Abstract
Aim
To assess the use of nasal high-frequency ventilation (HFV) to provide noninvasive ventilatory support for very low birthweight (VLBW) infants.
Study Design
VLBW infants, >7 days of age on nasal continuous positive airway pressure (CPAP), were placed on nasal HFV for 2 h using the Infant Star high-frequency ventilator (Mallinckrodt, Inc., St. Louis, MO, USA). Mean airway pressure was set to equal the previous level of CPAP, and amplitude was adjusted to obtain chest wall vibration. Capillary blood was sampled before starting HFV and after 2 h to determine change in pH and partial pressure of carbon dioxide (pCO2).
Results
Fourteen subjects were studied, 10 males and 4 females. Gestational age was 26–30 weeks (median 27). Age at study was 18–147 days (median 30). Median birth weight was 955 g; median weight at study was 1605 g. Nasal CPAP pressure was 4–7 cm H2O (mean 5). Amplitude was 30–60 (median 50). After 2 h, PCO2 (mean 45 torr) was significantly lower than initial PCO2 (mean 50 torr) (p = 0.01), and pH had increased significantly (7.40 vs. 7.37, p = 0.04).
Conclusions
Nasal HFV is effective in decreasing pCO2 in stable premature infants requiring nasal CPAP support. Long-term use of nasal HFV requires further study.
Keywords: CPAP, High-frequency ventilation, Nasal ventilation, Premature infant
INTRODUCTION
Premature infants often experience respiratory insufficiency due to lung immaturity, surfactant deficiency and immature respiratory control mechanisms. These infants often need respiratory support in the form of positive pressure ventilation. Current management techniques for these infants include endotracheal mechanical ventilation and nasal continuous positive airway pressure (CPAP). The smallest infants commonly require prolonged support including tracheal intubation and mechanical ventilation for days or weeks, followed by weeks or months of nasal CPAP. These infants commonly require reintubation after their initial ventilator course due to worsening respiratory insufficiency or apnoea of prematurity. Prolonged mechanical ventilation puts premature infants at risk for life-threatening complications including bronchopulmonary dysplasia (1) and nosocomial pneumonia (2). Furthermore, mechanical ventilation itself can cause lung injury, thereby slowing recovery from the underlying pulmonary problem (1,3).
Mechanical ventilation is typically provided via an endotracheal tube, using one of two modes: conventional synchronized intermittent mandatory ventilation (SIMV) or high-frequency ventilation (HFV). Endotracheal intubation carries risks, including those of subglottic stenosis (4), nosocomial pneumonia and pneumothorax (5). Avoiding tracheal intubation reduces these risks, as well as the risk of inherent lung injury associated with mechanical ventilation (1). Noninvasive nasal ventilatory modes represent a way to actively assist ventilation without the risks of tracheal intubation.
Synchronized nasal intermittent positive pressure ventilation (SNIPPV) is a form of noninvasive ventilation that has been successfully used to avoid the need for endotracheal intubation in preterm infants with respiratory failure. SNIPPV has been studied, and its safety and efficacy have been demonstrated (6–10). It has been shown to be particularly effective in decreasing reintubation when compared with nasal CPAP as an extubation mode (6,8,10).
SNIPPV has also been used as an alternative to continued endotracheal mechanical ventilation after surfactant therapy in 28- to 34-week gestation infants with respiratory distress syndrome (9). Infants extubated immediately to SNIPPV after surfactant administration were less likely to require reintubation, had decreased needs for supplemental oxygen during hospitalization and had shorter hospital stays than otherwise similar infants who remained on the ventilator following surfactant dosing. Work of breathing has also been shown to be decreased with the use of SNIPPV compared to nasal CPAP (11).
Nasal HFV has not yet been evaluated, except in a single case report and a clinical study with nonstandardized entry criteria and duration (12,13). There is rationale and support for the idea that high-frequency oscillation delivered into the nasopharynx may improve carbon dioxide elimination in infants. The earliest devices used to deliver CPAP to infants were constructed by venting the expiratory limb of a ventilatory circuit into a vessel containing water. There has been renewed interest in the use of this ‘bubble CPAP’ based on the idea that the resultant bubbling may create pressure variation of the gas in the circuit and that this variation may be transferred to the infant’s airway (14), thereby enhancing carbon dioxide elimination by one or more of the mechanisms active in endotracheal HFV. However, when studied in stable preterm infants, bubble CPAP did not change the level of partial pressure of carbon dioxide (pCO2) compared to conventional CPAP (15).
In premature infants, endotracheal HFV is believed to cause less lung injury than conventional ventilation, and SNIPPV is thought to be less injurious than endotracheal SIMV (16,17). This leads naturally to the question of whether the benefits of HFV and noninvasive (nasal) ventilation are synergistic. It is reasonable to conjecture that nasal HFV might improve carbon dioxide elimination compared with nasal CPAP alone, minimizing the need for intubation and mechanical ventilation, similar to SNIPPV.
We postulated that nasal HFV might provide an alternative method of noninvasive ventilation. Our aim was to investigate the short-term efficacy of nasal HFV in decreasing pCO2 values in stable preterm infants requiring nasal CPAP. As a first step in evaluating the therapeutic efficacy of nasal HFV, we tested the ability of this technique to lower pCO2 in premature infants recovering from lung disease who still required respiratory support with nasal CPAP. We chose a carefully specified population and performed a standard intervention on all subjects.
METHODS
We performed a prospective, nonrandomized pilot study of nasal HFV as a mode to reduce pCO2 in convalescing VLBW infants.
Inclusion/exclusion criteria
VLBW infants with a history of respiratory distress syndrome were enrolled in the study according to the following criteria: birth weight less than 1500 g, age more than 7 days, stable respiratory status on nasal CPAP, capillary pH above 7.25, capillary pCO2 above 43 torr and medically stable as determined by the treating medical team. Infants with severe congenital anomalies or grade III or IV intraventricular haemorrhage were excluded from participation. The pH value was chosen to exclude any patient with severe respiratory or metabolic acidosis, as these are signs of acute illness. The pCO2 value was chosen to reduce the risk of significant hypocarbia, should the intervention prove to be successful.
Intervention
All nasal CPAP and study nasal HFV was delivered via a single nasopharyngeal tube, positioned in the posterior nasopharynx (18). The study period for all subjects was 2 h. Written parental consent was obtained for all subjects and the study was approved by the University of Iowa Institutional Review Board.
Study protocol
On the study day, the subject’s morning blood gas was examined. If pH and pCO2 criteria were met, the study proceeded. The study intervention was timed to start immediately following the patient’s feeding, such that no subject received feedings during the study. The subject was then placed on nasal HFV for 2 h, using the Infrasonics Infant Star 950 high-frequency ventilator (Mallinckrodt, Inc., St. Louis, MO, USA). The positive end-expiratory pressure (PEEP) selected was the same as the nasal CPAP pressure, and the amplitude was adjusted to obtain visible chest wall or upper airway (anterior neck) vibration. Amplitude was increased every 30 min by 4–6 units if necessary to maintain clinically appropriate chest wall or anterior neck vibration. Frequency was fixed at 10 Hz throughout the study period for all subjects, representing an I:E ratio of 1:4.5, based on a fixed inspiratory time of 0.018 sec. The fraction of inspired oxygen (FiO2) was adjusted if necessary to keep saturation within physician-ordered parameters. After 2 h of nasal HFV, a capillary blood gas was obtained and subjects were returned to their prestudy nasal CPAP settings.
Monitoring
Ventilator settings (PEEP, amplitude, frequency, FiO2), heart rate, respiratory rate and oxygen saturation (by pulse oximetry) were recorded every 30 min. A chest radiograph was obtained after 1 h of nasal HFV to assess for air-trapping. A transcutaneous pCO2 (TCOM) monitor (Phillips Medical Systems, M1018A, New York, NY, USA) was attached to the patient’s skin and calibrated for 15 to 20 min prior to the initiation of nasal HFV. After confirming calibration with the morning blood gas value, this monitor remained in place for the duration of the study, with measurement recorded every 30 min during the intervention. The study was designed to be halted if the TCOM measurement was <30 torr or >80 torr at anytime.
Sample size and statistical analysis
Power calculation demonstrated that 14 infants were required to detect a decrease in pCO2 equal to two-thirds of the standard deviation in delta- pCO2, that is, sigma-to-delta ratio 0.67 with alpha (risk of type I error) 0.05 and beta (risk of type II error) 0.20. The delta-to-sigma ratio used in this calculation is based on the mean and standard deviation of the change in pCO2 seen in the study of van der Hoeven et al. (12). Pre- and postintervention pCO2, pH, were compared using the Wilcoxon signed-rank test. Trends in respiratory rate, heart rate, FiO2, TCOM reading and peripheral oxygen saturation (SpO2) over time during NHFV were examined using analysis of variance (ANOVA) for repeated measures. SPSS v14.0, Chicago, IL, USA was used for statistical analysis.
RESULTS
Patient characteristics
Fourteen infants were enrolled in the study (Table 1). There were 10 males and 4 females. Gestational age at birth ranged from 26 to 30 weeks (median 27 weeks). The subjects’ ages at the time of the study ranged from 18 to 147 days (median 30 days). The median birth weight was 955 g, and the median weight at the time of the study was 1605 g. All infants carried a diagnosis of respiratory distress syndrome at birth, and had been intubated and given surfactant in the first day of life.
Table 1.
Summary of study population characteristics
| Median (range) | |
|---|---|
| Gender | 10 male, 4 female |
| Birth weight (g) | 995 (438–1374) |
| Weight at study (g) | 1605 (1065–2870) |
| Gestational age (weeks) | 27 (25–30) |
| Postnatal age at study (days) | 39.5 (18–147) |
| Nasal CPAP pressure (cm H2O) | 5.5 (4–7) |
Respiratory outcomes
We found that nasal HFV significantly decreased the level of pCO2 in our population from a mean of 50 torr, to a mean of 45 torr (p = 0.01) (Fig. 1). PH was also significantly higher after 2 h of nasal HFV (7.40 vs. 7.37, p = 0.04). CPAP ranged from 4 to 7 cm H2O (mean 5 cm H2O), and remained constant throughout the study. Amplitude ranged from 29 to 60 (median 50) (Table 2).
Figure 1.
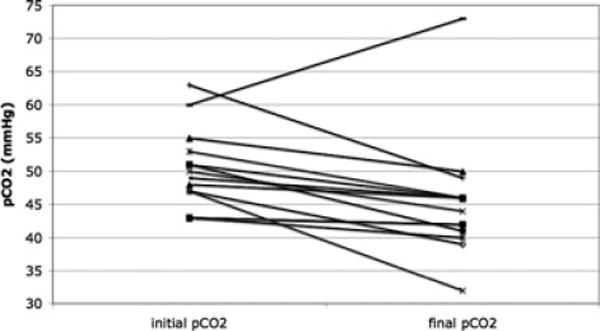
PCO2 before and after nasal HFV. Significant decrease in pCO2 after 2 h of nasal HFV (45 ± 9 torr) compared with before nasal HFV (50 ± 6 torr) (p = 0.011).
Table 2.
Summary of respiratory parameters before and during nasal HFV
| Study time, min
|
||||||
|---|---|---|---|---|---|---|
| Variable mean ± standard deviation | Pre-NHFV | 30 min | 60 min | 90 min | 120 min | p-value |
| Heart rate | 176 ± 15 | 169 ± 12 | 170 ± 12 | 166 ± 16 | 166 ± 15 | 0.084 |
| Respiratory rate | 49 ± 12 | 60 ± 18 | 53 ± 13 | 52 ± 16 | 51 ± 15 | 0.569 |
| FiO2, % | 23 ± 4 | 22 ± 3 | 22 ± 3 | 22 ± 3 | 23 ± 3 | 0.136 |
| SpO2, % | 96 ± 3 | 95 ± 4 | 95 ± 3 | 95 ± 4 | 96 ± 3 | 0.207 |
| TCOM, torr | 47 ± 5 | 48 ± 5 | 49 ± 10 | 46 ± 4 | 46 ± 5 | 0.003 |
| Amplitude, cm H2O | 41 ± 6 | 45 ± 5 | 47 ± 4 | 50 ± 6 | 51 ± 6 | <0.0001 |
| Capillary pCO2, torr | 50 ± 6 | NA | NA | NA | 45 ± 9 | 0.011 |
| Capillary pH, torr | 7.37 ± 0.04 | NA | NA | NA | 7.40 ± 0.06 | 0.043 |
Patient safety
There was no significant change in respiratory rate. However, heart rate showed a downward trend with a decrease of 10 beats per min (p = 0.08) during the study period (Table 2). FiO2 and oxygen saturations did not significantly change during NHFV (Table 2). Chest X-rays obtained after 1 h showed no evidence of air-trapping in any patient. TCOM readings were significantly lower at the end of the study than at the beginning (p = 0.003).
In one patient, the study was stopped early at 90 min due to a TCOM reading of 80. A blood gas obtained at this time showed a pCO2 of 46, indicating sensor malfunction, or poor perfusion to the skin beneath the electrode. This patient was analyzed with the rest of the group according to intention-to-treat principles. In another patient, pCO2 was higher after 2 h of NHFV (73 vs. 63 torr preintervention). At the conclusion of the study, the nasopharyngeal tube was found to be plugged with nasal secretions, which may explain the lack of effect in this patient.
DISCUSSION
We have demonstrated that short-term use of nasal HFV can lower pCO2 in convalescing stable preterm infants with no apparent adverse effects. This suggests that nasal HFV could provide an alternative method of noninvasive ventilation for VLBW infants. We have shown that CO2 elimination can be achieved with nasal HFV, whereas it has not been shown to be achieved with bubble CPAP alone (15).
Nasally administered HFV, using the Infant Star high-frequency ventilator, was previously investigated by van der Hoeven and colleagues in 1988. They studied 21 premature infants within the first 20 days of life. Nasal HFV was instituted in these patients due to the need for increased respiratory support and continued for periods ranging from hours to days. These investigators demonstrated a decrease in pCO2 from 62 torr (8.3 kPa) to 54 torr (7.2 kPa) within 2 h of nasal HFV. No air leak, sudden cardiorespiratory decompensation or other adverse event occurred (12). We have found similar results in our study within a chronic, stable population of VLBW infants. Hoehn and Krause also reported use of nasopharyngeal HFV in a 24-day-old, 24-week gestational age, 760 g infant. They used the Draeger Babylog 8000 ventilator (Draeger, Luebeck, Germany) for 24 h after extubation, and were able to significantly decrease the infant’s pCO2, preventing reintubation (13).
Our study’s strengths include a rigorous protocol. We exposed the subjects to a uniform intervention for a standardized period of time. We monitored vital signs and used transcutaneous CO2 monitoring to assess short-term safety of this intervention.
Our study is limited by its small size and brief use of nasal HFV. The 2-h exposure to nasal HFV was tolerated well by all patients without any adverse effects and was effective in reducing pCO2. However, the safety and efficacy of long-term nasal HFV are unknown. We did not test the ability of nasal HFV to provide ventilatory support in cases of acute respiratory failure or as a mode to be used immediately after extubation. This study does not provide support for the use of this mode to treat unstable or critically ill infants with severe hypercapnia. We did not measure blood gases after the subjects were returned to conventional CPAP, so it is unknown if the effect seen with NHFV would have been sustained or if values would return to preintervention levels, further strengthening the evidence that this therapy is effective. In addition, use of the TCOM as a safety device could introduce bias into the study, as we were not blinded to the TCOM results as we altered amplitude to maintain visible chest vibration. Although the difference between mean initial and final TCOM values was small (average 3 mmHg), it was significant (p = 0.007). Thus, although we did not use the TCOM readings to guide amplitude adjustment, our results may be influenced by its use.
The Infant Star ventilator is limited in the maximum amplitude it can generate, which limits the tidal volume delivered. The oscillations delivered were likely dampened due to the increased dead space of the pharynx as compared with intratracheally delivered HFV, thus requiring higher amplitudes than normally used for HFV delivered via an endotracheal tube. We did not attempt to minimize loss of pressure or volume through the mouth, via a chin strap, orally occlusive pacifier or other device. Such devices are not developmentally optimal, and also could potentially increase the risk of air-trapping. We found that relatively high amplitudes were required to achieve any visible shaking of the chest or neck. We did not measure intra-alveolar pressures, intra-oesophageal or intragastric pressures and are thus unable to directly assess the effect of these high amplitudes at the alveolar level, or on gastric distention. In addition, there can be a concern for development of expiratory flow limitation during endotracheal HFV when low mean airway pressures and high amplitudes are used, leading to air-trapping via a ‘choke point’ effect. However, we did not visualize any change in lung expansion on chest radiograph during HFV in any patient, and we did not see generally see elevation in pCO2, another indicator of air-trapping and inadequate gas exchange. As noted previously, pCO2 did increase in one patient whose nasopharyngeal tube was obstructed by secretions during the study.
Our results are also applicable only to a single nasopharyngeal tube delivery system. We did not test nasal HFV using short binasal prongs, which are also commonly used for nasal CPAP and SNIPPV. Furthermore, the Infant Star is also no longer in production and is becoming increasingly unavailable for use. Our study was limited to this device; we did not test nasal HFV using other high-frequency ventilators.
High-frequency oscillatory pulses have been shown to stimulate respiratory effort in adult patients with central sleep apnoea. Pulses were delivered to the upper airway by a nasal mask (19). This presents the intriguing possibility that nasal HFV may be a potential therapy for apnoea of prematurity as well as respiratory failure. This should be the subject of future investigation in premature infants.
Nasal HFV may provide another important tool to be used for noninvasive ventilatory support to minimize the need for endotracheal intubation and mechanical ventilation. This study provides a step towards evaluating the therapeutic efficacy of nasal HFV, as we have shown that this technique can lower pCO2 in premature infants recovering from lung disease who still require respiratory support in the form of nasal CPAP. Future studies of long-term nasal HFV should be undertaken to test the ability of nasal HFV to decrease extubation failure as compared to nasal CPAP alone and to prevent apnoea. Furthermore, the efficacy of nasal HFV should be tested using other ventilators capable of delivering noninvasive HFV.
Acknowledgments
This work was supported by grant M01 RR00059, from the General Clinical Research Centers Program, National Center for Research Resources, National Institutes of Health, USA and a scholarship (to U.M.M.Y.) from the Ministry of Higher Education of the Arab Republic of Egypt.
Abbreviations
- CPAP
continuous positive airway pressure
- SIMV
synchronized intermittent mechanical ventilation
- HFV
high-frequency ventilation
- SNIPPV
synchronized nasal intermittent positive pressure ventilation
- pCO2
partial pressure of carbon dioxide
- VLBW
very low birthweight
- PEEP
positive end-expiratory pressure
- FiO2
fraction of inspired oxygen
- TCOM
transcutaneous carbon dioxide monitor
- H2O
water
- SpO2
peripheral oxygen saturation
- ANOVA
analysis of variance
References
- 1.Ogata ES, Gregory GA, Kitterman JA, Phibbs RH, Tooley WH. Pneumothorax in the respiratory distress syndrome: incidence and effect on vital signs, blood gases, and pH. Pediatrics. 1976;58:177–83. [PubMed] [Google Scholar]
- 2.Carlo WA, Martin RJ, Fanaroff AA. Assisted ventilation and complications of respiratory distress. In: Fanaroff AA, Martin RJ, editors. Neonatal-perinatal medicine diseases of the fetus and infant. 7. Vol. 2. St. Louis, MO: Mosby; 2002. pp. 1011–25. [Google Scholar]
- 3.Jobe B. Bronchopulmonary dysplasia. Am J Respir Critl Care Med. 2001;163:1723–29. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 4.Donn SM, Sinha SK. Minimising ventilator induced lung injury in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2006;91:F226–30. doi: 10.1136/adc.2005.082271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ratner I, Whitfield J. Acquired subglottic stenosis in the very-low-birth-weight infant. Am J Dis Child. 1983;137:40–3. doi: 10.1001/archpedi.1983.02140270036013. [DOI] [PubMed] [Google Scholar]
- 6.Barrington KJ, Bull D, Finer NN. Randomized trial of nasal synchronized intermittent mandatory ventilation compared with continuous positive airway pressure after extubation of very low birth weight infants. Pediatrics. 2001;107:638–41. doi: 10.1542/peds.107.4.638. [DOI] [PubMed] [Google Scholar]
- 7.De Paoli AG, Davis PG, Lemyre B. Nasal continuous positive airway pressure versus nasal intermittent positive pressure ventilation for preterm neonates: a systematic review and meta-analysis. Acta Paediatrica. 2003;92:70–5. doi: 10.1111/j.1651-2227.2003.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 8.Khalaf MN, Brodsky N, Hurley J, Bhandari V. A prospective randomized, controlled trial comparing synchronized nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure as modes of extubation. Pediatrics. 2001;108:13–7. doi: 10.1542/peds.108.1.13. [DOI] [PubMed] [Google Scholar]
- 9.Santin R, Brodsky N, Bhandari V. A prospective observational pilot study of synchronized nasal intermittent positive pressure ventilation (SNIPPV) as a primary mode of ventilation in infants > or = 28 weeks with respiratory distress syndrome (RDS) J Perinatol. 2004;24:487–93. doi: 10.1038/sj.jp.7211131. [DOI] [PubMed] [Google Scholar]
- 10.Friedlich P, Lecart C, Posen R, Ramicone E, Chan L, Ramanathan R. A randomized trial of nasopharyngeal-synchronized intermittent mandatory ventilation versus nasopharyngeal continuous positive airway pressure in very low birth weight infants after extubation. J Perinat. 1999;19:413. doi: 10.1038/sj.jp.7200205. (Nature Publishing Group) [DOI] [PubMed] [Google Scholar]
- 11.Kiciman NM, Andréasson B, Bernstein G, Mannino FL, Rich W, Henderson C. Thoracoabdominal motion in newborns during ventilation delivered by endotracheal tube or nasal prongs. Pediatr Pulmonol. 1998;25:175–81. doi: 10.1002/(sici)1099-0496(199803)25:3<175::aid-ppul7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 12.Van Der Hoeven M, Brouwer E, Blanco CE. Nasal high frequency ventilation in neonates with moderate respiratory insufficiency. Arch Dis Child Fetal Neonatal Ed. 1998;79:F61–3. doi: 10.1136/fn.79.1.f61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoehn T, Krause MF. Effective elimination of carbon dioxide by nasopharyngeal high-frequency ventilation. Respir Med. 2000;94:1132–4. doi: 10.1053/rmed.2000.0889. [DOI] [PubMed] [Google Scholar]
- 14.Pillow JJ. High-frequency oscillatory ventilation: mechanisms of gas exchange and lung mechanics. Critical Care Medicine. 2005;33(Suppl 3):S135–41. doi: 10.1097/01.ccm.0000155789.52984.b7. [DOI] [PubMed] [Google Scholar]
- 15.Lee KS, Dunn MS, Fenwick M, Shennan AT. A comparison of underwater bubble continuous positive airway pressure with ventilator-derived continuous positive airway pressure in premature neonates ready for extubation. Biol Neonate. 1998;73:69–75. doi: 10.1159/000013962. [DOI] [PubMed] [Google Scholar]
- 16.Courtney SE, Durand DJ, Asselin JM, et al. High-frequency oscillatory ventilation versus conventional mechanical ventilation for very-low-birth-weight infants. [see comment] N Engl J Med. 2002;347:643–52. doi: 10.1056/NEJMoa012750. [DOI] [PubMed] [Google Scholar]
- 17.Henderson-Smart DJ, Bhuta T, Cools F, Offringa M. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. [update of Cochrane Database Syst Rev. 2003 (1):CD000104; PMID: 12535383] Cochrane Database Syst Rev. 2003:CD000104. doi: 10.1002/14651858.CD000104. [DOI] [PubMed] [Google Scholar]
- 18.Boros SJ, Reynolds JW. Prolonged apnea of prematurity: Treatment with continuous airway distending pressure delivered by nasopharyngeal tube. Clin Pediatr (Phila) 1976;15:123–34. doi: 10.1177/000992287601500203. [DOI] [PubMed] [Google Scholar]
- 19.Henke KG, Sullivan CE. Effects of high-frequency pressure waves applied to upper airway on respiration in central apnea. J Appl Physiol. 1992;73:1141–5. doi: 10.1152/jappl.1992.73.3.1141. [DOI] [PubMed] [Google Scholar]


