Abstract
Previous studies have established that incubation of low density lipoprotein (LDL) with cultured endothelial cells (EC) converts it to a new form (EC-modified LDL) that is now recognized by a specific receptor on macrophages (the acetyl LDL receptor) and is taken up and degraded 3-10 times more rapidly than native LDL (biological modification). The formation of EC-modified LDL depended on generation of free radicals with consequent peroxidation of LDL lipids and was accompanied by extensive hydrolysis of LDL phosphatidylcholine at the 2-position. The present studies show that p-bromophenacyl bromide, a site-specific irreversible inhibitor of phospholipase A2 activity, blocks this hydrolysis and, at the same time, the enhanced macrophage degradation. We show further that during EC modification the apoprotein B of LDL undergoes considerable modification and that this also is prevented by the phospholipase inhibitor. Finally, as reported previously, changes similar to those observed on incubation of LDL with EC can be induced by incubation in the absence of cells but in the presence of a sufficiently high concentration of Cu2+. This also is accompanied by hydrolysis of phosphatidylcholine at the 2-position and breakdown of apoprotein B. These changes are also inhibited by p-bromophenacyl bromide, suggesting the presence of a phospholipase A2 activity associated with LDL as it is isolated. A hypothesis is presented linking lipid peroxidation, phosphatidylcholine hydrolysis, and changes in the LDL apoprotein during EC modification.
Full text
PDF
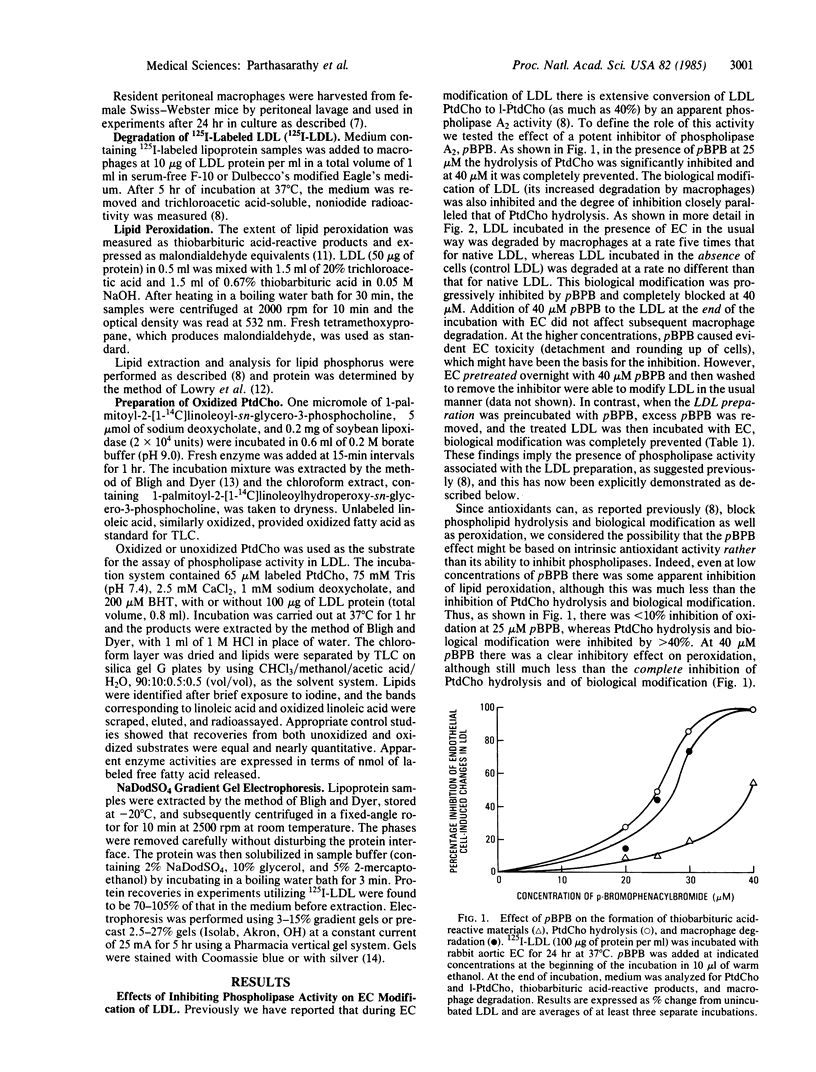
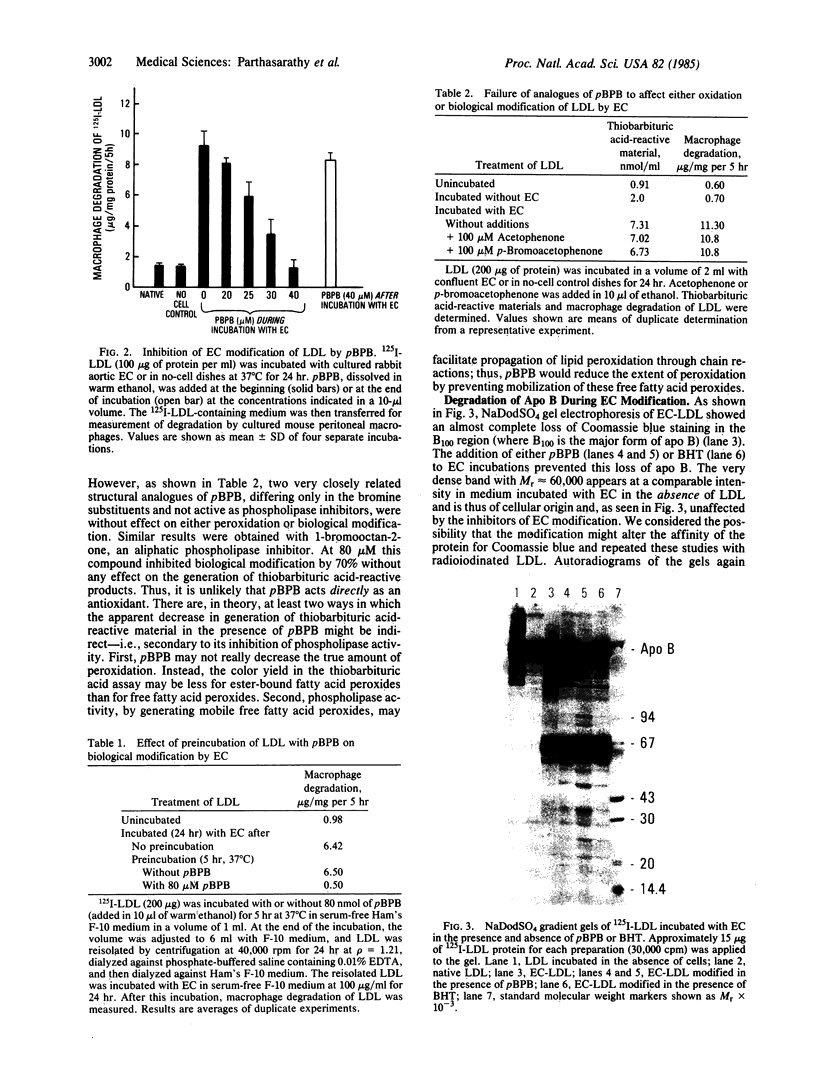
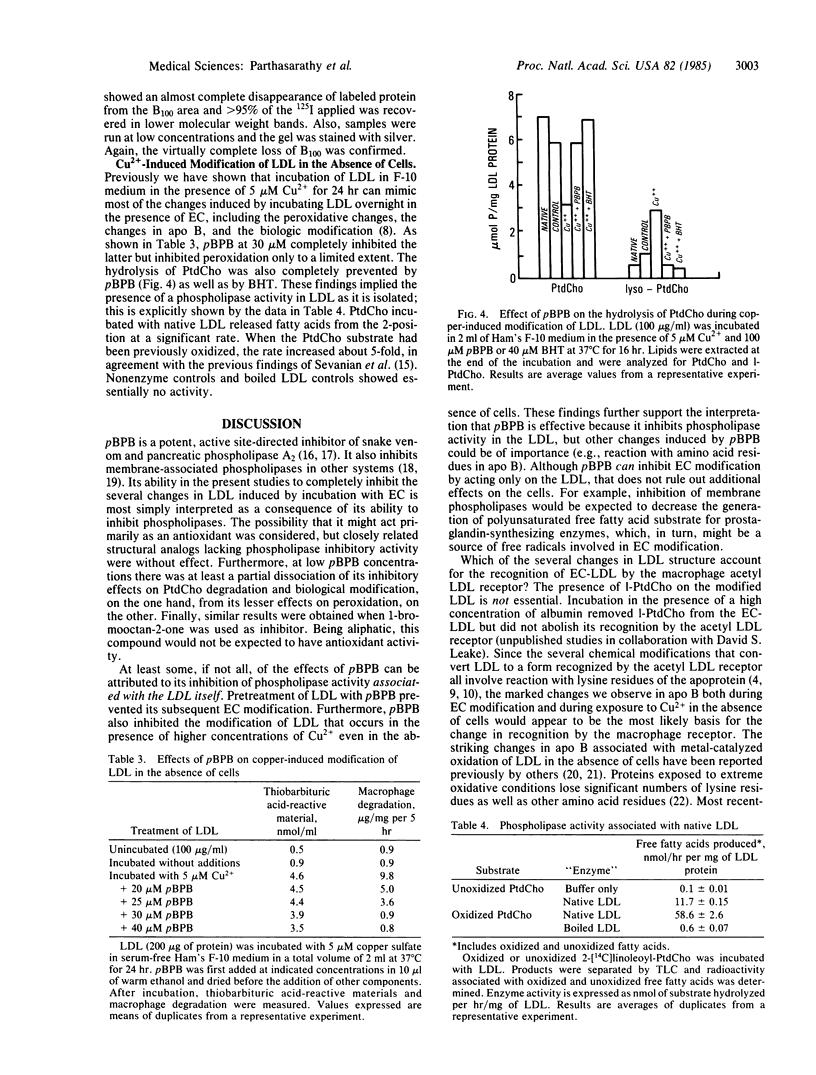

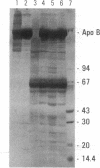
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Chio K. S., Tappel A. L. Inactivation of ribonuclease and other enzymes by peroxidizing lipids and by malonaldehyde. Biochemistry. 1969 Jul;8(7):2827–2832. doi: 10.1021/bi00835a020. [DOI] [PubMed] [Google Scholar]
- De Winter J. M., Vianen G. M., Van den Bosch H. Purification of rat liver mitochondrial phospholipase A2. Biochim Biophys Acta. 1982 Aug 18;712(2):332–341. doi: 10.1016/0005-2760(82)90351-4. [DOI] [PubMed] [Google Scholar]
- Fogelman A. M., Shechter I., Seager J., Hokom M., Child J. S., Edwards P. A. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2214–2218. doi: 10.1073/pnas.77.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinecke J. W., Rosen H., Chait A. Iron and copper promote modification of low density lipoprotein by human arterial smooth muscle cells in culture. J Clin Invest. 1984 Nov;74(5):1890–1894. doi: 10.1172/JCI111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Enhanced macrophage degradation of biologically modified low density lipoprotein. Arteriosclerosis. 1983 Mar-Apr;3(2):149–159. doi: 10.1161/01.atv.3.2.149. [DOI] [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6499–6503. doi: 10.1073/pnas.78.10.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Interactions of plasma lipoproteins with endothelial cells. Ann N Y Acad Sci. 1982;401:102–116. doi: 10.1111/j.1749-6632.1982.tb25711.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee D. M. Malondialdehyde formation in stored plasma. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1663–1672. doi: 10.1016/s0006-291x(80)80090-8. [DOI] [PubMed] [Google Scholar]
- Levine R. L., Oliver C. N., Fulks R. M., Stadtman E. R. Turnover of bacterial glutamine synthetase: oxidative inactivation precedes proteolysis. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2120–2124. doi: 10.1073/pnas.78.4.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Weisgraber K. B., Oh S. Y. Altered metabolism (in vivo and in vitro) of plasma lipoproteins after selective chemical modification of lysine residues of the apoproteins. J Clin Invest. 1979 Sep;64(3):743–750. doi: 10.1172/JCI109518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel D. W., Hessler J. R., Chisolm G. M. Low density lipoprotein cytotoxicity induced by free radical peroxidation of lipid. J Lipid Res. 1983 Aug;24(8):1070–1076. [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Roberts M. F., Deems R. A., Mincey T. C., Dennis E. A. Chemical modification of the histidine residue in phospholipase A2 (Naja naja naja). A case of half-site reactivity. J Biol Chem. 1977 Apr 10;252(7):2405–2411. [PubMed] [Google Scholar]
- Schaffner T., Taylor K., Bartucci E. J., Fischer-Dzoga K., Beeson J. H., Glagov S., Wissler R. W. Arterial foam cells with distinctive immunomorphologic and histochemical features of macrophages. Am J Pathol. 1980 Jul;100(1):57–80. [PMC free article] [PubMed] [Google Scholar]
- Schuh J., Fairclough G. F., Jr, Haschemeyer R. H. Oxygen-mediated heterogeneity of apo-low-density lipoprotein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3173–3177. doi: 10.1073/pnas.75.7.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevanian A., Stein R. A., Mead J. F. Metabolism of epoxidized phosphatidylcholine by phospholipase A2 and epoxide hydrolase. Lipids. 1981 Nov;16(11):781–789. doi: 10.1007/BF02535029. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar J. K., Sperelakis N., Pang D., Franson R. C. Characterization of phospholipase A2 activity in rat aorta smooth muscle cells. Biochim Biophys Acta. 1983 Jan 7;750(1):134–140. doi: 10.1016/0005-2760(83)90212-6. [DOI] [PubMed] [Google Scholar]
- Verheij H. M., Boffa M. C., Rothen C., Bryckaert M. C., Verger R., de Haas G. H. Correlation of enzymatic activity and anticoagulant properties of phospholipase A2. Eur J Biochem. 1980 Nov;112(1):25–32. doi: 10.1111/j.1432-1033.1980.tb04982.x. [DOI] [PubMed] [Google Scholar]
- Volwerk J. J., Pieterson W. A., de Haas G. H. Histidine at the active site of phospholipase A2. Biochemistry. 1974 Mar 26;13(7):1446–1454. doi: 10.1021/bi00704a020. [DOI] [PubMed] [Google Scholar]
- Weglicki W. B., Dickens B. F., Mak I. T. Enhanced lysosomal phospholipid degradation and lysophospholipid production due to free radicals. Biochem Biophys Res Commun. 1984 Oct 15;124(1):229–235. doi: 10.1016/0006-291x(84)90941-0. [DOI] [PubMed] [Google Scholar]
- Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976 Apr;15(2):212–216. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- Yasuda M., Fujita T. Effect of lipid peroxidation on phospholipase A2 activity of rat liver mitochondria. Jpn J Pharmacol. 1977 Jun;27(3):429–435. doi: 10.1254/jjp.27.429. [DOI] [PubMed] [Google Scholar]