2007;25(2):143–148. doi: 10.1007/s00345-007-0148-9. [DOI] [PubMed] [Google Scholar]
- 135.Gacci M, Ierardi A, Rose AD, et al. Vardenafil can improve continence recovery after bilateral nerve sparing prostatectomy: results of a randomized, double blind, placebo-controlled pilot study. Journal of Sexual Medicine. 2010;7(1):234–243. doi: 10.1111/j.1743-6109.2009.01471.x. [DOI] [PubMed] [Google Scholar]
- 136.Sacco E, Prayer-Galetti T, Pinto F, et al. Urinary incontinence after radical prostatectomy: incidence by definition, risk factors and temporal trend in a large series with a long-term follow-up. BJU International. 2006;97(6):1234–1241. doi: 10.1111/j.1464-410X.2006.06185.x. [DOI] [PubMed] [Google Scholar]
- 137.Lumen N, Ost P, van Praet C, et al. Developments in external beam radiotherapy for prostate cancer. Urology. 2013;82(1):5–10. doi: 10.1016/j.urology.2013.03.043. [DOI] [PubMed] [Google Scholar]
- 138.Lisbona A, Averbeck D, Supiot S, et al. IMRT combined to IGRT: increase of the irradiated volume. Consequences? Cancer Radiothérapie. 2010;14(6-7):563–570. doi: 10.1016/j.canrad.2010.07.227. [DOI] [PubMed] [Google Scholar]
- 139.Crehange G, Mirjolet C, Gauthier M, et al. Clinical impact of margin reduction on late toxicity and short-term biochemical control for patients treated with daily on-line image guided IMRT for prostate cancer. Radiotherapy and Oncology. 2012;103(2):244–246. doi: 10.1016/j.radonc.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 140.Pollack A, Zagars GK, Smith LG, et al. Preliminary results of a randomized radiotherapy dose-escalation study comparing 70 Gy with 78 Gy for prostate cancer. Journal of Clinical Oncology. 2000;18(23):3904–3911. doi: 10.1200/JCO.2000.18.23.3904. [DOI] [PubMed] [Google Scholar]
- 141.Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. International Journal of Radiation Oncology Biology Physics. 2008;70(4):1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 142.Jani AB, Parikh SD, Vijayakumar S, Gratzle J. Analysis of influence of age on acute and chronic radiotherapy toxicity in treatment of prostate cancer. Urology. 2005;65(6):1157–1162. doi: 10.1016/j.urology.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 143.Geinitz H, Zimmermann FB, Thamm R, Schumertl A, Busch R, Molls M. 3D conformal radiation therapy for prostate cancer in elderly patients. Radiotherapy and Oncology. 2005;76(1):27–34. doi: 10.1016/j.radonc.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 144.Pinkawa M, Gagel B, Piroth MD, et al. Erectile dysfunction after external beam radiotherapy for prostate cancer. European Urology. 2009;55(1):227–236. doi: 10.1016/j.eururo.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 145.Pardo Y, Guedea F, Aguiló F, et al. Quality-of-life impact of primary treatments for localized prostate cancer in patients without hormonal treatment. Journal of Clinical Oncology. 2010;28(31):4687–4696. doi: 10.1200/JCO.2009.25.3245. [DOI] [PubMed] [Google Scholar]
- 146.Rice K, Hudak J, Peay K, et al. Comprehensive quality-of-life outcomes in the setting of a multidisciplinary, equal access prostate cancer clinic. Urology. 2010;76(5):1231–1238. doi: 10.1016/j.urology.2010.03.087. [DOI] [PubMed] [Google Scholar]
- 147.Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109(11):2239–2247. doi: 10.1002/cncr.22676. [DOI] [PubMed] [Google Scholar]
- 148.Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. The New England Journal of Medicine. 2013;368(5):436–445. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.van der Wielen GJ, Hoogeman MS, Dohle GR, van Putten WLJ, Incrocci L. Dose-volume parameters of the corpora cavernosa do not correlate with erectile dysfunction after external beam radiotherapy for prostate cancer: results from a dose-escalation trial. International Journal of Radiation Oncology Biology Physics. 2008;71(3):795–800. doi: 10.1016/j.ijrobp.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 150.Mangar SA, Sydes MR, Tucker HL, et al. Evaluating the relationship between erectile dysfunction and dose received by the penile bulb: using data from a randomised controlled trial of conformal radiotherapy in prostate cancer (MRC RT01, ISRCTN47772397) Radiotherapy and Oncology. 2006;80(3):355–362. doi: 10.1016/j.radonc.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 151.Wernicke AG, Valicenti R, DiEva K, Houser C, Pequignot E. Radiation dose delivered to the proximal penis as a predictor of the risk of erectile dysfunction after three-dimensional conformal radiotherapy for localized prostate cancer. International Journal of Radiation Oncology Biology Physics. 2004;60(5):1357–1363. doi: 10.1016/j.ijrobp.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 152.Roach M, Winter K, Michalski JM, et al. Penile bulb dose and impotence after three-dimensional conformal radiotherapy for prostate cancer on RTOG 9406: findings from a prospective, multi-institutional, phase I/II dose-escalation study. International Journal of Radiation Oncology Biology Physics. 2004;60(5):1351–1356. doi: 10.1016/j.ijrobp.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 153.Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. International Journal of Radiation Oncology, Biology, Physics. 2009;43(5):1095–1101. doi: 10.1016/s0360-3016(98)00438-6. [DOI] [PubMed] [Google Scholar]
- 154.Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5, 969 patients in seven international institutional datasets: α/β = 1.4 (0.9–2.2) . International Journal of Radiation Oncology Biology Physics. 2012;82(1):e17–e24. doi: 10.1016/j.ijrobp.2010.10.075. [DOI] [PubMed] [Google Scholar]
- 155.Quon H, Cheung CF, Loblaw DA. Quality of life after hypofractionated concomitant intensity-modulated radiotherapy boost for high-risk prostate cancer. International Journal of Radiation Oncology Biology Physics. 2012;83(2):617–623. doi: 10.1016/j.ijrobp.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 156.Namiki S, Ishidoya S, Tochigi T, et al. Health-related quality of life after intensity modulated radiation therapy for localized prostate cancer: comparison with conventional and conformal radiotherapy. Japanese Journal of Clinical Oncology. 2006;36(4):224–230. doi: 10.1093/jjco/hyl002. [DOI] [PubMed] [Google Scholar]
- 157.Little DJ, Kuban DA, Levy LB, Zagars GK, Pollack A. Quality-of-life questionnaire results 2 and 3 years after radiotherapy for prostate cancer in a randomized dose-escalation study. Urology. 2003;62(4):707–713. doi: 10.1016/s0090-4295(03)00504-1. [DOI] [PubMed] [Google Scholar]
- 158.Kupelian PA, Reddy CA, Klein EA, Willoughby TR. Short-course intensity-modulated radiotherapy (70 GY at 2.5 GY per fraction) for localized prostate cancer: preliminary results on late toxicity and quality of life. International Journal of Radiation Oncology Biology Physics. 2001;51(4):988–993. doi: 10.1016/s0360-3016(01)01730-8. [DOI] [PubMed] [Google Scholar]
- 159.Dieperink KB, Hansen S, Wagner L, et al. Living alone, obesity and smoking: important factors for quality of life after radiotherapy and androgen deprivation therapy for prostate cancer. Acta Oncologica. 2012;51(6):722–729. doi: 10.3109/0284186X.2012.682627. [DOI] [PubMed] [Google Scholar]
- 160.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. The Lancet. 2002;360(9327):103–108. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 161.Cooperberg MR, Grossfeld GD, Lubeck DP, Carroll PR. National practice patterns and time trends in androgen ablation for localized prostate cancer. Journal of the National Cancer Institute. 2003;95(13):981–989. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wibowo E, Wassersug RJ. The effect of estrogen on the sexual interest of castrated males: implications to prostate cancer patients on androgen-deprivation therapy. Critical Reviews in Oncology/Hematology. 2013;87(3):224–238. doi: 10.1016/j.critrevonc.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 163.Tsujimura A. Role of androgen in the elderly. Problems of androgen deprivation therapy. Clinical Calcium. 2013;23(8):1185–1190. [PubMed] [Google Scholar]
- 164.Fossa SD, Woehre H, Kurth K-H, et al. Influence of urological morbidity on quality of life in patients with prostate cancer. European Urology. 1997;31(3):3–8. doi: 10.1159/000474553. [DOI] [PubMed] [Google Scholar]
- 165.Dacal K, Sereika SM, Greenspan SL. Quality of life in prostate cancer patients taking androgen deprivation therapy. Journal of the American Geriatrics Society. 2006;54(1):85–90. doi: 10.1111/j.1532-5415.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 166.Joly F, Alibhai SMH, Galica J, et al. Impact of androgen deprivation therapy on physical and cognitive function, as well as quality of life of patients with nonmetastatic prostate cancer. Journal of Urology. 2006;176(6):2443–2447. doi: 10.1016/j.juro.2006.07.151. [DOI] [PubMed] [Google Scholar]
- 167.Lubeck DP, Grossfeld GD, Carroll PR. The effect of androgen deprivation therapy on health-related quality of life in men with prostate cancer. Urology. 2001;58(2):94–99. doi: 10.1016/s0090-4295(01)01250-x. [DOI] [PubMed] [Google Scholar]
- 168.Holzbeierlein JM, McLaughlin MD, Thrasher JB. Complications of androgen deprivation therapy for prostate cancer. Current Opinion in Urology. 2004;14(3):177–183. doi: 10.1097/00042307-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 169.Descazeaud A, Chaskalovic J, Debré B, Zerbib M, Peyromaure M. Sexual function after radical prostatectomy does not affect global patient satisfaction. Progres en Urologie. 2004;14(6):1177–1180. [PubMed] [Google Scholar]
- 170.Spry NA, Kristjanson L, Hooton B, et al. Adverse effects to quality of life arising from treatment can recover with intermittent androgen suppression in men with prostate cancer. European Journal of Cancer. 2006;42(8):1083–1092. doi: 10.1016/j.ejca.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 171.Denham JW, Wilcox C, Joseph D, et al. Quality of life in men with locally advanced prostate cancer treated with leuprorelin and radiotherapy with or without zoledronic acid (TROG 03.04 RADAR): secondary endpoints from a randomised phase 3 factorial trial. The Lancet Oncology. 2012;13(12):1260–1270. doi: 10.1016/S1470-2045(12)70423-0. [DOI] [PubMed] [Google Scholar]
- 172.Schellhammer PF. An evaluation of bicalutamide in the treatment of prostate cancer. Expert Opinion on Pharmacotherapy. 2002;3(9):1313–1328. doi: 10.1517/14656566.3.9.1313. [DOI] [PubMed] [Google Scholar]
- 173.Stav SY, Segal G. The role of bicalutamide in the treatment of prostate cancer. Harefuah. 2002;141(4):379–407. [PubMed] [Google Scholar]
- 174.Iversen P, Tyrrell CJ, Kaisary AV, et al. Casodex (bicalutamide) 150-mg monotherapy compared with castration in patients with previously untreated nonmetastatic prostate cancer: results from two multicenter randomized trials at a median follow-up of 4 years. Urology. 1998;51(3):389–396. doi: 10.1016/s0090-4295(98)00004-1. [DOI] [PubMed] [Google Scholar]


