Abstract
Oxidative stress (OS) is known to be strongly involved in a large number of fetal, neonatal, and adult diseases, including placental disorders, leading to pregnancy loss and stillbirths. A growing body of research links OS to preeclampsia, gestational diabetes, obesity, spontaneous abortion, recurrent pregnancy, preterm labor, and intrauterine growth restriction. While a considerable number of miRNAs have been related to physiological functions and pathological conditions of the placenta, a direct link among these miRNAs, placental functions, and OS is still lacking. This review summarizes data describing the role of miRNAs in placental pathophysiological processes and their possible impact on OS damaging responses. As miRNAs can be found in circulation, improving our understanding on their role in the pathogenesis of pregnancy related disorders could have an important impact on the diagnosis and prognosis of these diseases.
1. Introduction
Oxidative stress (OS) occurs when the production of free radicals exceeds the capacity of antioxidant defenses. It represents an imbalance between the production of reactive species and the biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage. Each cell is characterized by a particular concentration of electrons stored in many cellular constituents and the redox state of a cell with its oscillation determines cellular functioning [1]. Disturbances in the normal redox state of tissues can cause toxic effects through the production of peroxides and free radicals (FRs) that damage all components of the cell, including proteins, lipids, RNA, and DNA. Some reactive oxygen and nitrogen species (ROS and RNS) can even act as messengers: at low levels, they are signaling molecules and at high levels, they can damage organelles, particularly mitochondria.
Oxidative damage and associated mitochondrial dysfunction may result in energy depletion, accumulation of cytotoxic mediators, and cell death. OS is known to be associated with numerous diseases in humans, such as atherosclerosis, inflammation, cancer, type-2 diabetes, Parkinson's disease, and Alzheimer's disease [2, 3], as well as in various pregnancy related disorders and placenta related alterations [4], such as preeclampsia and intra-uterine growth restriction. However, most mechanisms involved in OS-related pathophysiology in placenta alterations remain unknown. For obvious reasons, pregnancy makes scientific investigation complicated, and most of the knowledge we have on the role of OS during pregnancy is derived from animal studies.
MicroRNAs (miRNAs) are a group of small noncoding RNAs that, after a process of maturation, consist of about 18–25 nucleotides. MiRNAs are known to perform a unique role in posttranscriptional gene regulation. Since their discovery, miRNAs have been known to regulate the expression of a large number of proteins and it is supposed that they could regulate up to 30% (or even more) of the human genome [5]. Indeed, depending on the degree of complementarity, miRNAs can block protein synthesis or even induce mRNA degradation [5]. In addition, members of a microRNA family are clustered and exhibit overlying roles since they share the same sequence for the binding to the mRNA target [6]. At present, the literature describes the importance of miRNAs in the regulation of physiological as well as pathological, cellular, and tissue processes [7, 8]. MiRNAs have been shown to modulate most biological processes, even cellular responses to redox imbalance. In particular, miR-200 family members play a crucial role in OS dependent endothelial dysfunction, as well as in vascular complications of diabetes and obesity [6]. In addition, different miRNAs, such as miR-210, have been shown to play a key role in mitochondrial metabolism, therefore modulating ROS production and sensitivity [9].
MiRNAs have also promising applications in the diagnosis and prognosis of many pathologies, as their quali-quantitative expression varies depending on disease condition [10]. Furthermore, a number of miRNA have already been described as important for the normal placental development and for placenta related alterations [11, 12].
This review will focus on the miRNAs related to placental alterations caused by OS.
2. Placental Alterations and Oxidative Stress
The placenta is a vital organ forming a connection between mother and fetus. It mediates nutrient, oxygen, and hormone exchanges between mother and fetus and operates as an immune protective barrier. Placentation starts with trophoblastic invasion of the maternal spiral arteries and changes in the placental vasculature to ensure optimal maternal vascular perfusion [13]. To ensure a normal fetus growth, equilibrium among trophoblast proliferation, invasion, migration, fusion, and apoptosis is required [14]. However, the regulation and progression of these cellular events remain largely unknown [14]. Several differentiation processes are known to be oxygen regulated via the expression of hormones or growth factors (e.g., the induction of the vascular endothelial growth factor (VEGF) which is triggered via the hypoxia-induced factor) [14]. Furthermore, physiological hypoxia is observable prior to unplugging the maternal spiral arteries by trophoblastic plugs due to the low O2 tension in early pregnancy [14]. Unplugging the maternal spiral arteries is followed by an increase of the O2 tension with increased ROS production and placental OS [15]. The increase of ROS production maintains important physiological roles and it induces the transcription of several genes related to cell differentiation and proliferation (e.g., HIF1A, CREB1, and NFKB1) [16] and mediates cytokine-induced trophoblast apoptosis [14]. ROS generation leads consequently to a protective up-regulation of antioxidant gene expression and activity of heme oxygenases (HO-1 and HO-2), Cu,Zn-superoxide dismutase (Cu,Zn-SOD), catalase, and glutathione peroxidase (GPx) [17]. However, the high metabolic demands and elevated requirements for tissue oxygen may lead to overproduction of ROS and OS even in normal pregnancies. The loss of the balance of ROS production and antioxidant systems may in fact contribute to a number of serious complications and diseases.
Preeclampsia is a multisystem disorder, that affects 5–14% of pregnancies and is a leading cause of maternal and fetal morbidity and mortality worldwide [18–20]. It manifests itself with focal vasospasm and a porous vascular tree that transfers fluid from the intravascular to the extravascular space causing OS [21]. This state seems to originate from insufficient placental perfusion and a limited enzymatic antioxidant capacity through the reduction of placental Cu,Zn-SOD, GPx and glucose 6-phosphate-dehydrogenase activity and reduced vitamin E tissue levels [22].
Mechanisms that induce OS seem to be one of the causes of spontaneous abortion. Actually, intraplacental circulation is formed 2–4 weeks earlier than under normal conditions [23]. Under such circumstances, the antioxidant enzymes seem not to be present in a sufficient concentration to withstand the OS induced by ROS. This theory is also supported by the presence of increased levels of lipid peroxidation markers and reduced levels of GSH and vitamin E [24].
Recurrent pregnancy loss is a condition that occurs with an incidence of around 3%. A number of studies support the role of OS in the pathophysiology of recurrent pregnancy loss through the increased presence of endometrial NK cells that cause precocious angiogenesis and intraplacental circulation, with a resulting increase in OS [25, 26].
Intrauterine growth restriction (IUGR) refers to a birth weight below the 10th percentile. An important cause of IUGR seems to be preeclampsia, through ischemic mechanisms caused in the placenta and following ROS production [27, 28]. Studies also indicate increased markers of lipid peroxidation in pregnancies with IUGR fetuses [29].
Preterm labor is the leading cause of perinatal morbidity and mortality worldwide with an incidence of up to 12%. Its pathogenic mechanisms include changes in the chorioamniotic membranes with following inflammatory responses [30]. Preterm labor is associated with a ROS-induced reduction of antioxidant defenses (e.g., GPx, GSH) leading to OS, tissue injury, and an increased risk of preterm birth [31, 32].
Obesity during pregnancy is known to be associated with hypertension, diabetes, an increased rate of cesarean section, prematurity, stillbirth, and macrosomia [33]. These complications may be related to OS due to the increased production of inflammatory cytokines, which in turn favor ROS and RNS generation [34].
Gestational diabetes is related to insulin resistance and occurs in 0.3–0.5% of pregnant women [35]. It develops during the second half of pregnancy. Patients with gestational diabetes present a higher risk of spontaneous abortions, perinatal death, congenital anomalies, and disturbances of fetal growth. Oxidative stress seems to be related to gestational diabetes and different markers of OS (e.g., malondialdehyde, Cu,Zn-SOD, and GSH) have been described as being altered under this condition [36].
Since OS has an important impact on placental alterations, a better understanding of the regulatory mechanisms triggered by ROS could be relevant to future research on placental alterations and related complications.
3. MicroRNAs Related to Placental Development and Pregnancy
There is increasing evidence of the importance of miRNAs in placental development [37–39]. A large number of miRNAs and miRNA related proteins (e.g., Drosha, Exportin 5, Dicer, Argonaute 2, and DP103) have been identified in human placental tissues [40, 41]. Additionally, a number of in vitro studies [42, 43] and clinical investigations [44, 45] have described the important role of miRNAs in placental development and its alterations. Many miRNAs are known to be specifically expressed in the placenta and three miRNA clusters are known to be specifically associated with placental development.
The chromosome 14 miRNA cluster (C14MC) is the largest described miRNA cluster known, comprising 52 miRNAs. It is located at the human 14q32 chromosome and is expressed from the maternally inherited chromosome. Some miRNAs of the C14MC cluster are predominantly expressed in the placenta. Furthermore, the C14MC seems to play an important role in embryonic development, neurogenesis, and RNA metabolism [46].
The chromosome 19 microRNA cluster (C19MC) is primate specific and comprises 46 miRNAs located at the human 19q13.41 chromosome. C19MC is only expressed from the paternally inherited chromosome and is mainly related to the placenta. Therefore, C19MC may be related to human embryonal development [46].
The miR-371-3 cluster consists of hsa-miR-371a-3p, has-miR-371b-3p, hsa-miR-371-5p, hsa-miR-372, hsa-miR-373-3p, and hsa-miR-373-5p. This cluster is adjacent to the C19MC cluster and is known to be expressed in the placenta. Particularly, the miRNAs of this cluster are highly expressed in human embryonic stem cells (ESCs), while their levels decrease during development. The miR-371-3 cluster seems to regulate cell cycle, proliferation, and apoptosis [46].
Recent studies have revealed that a number of miRNAs act on placental development. Trophoblast proliferation can be promoted bymiR-378a-5p [47], miR-376c [48], and miR-141 [49], while miR-155 [43] and miR-675 [50] have been shown to inhibit trophoblast cell proliferation. MiR-29b has been shown to induce [42] and miR-182 to inhibit apoptosis in trophoblast cells [51]. MiR-195 [52], miR-376c [48], and miR-378a-5p [47] have been reported to enhance and miR-210 [53], miR-34a [54, 55], and miR-29b [42, 54] to inhibit trophoblast migration and invasion. MiR-29b [52] and miR-16 [56] have shown an inhibitory effect on angiogenesis through vascular endothelial growth factor (VEGFA) suppression. Furthermore, a number of miRNAs have been described as being specifically altered in a number of placenta related diseases. Recent studies suggest that miRNAs are involved in the development of preeclampsia [37, 39, 43, 57]. Most notably miR-210 [51, 53], miR-20a [57], miR-20b [57], miR-29b [42], miR-16 [44], miR-155 [58], and miR-675 [50] have shown to be upregulated and are suggested to inhibit angiogenesis, trophoblast cell proliferation, and migration, while miR-378a-5p [47], miR-376c [48], and miR-195 [52] have been shown to be downregulated and to promote trophoblast cell proliferation, survival, and invasion. A study suggests that miR-518b, miR-1323, miR-516b, miR-515-5p, miR-520 h, miR-519d, and miR-526b are downregulated in IUGR [59]. MiR-132, miR-29a, and miR-222 [60] have been described as being underexpressed in gestational diabetes and are suggested as biomarkers of the disease, as their levels are retrievable from circulation. Finally miR-25, miR-338, miR-101, miR-449, miR-154, miR-135a, miR-142-3p, miR-202*, miR-199a*, and miR-136 [61] have been shown to be upregulated in preterm birth. As pointed out, the regulatory mechanism exerted by miRNAs in placental development and alterations is essential but still needs to be more investigated. This includes trophoblast proliferation, differentiation, migration, invasion, and apoptosis as well as angiogenesis and the expression of antioxidant genes. Since different placental alterations seem to show specific miRNA patterns, it is possible to hypothesize that these patterns could be used as biomarkers for these alterations.
4. MicroRNAs Modulated by ROS and Probably Related to Placental Alterations
Some studies suggest that oxygen tension and hypoxia are important regulators of placental miRNA expression [44, 45]. Furthermore, a number of miRNAs previously described as being altered under various placental alterations have been associated with ROS and OS under various experimental conditions. For example, among the miRNAs up-regulated in preeclampsia, miR-210, miR-144*, miR-451, miR-146b-5p, miR-126*, miR-16, miR-29b, miR-26b, miR-335, miR-182, miR-155, and miR-20a [44, 45, 51, 58, 62–64] have been described as being related to OS. MiR-210 was found to be induced by the hypoxia-inducible factor 1-α (HIF-1 α) [65] and to modulate the adaptive mechanisms involved in acute peripheral ischemia, regulating oxidative metabolism, OS [66], mitochondrial metabolism, angiogenesis, DNA repair, and cell survival [67]. MiR-144 and miR-451 have been found dysregulated under ischaemic conditions in mice and to protect erythrocytes against OS [68]. MiR-144 is known to modulate OS tolerance through down-regulation of the nuclear factor-erythroid 2-related factor 2 (Nrf2), a central regulator of cellular response to OS [69] while miR-451 suppresses the production of 14-3-3zeta (KCIP-1), phosphoserine/threonine-binding protein that inhibits the nuclear accumulation of the transcription factor FoxO3, a positive regulator of erythroid antioxidant genes [70]. MiR-146b-5p has been shown to be downregulated in monocytes of obese subjects and its decrease has been shown to be associated with increased mitochondrial ROS generation and increased NFκB p65 DNA binding activity [71]. A study on the effects of resveratrol on hydrogen peroxide CRL-1730 treated cells showed a downregulation of miR-126 [72]. The induction of OS with N-(4-hydroxyphenyl)-retinamide (4HPR) has been shown to increase the expression of miR-16 and miR-26b in ARPE-19 cells [73]. Chronic OS induces significant downregulation of miR-29b in human trabecular meshwork cells resulting in an increased expression of various extracellular matrix genes [74]. MiR-335 has shown to induce premature senescence of young mesangial cells via suppression of SOD2 and ROS increase [75]. MiR-182 has been shown to be a negative regulator of FoxO1, a protein widely known for its role in protecting diverse cells from ROS [76]. MiR-155 and miR-16 have been found to be altered in their expression in AG01522 primary human fibroblasts (incubated for 1 hour in the logarithmic growth with 25 mM H2O2) [77]. Interestingly, among these miRNAs, miR-210, miR-155, miR-16, and miR-29b are known for their important roles in normal placental development. MiR-210 is known for its inhibiting role in trophoblast migration, iron metabolism, mitochondrial respiration, and steroid metabolism [53, 62, 63, 78]. MiR-155 regulates negatively trophoblast proliferation and migration, miR-16 inhibits trophoblast invasion, proliferation and angiogenesis, and finally miR-29b inhibits trophoblast invasion and angiogenesis and promotes apoptosis [42, 56, 58]. Among the miRNAs downregulated in preeclampsia, miR-204, miR-195, and miR-1 [45] have been described as being related to OS. MiR-204 has been shown to be an important modulator of OS response in human trabecular meshwork cells, leading to increased levels of apoptosis, decreased viability, and increased accumulation of oxidized proteins [79]. MiR-1, known to occur in ischemic myocardium, has been shown to be upregulated by ROS but downregulated by insulin [80]. A recent study, evaluating the effect of curcumin on altered miRNA expression induced with H2O2 in ARPE-19 cells, showed also upregulation of miR-195 [81], which has been shown to promote trophoblast invasion [52]. Also miR-338, known to be upregulated in preterm labor, has been described to control axonal ROS levels [82]. MiR-132, downregulated in gestational diabetes [60], has been shown to bear neuroprotective functions against OS through the regulation of PTEN, FOXO3a, and P300, which are all key elements of AKT signaling pathway [83]. The downregulation of miR-21 (as well as the downregulation of the previously mentioned miR-16 and the upregulation of miR-210) has been associated with reduced fetal growth. MiR-21, in addition, has been shown to be upregulated by ROS and to be associated with gastric and colon cancer [84, 85]. Interestingly, miR-21 has also been defined as a functional miRNA since it promotes trophoblast proliferation and invasion [86, 87]. It should be noted that miR-21 and miR-132 have also been found in the maternal circulation [60, 88, 89], highlighting the possibility of using miRNA as diagnostic and/or prognostic tools. OS-related miRNAs deregulated in placental alterations are summarized in Figure 1.
Figure 1.
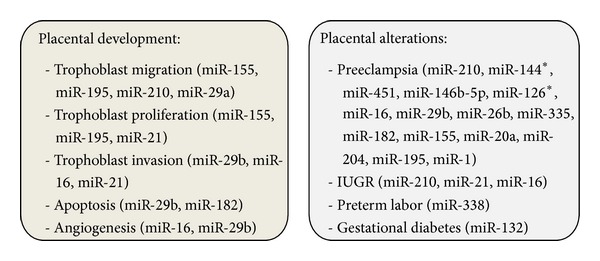
MicroRNAs modulated by oxidative stress. The microRNAs indicated in the figure are involved in placental development/alterations and in pregnancy related disorders.
5. Concluding Remarks
ROS production and OS seem to play a key role in placental physiological and pathological conditions. Several miRNAs have been reported to be specifically expressed in the placenta, where they could mediate key processes in its development, like trophoblast proliferation, differentiation, migration, invasion, and apoptosis, as well as angiogenesis and expression of antioxidant enzymes. Interestingly, the expression of some of these miRNAs is altered under various placental alterations, like preeclampsia, gestational diabetes, and other pregnancy related disorders. Some of these miRNAs, such as miR-210, miR-155, miR-16, miR-195, miR-21, and miR-29b, are related to ROS and OS. Considering the important role of OS in placental physiological and pathological conditions, the study of miRNA regulatory mechanisms in this context needs to be intensified especially in the light of the fact that some miRNAs could have an important impact on diagnosis and prognosis of placenta related disorders, as they can be found in circulation. Two promising candidates in this context are miR-21 and miR-132.
Acknowledgments
The authors are very grateful to EURAIBI (EURope Against Infant Brain Injury) ONLUS Foundation for its support. This work was supported by grants from the Tuscany Region and the Ministry of Health, General Directorate of Scientific and Technological Research for the 2011–2014 Project “Early identification of newborn at high risk of brain injury through a validated biomarkers profile and specific neuro-imaging patterns. A follow-up study of clinical and neurodevelopmental outcome in a large cohort study,” RF-2009-1499651.
Conflict of Interests
The authors declare that they have no conflict of interests regarding the publication of this paper.
References
- 1.Buonocore G, Perrone S, Tataranno ML. Oxygen toxicity: chemistry and biology of reactive oxygen species. Seminars in Fetal and Neonatal Medicine. 2010;15(4):186–190. doi: 10.1016/j.siny.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry & Cell Biology. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Dlasková A, Hlavatá L, Ježek P. Oxidative stress caused by blocking of mitochondrial complex I H+ pumping as a link in aging/disease vicious cycle. The International Journal of Biochemistry & Cell Biology. 2008;40:1792–1805. doi: 10.1016/j.biocel.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Gupta S, Agarwal A, Banerjee J, Alvarez JG. The role of oxidative stress in spontaneous abortion and recurrent pregnancy loss: a systematic review. Obstetrical and Gynecological Survey. 2007;62(5):335–347. doi: 10.1097/01.ogx.0000261644.89300.df. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan T, Sudarsanam D. RNAi: an innate gene Knockdown mechanism. European Journal of Applied Sciences. 2010;2(1):6–9. [Google Scholar]
- 6.Magenta A, Greco S, Gaetano C, et al. Oxidative stress and microRNA in vascular diseases. International Journal of Molecular Sciences. 2013;14(9):17319–17346. doi: 10.3390/ijms140917319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magenta A, Cencioni C, Fasanaro P, et al. MiR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death and Differentiation. 2011;18(10):1628–1639. doi: 10.1038/cdd.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simone NL, Soule BP, Ly D, et al. Ionizing radiation-induced oxidative stress alters miRNA expression. PLoS ONE. 2009;4(7) doi: 10.1371/journal.pone.0006377.e6377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cicchillitti L, Di Stefano V, Isaia E, et al. Hypoxia-inducible factor 1-alpha induces miR-210 in normoxic differentiating myoblasts. Journal of Biological Chemistry. 2012;287:44761–44771. doi: 10.1074/jbc.M112.421255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim VN, Nam J-W. Genomics of microRNA. Trends in Genetics. 2006;22(3):165–173. doi: 10.1016/j.tig.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Saito J, Hirota T, Furuta S, et al. Association between DNA methylation in the miR-328 5-flanking region and inter-individual differences in miR-328 and BCRP expression in human placenta. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0072906.e72906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleinrouweler CE, van Uitert M, Moerland PD, et al. Differentially expressed genes in the pre-eclamptic placenta: a systematic review and meta-analysis. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0068991.e68991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webster RP, Roberts VHJ, Myatt L. Protein nitration in placenta—functional significance. Placenta. 2008;29(12):985–994. doi: 10.1016/j.placenta.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jauniaux E, Watson A, Burton G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks’ gestation. American Journal of Obstetrics & Gynecology. 2001;184(5):998–1003. doi: 10.1067/mob.2001.111935. [DOI] [PubMed] [Google Scholar]
- 15.Myatt L, Cui X. Oxidative stress in the placenta. Histochemistry and Cell Biology. 2004;122(4):369–382. doi: 10.1007/s00418-004-0677-x. [DOI] [PubMed] [Google Scholar]
- 16.Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. Journal of Anatomy. 2009;215(1):27–35. doi: 10.1111/j.1469-7580.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura M, Sekizawa A, Purwosunu Y, et al. Cellular mRNA expressions of anti-oxidant factors in the blood of preeclamptic women. Prenatal Diagnosis. 2009;29(7):691–696. doi: 10.1002/pd.2278. [DOI] [PubMed] [Google Scholar]
- 18.Wisdom SJ, Wilson R, McKillop JH, Walker JJ. Antioxidant systems in normal pregnancy and in pregnancy-induced hypertension. American Journal of Obstetrics & Gynecology. 1991;165(6, part 1):1701–1704. doi: 10.1016/0002-9378(91)90018-m. [DOI] [PubMed] [Google Scholar]
- 19.Walsh SW, Wang Y. Secretion of lipid peroxides by the human placenta. American Journal of Obstetrics & Gynecology. 1993;169(6):1462–1466. doi: 10.1016/0002-9378(93)90419-j. [DOI] [PubMed] [Google Scholar]
- 20.Reslan OM, Khalil RA. Molecular and vascular targets in the pathogenesis and management of the hypertension associated with preeclampsia. Cardiovascular & Hematological Agents in Medicinal Chemistry. 2010;8(4):204–226. doi: 10.2174/187152510792481234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burton GJ, Yung H-W, Cindrova-Davies T, Charnock-Jones DS. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta. 2009;30:S43–S48. doi: 10.1016/j.placenta.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Walsh SW. Antioxidant activities and mRNA expression of superoxide dismutase, catalase, and glutathione peroxidase in normal and preeclamptic placentas. Journal of the Society for Gynecologic Investigation. 1996;3(4):179–184. [PubMed] [Google Scholar]
- 23.Jauniaux E, Watson AL, Hempstock J, Bao Y-P, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress: a possible factor in human early pregnancy failure. American Journal of Pathology. 2000;157(6):2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polak G, Kozioł-Montewka M, Tarkowski R, Kotarski J. Peritoneal fluid and plasma 4-hydroxynonenal and malonyldialdehyde concentrations in infertile women. Ginekologia Polska. 2001;72(12 A):1316–1320. [PubMed] [Google Scholar]
- 25.Poston L, Raijmakers MTM. Trophoblast oxidative stress, antioxidants and pregnancy outcome—a review. Placenta. 2004;25:S72–S78. doi: 10.1016/j.placenta.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Quenby S, Nik H, Innes B, et al. Uterine natural killer cells and angiogenesis in recurrent reproductive failure. Human Reproduction. 2009;24(1):45–54. doi: 10.1093/humrep/den348. [DOI] [PubMed] [Google Scholar]
- 27.Biri A, Bozkurt N, Turp A, Kavutcu M, Himmetoglu Ö, Durak I. Role of oxidative stress in intrauterine growth restriction. Gynecologic and Obstetric Investigation. 2007;64(4):187–192. doi: 10.1159/000106488. [DOI] [PubMed] [Google Scholar]
- 28.Scifres CM, Nelson DM. Intrauterine growth restriction, human placental development and trophoblast cell death. Journal of Physiology. 2009;587(14):3453–3458. doi: 10.1113/jphysiol.2009.173252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longini M, Perrone S, Kenanidis A, et al. Isoprostanes in amniotic fluid: a predictive marker for fetal growth restriction in pregnancy. Free Radical Biology and Medicine. 2005;38(11):1537–1541. doi: 10.1016/j.freeradbiomed.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Haddad R, Tromp G, Kuivaniemi H, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. American Journal of Obstetrics & Gynecology. 2006;195(2):394.e12–405.e12. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan RN, Matharoo-Ball B, Shaw RW. Antioxidant enzyme expression, lipid peroxidation, and protein oxidation in human myometrium with parturition. Reproductive Sciences. 2010;17(1):78–84. doi: 10.1177/1933719109348027. [DOI] [PubMed] [Google Scholar]
- 32.Mustafa MD, Pathak R, Ahmed T, et al. Association of glutathione S-transferase M1 and T1 gene polymorphisms and oxidative stress markers in preterm labor. Clinical Biochemistry. 2010;43(13-14):1124–1128. doi: 10.1016/j.clinbiochem.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 33.Rode L, Nilas L, Wøjdemann K, Tabor A. Obesity-related complications in Danish single cephalic term pregnancies. Obstetrics and Gynecology. 2005;105(3):537–542. doi: 10.1097/01.AOG.0000152304.39492.1c. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. American Journal of Clinical Nutrition. 2006;83(2):461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 35.Weintrob N, Karp M, Moshe H. Short- and long-range complications in offspring of diabetic mothers. Journal of Diabetes and its Complications. 1996;10(5):294–301. doi: 10.1016/1056-8727(95)00080-1. [DOI] [PubMed] [Google Scholar]
- 36.Kinalski M, Sledziewski A, Telejko B, Kowalska I, Kretowski A, Kinalska I. Evaluation of lipid peroxidation and acid-base status in cord blood of newborns after diabetes in pregnancy. Przeglad Lekarski. 2001;58(3):120–123. [PubMed] [Google Scholar]
- 37.Mayor-Lynn K, Toloubeydokhti T, Cruz AC, Chegini N. Expression profile of microRNAs and mRNAs in human placentas from pregnancies complicated by preeclampsia and preterm labor. Reproductive Sciences. 2011;18(1):46–56. doi: 10.1177/1933719110374115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mouillet J-F, Chu T, Sadovsky Y. Expression patterns of placental microRNAs. Birth Defects Research A. 2011;91(8):737–743. doi: 10.1002/bdra.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Enquobahrie DA, Abetew DF, Sorensen TK, Willoughby D, Chidambaram K, Williams MA. Placental microRNA expression in pregnancies complicated by preeclampsia. American Journal of Obstetrics & Gynecology. 2011;204(2):178.e12–178.e21. doi: 10.1016/j.ajog.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barad O, Meiri E, Avniel A, et al. MicroRNA expression detected by oligonucleotide microarrays: system establishment and expression profiling in human tissues. Genome Research. 2004;14(12):2486–2494. doi: 10.1101/gr.2845604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donker RB, Mouillet J-F, Nelson DM, Sadovsky Y. The expression of Argonaute2 and related microRNA biogenesis proteins in normal and hypoxic trophoblasts. Molecular Human Reproduction. 2007;13(4):273–279. doi: 10.1093/molehr/gam006. [DOI] [PubMed] [Google Scholar]
- 42.Li P, Guo W, Du L, et al. MicroRNA-29b contributes to pre-eclampsia through its effects on apoptosis, invasion and angiogenesis of trophoblast cells. Clinical Science. 2013;124(1):27–40. doi: 10.1042/CS20120121. [DOI] [PubMed] [Google Scholar]
- 43.Dai Y, Qiu Z, Diao Z, et al. MicroRNA-155 inhibits proliferation and migration of human extravillous trophoblast derived HTR-8/SVneo cells via down-regulating cyclin D1. Placenta. 2012;33(10):824–829. doi: 10.1016/j.placenta.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Hu Y, Li P, Hao S, Liu L, Zhao J, Hou Y. Differential expression of microRNAs in the placentae of Chinese patients with severe pre-eclampsia. Clinical Chemistry and Laboratory Medicine. 2009;47(8):923–929. doi: 10.1515/CCLM.2009.228. [DOI] [PubMed] [Google Scholar]
- 45.Zhu X-M, Han T, Sargent IL, Yin G-W, Yao Y-Q. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. American Journal of Obstetrics & Gynecology. 2009;200(6):661.e1–661.e7. doi: 10.1016/j.ajog.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 46.Morales-Prieto DM, Ospina-Prieto S, Chaiwangyen W, et al. Pregnancy-associated miRNA-clusters. Journal of Reproductive Immunology. 2013;97:51–61. doi: 10.1016/j.jri.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Luo L, Ye G, Nadeem L, et al. MicroRNA-378a-5p promotes trophoblast cell survival, migration and invasion by targeting Nodal. Journal of Cell Science. 2012;125:3124–3132. doi: 10.1242/jcs.096412. [DOI] [PubMed] [Google Scholar]
- 48.Fu G, Ye G, Nadeem L, et al. MicroRNA-376c impairs transforming growth factor-beta and nodal signaling to promote trophoblast cell proliferation and invasion. Hypertension. 2013;61:864–872. doi: 10.1161/HYPERTENSIONAHA.111.203489. [DOI] [PubMed] [Google Scholar]
- 49.Bentwich I, Avniel A, Karov Y, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nature Genetics. 2005;37(7):766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- 50.Gao WL, Liu M, Yang Y, et al. The imprinted H19 gene regulates human placental trophoblast cell proliferation via encoding miR-675 that targets Nodal Modulator 1 (NOMO1) RNA Biology. 2012;9(7):1002–1010. doi: 10.4161/rna.20807. [DOI] [PubMed] [Google Scholar]
- 51.Pineles BL, Romero R, Montenegro D, et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. American Journal of Obstetrics & Gynecology. 2007;196(3):261.e1–261.e6. doi: 10.1016/j.ajog.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Bai Y, Yang W, Yang H, et al. Downregulated miR-195 detected in preeclamptic placenta affects trophoblast cell invasion via modulating ActRIIA expression. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0038875.e38875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Fei M, Xue G, et al. Elevated levels of hypoxia-inducible microRNA-210 in pre-eclampsia: new insights into molecular mechanisms for the disease. Journal of Cellular and Molecular Medicine. 2012;16(2):249–259. doi: 10.1111/j.1582-4934.2011.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Umemura K, Ishioka S, Endo T, et al. Roles of microRNA-34a in the pathogenesis of placenta accrete. Journal of Obstetrics and Gynaecology Research. 2013;39(1):67–74. doi: 10.1111/j.1447-0756.2012.01898.x. [DOI] [PubMed] [Google Scholar]
- 55.Pang RTK, Leung CON, Ye T-M, et al. MicroRNA-34a suppresses invasion through downregulation of Notch1 and Jagged1 in cervical carcinoma and choriocarcinoma cells. Carcinogenesis. 2010;31(6):1037–1044. doi: 10.1093/carcin/bgq066. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Fan H, Zhao G, et al. MiR-16 inhibits the proliferation and angiogenesis-regulating potential of mesenchymal stem cells in severe pre-eclampsia. FEBS Journal. 2012;289:4510–4524. doi: 10.1111/febs.12037. [DOI] [PubMed] [Google Scholar]
- 57.Wang W, Feng L, Zhang H, et al. Preeclampsia up-regulates angiogenesis-associated microRNA (i.e., miR-17, -20a, and -20b) that target ephrin-B2 and EPHB4 in human placenta. Journal of Clinical Endocrinology and Metabolism. 2012;97:E1051–E1059. doi: 10.1210/jc.2011-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Diao Z, Su L, et al. MicroRNA-155 contributes to preeclampsia by down-regulating CYR61. American Journal of Obstetrics & Gynecology. 2010;202:466.e1–466.e7. doi: 10.1016/j.ajog.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 59.Higashijima A, Miura K, Mishima H, et al. Characterization of placenta-specific microRNAs in fetal growth restriction pregnancy. Prenatal Diagnosis. 2013;33(3):214–222. doi: 10.1002/pd.4045. [DOI] [PubMed] [Google Scholar]
- 60.Zhao C, Dong J, Jiang T, et al. Early second-trimester serum miRNA profiling predicts gestational diabetes mellitus. PLoS ONE. 2011;6(8) doi: 10.1371/journal.pone.0023925.e23925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Montenegro D, Romero R, Kim SS, et al. Expression patterns of microRNAs in the chorioamniotic membranes: a role for microRNAs in human pregnancy and parturition. Journal of Pathology. 2009;217(1):113–121. doi: 10.1002/path.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takizawa T, Ishibashi O, Ohkuchi A, et al. Hydroxysteroid (17-β) dehydrogenase 1 is dysregulated by miR-210 and miR-518c that are aberrantly expressed in preeclamptic placentas: a novel marker for predicting preeclampsia. Hypertension. 2012;59(2):265–273. doi: 10.1161/HYPERTENSIONAHA.111.180232. [DOI] [PubMed] [Google Scholar]
- 63.Lee D-C, Romero R, Kim J-S, et al. MiR-210 targets iron-sulfur cluster scaffold homologue in human trophoblast cell lines: siderosis of interstitial trophoblasts as a novel pathology of preterm preeclampsia and small-for-gestational-age pregnancies. The American Journal of Pathology. 2011;179(2):590–602. doi: 10.1016/j.ajpath.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gandhy SU, Kim K, Larsen L, et al. Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp)transcription factors by targeting microRNAs. BMC Cancer. 2012;12, article 564 doi: 10.1186/1471-2407-12-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cicchillitti L, Di Stefano V, Isaia E, et al. Hypoxia-inducible factor 1-α induces miR-210 in normoxic differentiating myoblasts. Journal of Biological Chemistry. 2012;287(53):44761–44771. doi: 10.1074/jbc.M112.421255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zaccagnini G, Maimone B, di Stefano V, et al. Hypoxiainduced miR-210 modulates tissue response to acute peripheral ischemia. Antioxidants & Redox Signaling. 2013 doi: 10.1089/ars.2013.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Devlin C, Greco S, Martelli F, Ivan M. MiR-210: more than a silent player in hypoxia. IUBMB Life. 2011;63(2):94–100. doi: 10.1002/iub.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X, Zhu H, Zhang X, et al. Loss of the miR-144/451 cluster impairs ischaemic preconditioning-mediated cardioprotection by targeting Rac-1. Cardiovascular Research. 2012;94(2):379–390. doi: 10.1093/cvr/cvs096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sangokoya C, Telen MJ, Chi J-T. microRNA miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood. 2010;116(20):4338–4348. doi: 10.1182/blood-2009-04-214817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu D, dos Santos CO, Zhao G, et al. miR-451 protects against erythroid oxidant stress by repressing 14-3-3zeta. Genes & Development. 2010;24(15):1620–1633. doi: 10.1101/gad.1942110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hulsmans M, van Dooren E, Mathieu C, Holvoet P. Decrease of miR-146b-5p in monocytes during obesity is associated with loss of the anti-inflammatory but not insulin signaling action of adiponectin. PLoS ONE. 2012;7(2) doi: 10.1371/journal.pone.0032794.e32794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sui XQ, Xu ZM, Xie MB, Pei DA. Resveratrol inhibits hydrogen peroxide-induced apoptosis in endothelial cells via the activation of PI3K/Akt bymiR-126. Journal of Atherosclerosis and Thrombosis. 2013 doi: 10.5551/jat.19257.19257 [DOI] [PubMed] [Google Scholar]
- 73.Krishnan Kutty R, Samuel W, Jaworski C, et al. MicroRNA expression in human retinal pigment epithelial (ARPE-19) cells: increased expression of microRNA-9 by N-(4-Hydroxyphenyl)retinamide. Molecular Vision. 2010;16:1475–1486. [PMC free article] [PubMed] [Google Scholar]
- 74.Luna C, Li G, Qiu J, Epstein DL, Gonzalez P. Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress. Molecular vision. 2009;15:2488–2497. [PMC free article] [PubMed] [Google Scholar]
- 75.Bai X-Y, Ma Y, Ding R, Fu B, Shi S, Chen X-M. miR-335 and miR-34a promote renal senescence by suppressing mitochondrial antioxidative enzymes. Journal of the American Society of Nephrology. 2011;22(7):1252–1261. doi: 10.1681/ASN.2010040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim KM, Park SJ, Jung SH, et al. miR-182 is a negative regulator of osteoblast proliferation, differentiation, and skeletogenesis through targeting FoxO1. Journal of Bone and Mineral Research. 2012;27(8):1669–1679. doi: 10.1002/jbmr.1604. [DOI] [PubMed] [Google Scholar]
- 77.Simone NL, Soule BP, Ly D, et al. Ionizing radiation-induced oxidative stress alters miRNA expression. PLoS ONE. 2009;4(7) doi: 10.1371/journal.pone.0006377.e6377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muralimanoharan S, Maloyan A, Mele J, et al. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta. 2012;33(10):816–823. doi: 10.1016/j.placenta.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Role of miR-204 in the regulation of apoptosis, endoplasmic reticulum stress response, and inflammation in human trabecular meshwork cells. Investigative Ophthalmology & Visual Science. 2011;52(6):2999–3007. doi: 10.1167/iovs.10-6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen T, Ding G, Jin Z, et al. Insulin ameliorates miR-1-induced injury in H9c2 cells under oxidative stress via Akt activation. Molecular and Cellular Biochemistry. 2012;369(1-2):167–174. doi: 10.1007/s11010-012-1379-7. [DOI] [PubMed] [Google Scholar]
- 81.Howell JC, Chun E, Farrell AN, et al. Global microRNA expression profiling: curcumin (diferuloylmethane) alters oxidative stress-responsivemicroRNAs in human ARPE-19 cells. Molecular Vision. 2013;19:544–560. [PMC free article] [PubMed] [Google Scholar]
- 82.Aschrafi A, Kar AN, Natera-Naranjo O, et al. MicroRNA-338 regulates the axonal expression of multiple nuclear-encoded mitochondrial mRNAs encodingsubunits of the oxidative phosphorylation machinery. Cellular and Molecular Life Sciences. 2012;69(23):4017–4027. doi: 10.1007/s00018-012-1064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wong HK, Veremeyko T, Patel N, et al. De-repression of FOXO3a death axis by microRNA-132 and -212 causes neuronal apoptosis in Alzheimer’s disease. Human Molecular Genetics. 2013;22(15):3077–3092. doi: 10.1093/hmg/ddt164. [DOI] [PubMed] [Google Scholar]
- 84.Tu H, Sun H, Lin Y, et al. Oxidative stress upregulates PDCD4 expression in patients with gastric cancer via miR-21. Current Pharmaceutical Design. 2013 doi: 10.2174/13816128113199990547. [DOI] [PubMed] [Google Scholar]
- 85.Saxena A, Tammali R, Ramana KV, et al. Aldose reductase inhibition prevents colon cancer growth by restoring phosphatase and tensin homolog through modulation of miR-21 and FOXO3a. Antioxidants & Redox Signaling. 2013;18(11):1249–1262. doi: 10.1089/ars.2012.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morales-Prieto DM, Chaiwangyen W, Ospina-Prieto S, et al. MicroRNA expression profiles of trophoblastic cells. Placenta. 2012;33:725–734. doi: 10.1016/j.placenta.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 87.Maccani MA, Padbury JF, Marsit CJ. miR-16 and miR-21 expression in the placenta is associated with fetal growth. PLoS ONE. 2011;6(6) doi: 10.1371/journal.pone.0021210.e21210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chim SSC, Shing TKF, Hung ECW, et al. Detection and characterization of placental microRNAs in maternal plasma. Clinical Chemistry. 2008;54(3):482–490. doi: 10.1373/clinchem.2007.097972. [DOI] [PubMed] [Google Scholar]
- 89.Luo S-S, Ishibashi O, Ishikawa G, et al. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biology of Reproduction. 2009;81(4):717–729. doi: 10.1095/biolreprod.108.075481. [DOI] [PubMed] [Google Scholar]


