Abstract
RhoA is one of the more extensively studied members of the Rho family of small GTPase where it is most readily recognized for its contributions to actin-myosin contractility and stress fiber formation. Accordingly, RhoA function during cell migration has been relegated to the rear of the cell where it mediates retraction of the trailing edge. However, RhoA can also mediate membrane ruffling, lamellae formation and membrane blebbing, thus suggesting an active role in membrane protrusions at the leading edge. With the advent of fluorescence resonance energy transfer (FRET)-based Rho activity reporters, RhoA has been shown to be active at the leading edge of migrating cells where it precedes Rac and Cdc42 activation. These observations demonstrate a remarkable versatility to RhoA signaling, but how RhoA function can switch between contraction and protrusion has remained an enigma. This review highlights recent advances regarding how the cooperation of Rho effector Rhotekin and S100A4 suppresses stress fiber generation to permit RhoA-mediated lamellae formation.
Keywords: ROCK, breast, carcinoma, chemotaxis, fiber, growth factor, invasive growth, mDia, stress, tumor progression
Introduction
Rho family small GTPases mediate multiple aspects of tumor progression including cell transformation, cytokinesis, angiogenesis, extracellular matrix deposition and tumor cell dissemination. Rho GTPases belong to the Ras superfamily and consist of more than 20 members of 20–30 KDa GTP-binding proteins in mammals. Like Ras, Rho GTPases act as molecular switches by cycling from GTP bound active state to GDP bound inactive state. The cycling between these two states is positively controlled by guanine nucleotide-exchange factors (GEFs), and negatively regulated by its intrinsic GTPase activity, GTPase activating proteins (GAPs) and guanine nucleotide-dissociation inhibitors (GDIs).1-3 The major function of Rho small GTPases is the coordination of actin cytoskeleton reorganization in response to receptor activation (including growth factor, cytokine and adhesion receptors), which in turn regulates GEF and GAP activities.3,4
Most notably, the members of the Rho family of small GTPases are renowned for their contributions to actin cytoskeletal reorganization that drive cell motility and invasion. These concepts were brought to the forefront based on landmark findings by Ridley, Hall and colleagues in 1992 when they documented that Rac stimulated the formation of lamellae5 while RhoA mediated stress fiber formation.6 In the intervening two decades, our vision of Rac mediating lamellae formation and its importance to cell motility remains constant while many of the details of how these processes are regulated has been elucidated (reviewed in refs. 3 and 4). In contrast, the literature regarding RhoA’s role in the migration and invasion is more conflicting, perhaps due to the greater versatility to RhoA functions.
The Rho subgroup of Rho GTPases, including RhoA, RhoB and RhoC, share about 85% amino acid sequence identity where the primary differences are found in the C-terminal hypervariable region.3 Given that Rho proteins play important roles in cell migration, actin cytoskeleton reorganization, and focal adhesion, it is well accepted that Rho signaling should contribute to tumor invasion and metastasis. Indeed, RhoA and RhoC have been shown to be involved in different stages of tumor progression such as loss of apical-basal polarity and cell junctions, intravasation and vascularization.7 There is substantial evidence to support the involvement of aberrant expression of Rho, especially RhoC in the metastatic capacity of different types of cancers, such as breast, colon, prostate, lung, head and neck and pancreatic.7,8 In contrast, most studies suggest that RhoB acts as a tumor suppressor and is generally downregulated in cancers.3,8 RhoA and RhoC are equally capable of mediating stress fiber formation and generating contractile force needed for retraction of the trailing edge during migration. However, recent studies utilizing Rho activity biosensors suggest that RhoA is also activated at the leading edge of the migrating cells9,10 and, thus, validate several reports that demonstrate that RhoA functions in membrane ruffling and lamellae formation.9,11-13 Additionally, RhoA has been implicated in membrane blebbing, which has been implicated in amoeboid-like motility (reviewed in ref. 14). In light of these observations, our perceptions of the role of Rho GTPases in cell migration, tumor cell invasion and metastasis are changing.
This mini-review focuses on recent studies that shed light on how conditional signaling can influence the functional output of RhoA signaling. Specifically, we will discuss the mechanisms of how RhoA signaling, in conjunction with the Rho effector Rhotekin and the pro-metastatic calcium binding protein S100A4, can promote membrane protrusions such as lamellipodial ruffles in lieu of stress fibers. We will further discuss how these RhoA functions associate with cell migration and invasion in two-dimensional (2D) and three-dimensional (3D) environments.
RhoA in Cell Migration
The importance of Rho proteins in cell migration and invasion is now well established; however, it has not always been so. The role of RhoA in cell migration at one time was considered by many to be dispensable or inhibitory to cell migration. Several factors lead to the early conclusion. Primarily the observation that RhoA promotes stress fibers and strong adhesion through focal adhesion formation guided the path to this deduction (reviewed in refs. 4 and 15). There also exists a reciprocal relationship between RhoA and Rac1 in which high Rac activity leads to the reduction of Rho and vice versa.16,17 Since Rac is instrumental for lamellae formation and cellular protrusions it seemed logical that RhoA would be inhibitory to these processes. Finally, the involvement of p190RhoGAP in cell spreading and migration,18 as well as the induction of RhoC in the metastatic process3 cemented this concept that RhoA might be detrimental to, or at least dispensable for, cell migration and invasion.
However, many studies demonstrating a positive role for RhoA in migration prompted to the concept that Rac and RhoA were spatially separated during cell migration such that Rac was activated at the leading edge and RhoA was activated at the trailing edge.19 With the advent of FRET-based Rho GTPase activity biosensors, the hypotheses regarding the small GTPases in cell migration began to evolve. RhoA was found to be active at the leading edge of migrating cells. Importantly, the three major Rho small GTPases (Rac1, Cdc42 and RhoA) were all activated at the front of the migrating cells in a spatial and temporal manner, such that RhoA activation preceded that of Rac and Cdc42.20,21 These studies added validity to previous studies,9,11,12,22 including our own,13,23 that implicated RhoA in membrane ruffling and lamellae formation and, therefore, an important role in the protrusive events at the leading edge that drive cell motility.
How RhoA switches from stress fibers to lamellae formation is unclear. It is tempting to speculate that the choice of one effector controls this fate; however, both membrane ruffling and stress fiber formation are mediated through the Rho effectors ROCK and mammalian homolog of Drosophila diaphanous (mDia).24 To understand how this switch occurs, we will first discuss what we know about RhoA effectors and stress fiber formation.
Rho Effectors and Stress Fiber Formation
The effectors of small Rho GTPases comprise of a variety of proteins including lipid kinases, scaffold proteins, and serine/threonine kinases that can be classified into discrete classes based on how they bind the Rho binding domain.22,25 Although RhoA, RhoB and RhoC share overlapping effectors, whether the preference of each isoform for different effectors contributes to distinct effect on cell behavior has not been fully elucidated. Among these effectors, however, ROCK and mDia have been most extensively studied and their role in stress fiber formation well documented.
ROCK is a major mediator of Rho function. Inhibition of ROCK blocks the formation of most Rho-mediated actin cytoskeletal structures, including stress fibers. ROCK inactivates myosin phosphatase by phosphorylation of its myosin-binding subunit as well as direct phosphorylation and activation of myosin light chain. As a consequence, ROCK enhances actomyosin contractility.22 The resulting contraction on the actin filaments leads to the bundling of actin fibers and the clustering integrins into focal adhesions.15,26 However, constitutive activation of ROCK is insufficient to promote stress fiber formation, suggesting that ROCK is necessary but not sufficient. Notably, actin polymerization is also required. As shown in Figure 1, other effectors downstream of RhoA including phosphotidylinositide 4P-5 kinase (PI4P-5K) and mDia have been shown to stimulate actin polymerization.24,27,28 Furthermore, ROCK-mediated phosphorylation and activation of LIM kinase (LIMK) facilitates actin polymerization by stabilizing actin filaments by inactivating the actin severing functions of cofilin29 (Fig. 1). These observations support the cooperation of ROCK and mDia in stress fiber formation.
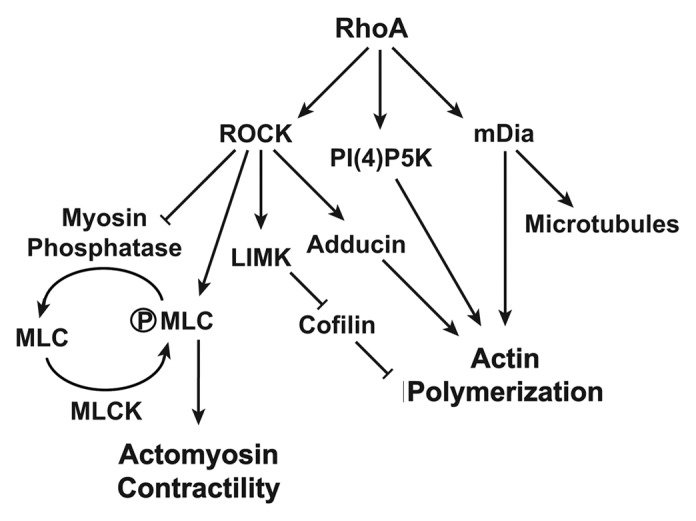
Figure 1. RhoA mediated pathways to actin polymerization and actomyosin contractility. RhoA facilitates actin polymerization by positively regulating multiple effectors and kinases (arrows) as well as through the negative regulation of cofilin by the ROCK-LIMK pathway (blunted lines). Through parallel pathways, the RhoA-ROCK pathway also leads to myosin mediated actin contraction by inhibition of myosin phosphate or through the direct phosphorylation of MLC.
During their formation, stress fibers lead to the generation of focal adhesions and, in the absence of adequate focal adhesion turnover, are associated with non-motile cells. Constitutive activation of RhoA has been demonstrated to negatively regulate cell migration due to excess stress fiber formation and adhesion forces.30,31 Inhibition of ROCK under conditions where RhoA activity is high or altering the ratio of ROCK to mDia can reduce stress fiber thickness and favor cell migration.15,22 These studies support the concept that the contractility downstream of RhoA activity must be tempered in order for membrane protrusions to dominate. Notably, most advanced carcinomas do not form true stress fibers, but rather thinner contractile filaments (which are often referred to as stress fibers for lack of a better term) that are conducive to cell migration. These observations suggest that advanced carcinoma cells acquire a mechanism to temper RhoA-ROCK mediated contractility to permit the protrusive events downstream to predominate.
RhoA Function at the Leading Edge
The membrane protrusions at the leading edge, including filopodia and lamellae, are well known to be regulated by Cdc42 and Rac, respectively. However, in cells with epithelial origin, RhoA is active in the leading edge, as shown by using fluorescence-based Rho biosensors,9,10,20 where it promotes membrane ruffling and facilitates cell motility.9,11-13 In 2000, we were the first to publish that RhoA could promote the formation of lamellae in the absense of Rac1. We showed that the engagement of the integrin α6β4 with laminin in Clone A colon carcinoma cells produced RhoA-dependent membrane ruffles and lamellae that were instrumental for haptotaxis of these cells.13 While quite heretical at the time, the concept that RhoA activity can localize at the leading edge to drive migration was validated using RhoA biosensors which demonstrated that RhoA activity localized to the leading edge of fibroblasts. Shortly thereafter, Kurokawa et al. found that RhoA is not only active in the leading edge but also in the rear of HeLa cells during random migration on collagen. They further demonstrated that RhoA activity persists in membrane ruffles upon growth factor stimulation in Cos1 and NIH3T3 cells and that RhoA activity was required for the induction of membrane ruffles.9
These concepts led to confusion regarding the timing of activation and the relationship among the small GTPases at the leading edge. To answer this question, collaborative efforts between the Hahn and Danuser labs assessed the activation of RhoA, Rac1 and Cdc42 in the same cells under growth factor stimulation. They found that the activation of RhoA synchronized with protrusions, was restricted to within 2 μm of the leading edge, and preceded the activation of Rac and Cdc42.20 This restriction and slight separation of Rac and Rho activities from each other helps to explain how integrin- and Rac-activated GAP activities could co-exist with RhoA at the leading edge. Furthermore, it highlights that RhoA activity at the leading edge must be delicately regulated by positive and negative regulators in order for RhoA to promote membrane protrusions.
How RhoA regulates two very different processes such as stress fibers and membrane ruffling is puzzling. A mechanism for switching these two functions must exist. As mentioned above, altering the ratio of ROCK to mDia can influence these processes. Additional mechanisms to regulate actin polymerization are present downstream of RhoA (Fig. 2). Notably, adducin phosphorylation by ROCK has been shown to be an instrumental aspect of the pro-ruffling features of ROCK signaling.11 However, these effectors offer more of a sliding scale than a discrete switch.

Figure 2. RhoA signaling toward actin polymerization and actomyosin contractility is delicately balanced. While RhoA signals to actin polymerization as well as myosin-mediated actin filament contraction, tipping this balance toward more actin polymerization facilitates membrane ruffling and lamellae formation, while higher contractile forces lead to stress fiber formation.
Work from our lab has shown that integrin α6β4 promotes membrane ruffling and lamellae formation in carcinoma cells, which are mediated by RhoA.13,23,32 Notably, other reports implicating RhoA in membrane ruffling came from cells of epithelial origin9,11,12 that also express integrin α6β4. The most dramatic example of this concept is seen in the MDA-MB-435 cells. In the absence of integrin α6β4 expression, these cells utilize RhoC for migration and do not form lamellae in response to LPA. However, integrin α6β4 signaling facilitates RhoA activation and RhoA-dependent membrane ruffles and lamellae with LPA stimulation, which in turn dramatically enhances cell migration and invasion.23,32 These observations suggest that integrin α6β4 may hold the key to how the function of RhoA is switched from stress fibers to lamellae formation. Through our transcriptome studies on integrin α6β4 in breast, we found that integrin α6β4 controls the expression of the pro-metastatic gene S100A4.33 In the next section, we discuss our recent finding that S100A4 binds the Rho effector Rhotekin to form a complex with RhoA, which in turns changes RhoA function to permit this GTPase to stimulate membrane ruffling in lieu of stress fibers.
Rhotekin and S100A4 Navigate the Switch
Rhotekin is a scaffold protein that was initially identified as a putative target for Rho that interacts with both RhoA and RhoC.34 The search for Rhotekin interacting proteins focused on the C-terminal domain since it contains a consensus binding motif for Class I PDZ proteins. Rhotekin was found to interact with vinexin, Lin7B, PIST and septin, which are considered to play roles in cell polarity, focal adhesion and septin organization.35-37 Rhotekin was also found to be overexpressed in metastatic colon cancer38 and gastric adenocarcinoma cells and confers resistance to apoptosis through activation of NF-κB.39 Further impact on transcription was shown through the interaction of Rhotekin and TIP-1 with active Rho which strongly activate SRE (serum response element).37 A recent study revealed that Rhotekin is a substrate of Protein kinase D (PKD). Although there is no physical interaction between Rhotekin and PKD, the authors found that PKD induced Rhotekin phosphorylation at serine 435. This phosphorylation event significantly increase membrane anchoring of RhoA as well as RhoA activity, thereby promoting actin stress fiber formation in NIH-3T3 cells.40 Despite these findings, the role of Rhotekin in Rho-mediated downstream signal transduction leading to actin cytoskeleton reorganization remained largely unknown. This may be because the domain of Rhotekin that influences the cytoskeleton is the Rho binding domain (RBD). Based on our recent serendipitous finding that S100A4 can bind the RBD of Rhotekin, we have uncovered a new function for Rhotekin that may help explain its role in tumor progression.
S100A4 is a calcium binding EF-hand protein that belongs to the S100 superfamily that contains at least 21 family members. It was cloned independently from various cell types under different names including metastasin-1 (mts1), CAPL and fibroblast specific protein (FSP1), 18A2, pEL98, p9Ka, 42A and calvasculin.41,42 S100A4 is associated with the progression of a variety of cancers, including breast, prostate, pancreatic, gallbladder, colon, gastric, lung and melanoma41-44 and has been considered as a valuable prognostic marker for several tumors including breast and colon.45,46 The role of S100A4 in tumor progression, and specifically on tumor metastasis, was also documented in several types of cancer by experimental metastasis and genetically-modified mouse models.41,42 Although S100A4 was initially identified as a fibroblast marker,47 investigations on S100A4 expression demonstrated that it is expressed in highly motile cell types, including T-lymphocytes, neutrophils, macrophages, platelets, endothelial cells, fibroblast and carcinoma cells.41,42,48 Notably, cell motility has been implicated as a major function controlled by S100A4.43 Intracellularly, S100A4 interacts with target proteins such as heavy chain of non-muscle myosin IIA (MHC-IIA),49 tropomyosin50 and liprin β1.51 Most notably, the interaction of S100A4 with myosin IIA heavy chain inhibits myosin IIA phosphorylation, promotes myosin disassembly and reduces the contractility of myosin; this well-defined feature of S100A4 represents a major mechanism of how S100A4 mediates cell motility and invasion.41,52,53
In a recent study from our group,54 we found that S100A4 specifically bound the RBD of Rhotekin, but not the RBDs of other class I effectors or critical Rho effectors such as ROCK or mDia. We further determined that S100A4 bound a region of the RBD distinct from where RhoA bound. This observation led to the discovery that active RhoA-Rhotekin and S100A4 could form a complex. Despite the proposed role of Rhotekin in maintaining Rho in an active conformation, we saw no changes in RhoA activity with Rhotekin and/or S100A4 knockdown (unpublished observation). Instead, we discovered a functional change in RhoA functional output. Using MDA-MB-231 stimulated with EGF as a model, we found that RNAi-mediated suppression of S100A4 and Rhotekin switched Rho from mediating membrane ruffling and lamellae to thick contractile stress fibers.
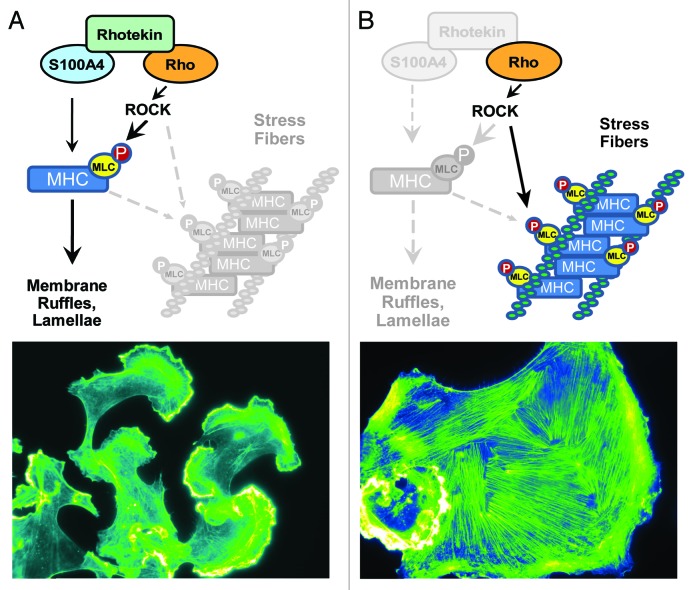
In Figure 3, we depict our working model of this concept of how S100A4 and Rhotekin cooperate to alter RhoA function. Central to this concept is the fact that S100A4 binds to the myosin IIA heavy chain to prevent its oligomerization and temper contractility. As shown in Figure 3A, we propose that when cells express both Rhotekin and S100A4, growth factor stimulation of Rho activity leads to the coupling of Rho to S100A4. Under these conditions, myosin II oligomerization is restricted within close proximity to active Rho, thus limiting stress fiber formation. The inhibition of myosin-mediated actin contractility then permits membrane ruffling and lamellae formation to predominate downstream of Rho effectors such as ROCK. In the absence of S100A4 and Rhotekin, RhoA activation and non-oligomeric myosin do not colocalize, therefore the contractility events downstream of RhoA signaling predominate leading to stress fiber formation (Fig. 3B).
Figure 3. Mechanisms of S100A4-Rhotekin-RhoA crosstalk in mediating membrane ruffling. (A) We propose that S100A4-mediated inhibition of myosin IIA heavy chain oligomerization limits the contractility of pMLC-myosin IIA complex. Under this condition, the actin polymerization functions of ROCK (shown here) and other effectors such as mDia (not shown) predominate, thus permitting the formation of lamellae. The lower panel depicts MDA-MB-231 cells stimulated with EGF for 5 min and then stained with phalloidin. (B) In the absence of S100A4 and Rhotekin, Rho/ROCK-mediated MLC phosphorylation in the presence of oligomers of myosin IIA facilitates the contractility required for stress fiber formation, while preventing membrane ruffles downstream of RhoA from forming. The lower panel represents an extreme phenotype of MDA-MB-231 cells with RNAi-mediated reduction of S100A4 and Rhotekin that were stimulated with EGF for 5 min and then stained with phalloidin.
Contractility that limits the rate of membrane ruffling and protrusions occurs beyond the lamellipodium into the lamella where myosin IIA mediates retrograde actin flow. Notably, the rate of cellular protrusions is inversely correlated with retrograde actin flow within lamellae such that blocking myosin IIA by siRNA or blebbistatin can increase membrane protrusions.55,56 If the modulation of actomyosin contractility by S100A4 extends beyond the lamellipodium (where RhoA is localized and signals) into the lamella (where MLCK has been shown to be more active57), it would suggest that that S100A4 could facilitate RhoA signaling and membrane protrusion by restricting retrograde flow within the lamella through the modulation of myosin IIA contractility regulated by other pathways. However, to determine if these mechanisms are in fact coupled and coordinated in such a manner will require further analysis.
RhoA in 3D Invasion
While RhoA has been shown to function in 2D migration systems, there are clearly conditions in which RhoA is dispensable for or inhibitory to cell migration. However, with the use of more physiological assessments of tumor cell invasion and 3D invasive growth, RhoA becomes much more influential. The mechanisms governing invasion of carcinoma cells in 3D and in vivo differ greatly from those in 2D culture. First and foremost, the tension supplied in 2D cultures comes from the glass or plastic support on which cells are plated. In 3D cultures and in vivo, the tension in the matrix must be supplied by the tumor cells themselves or from nearby stromal cells. Alignment of the collagen fibers found in the stroma is diagnostic for tumor aggressiveness58 and has been shown by the Condeelis and Segall groups to facilitate tumor cell migration along these filaments in vivo.59 The concept that Rho contributes to tension within the tumor microenvironment has been validated by Provensano and Keely where they elegantly showed that Rho signaling in this context to be a major contributor to tumor aggressiveness.60-62
Certainly the role of Rho proteins in 3D invasive growth is more complex than the tension placed on the extracellular matrices. In our study,54 we found that simultaneous reduction of Rhotekin and S100A4 led to the collapse of invasive structures thus limiting cells to the formation of acinar structures in 3D breast carcinoma model. If our hypotheses are correct, cells without S100A4 and Rhotekin would exert greater tension on the matrix, yet still do not demonstrate invasive growth. Clearly the concept of balancing protrusive events and contractility remains relevant to the 3D environment. Perhaps in the absence of protrusion-promoting signals, the default is to form tighter cell:cell adhesion and an acinar structure. To fully understand the role of Rho proteins in 3D invasive growth and the invasive process in vivo, our concepts must evolve as we improve our understanding of how cells interact with their microenvironment under more physiological conditions.
RhoA Cooperation with Other Small GTPases
There is still much to be deciphered regarding how RhoA promotes membrane ruffling. Despite the abilities of RhoA to stimulate actin polymerization through multiple effectors, it rarely works alone in this process. In many cell types, either Rac or Cdc42 is activated in conjunction with RhoA.9,21,23 Both Rac and Cdc42 signal through Pak1 to stimulate LIMK, which then phosphorylates and inactivates cofilin to prevent cleavage of actin fibers. This represents a convergence point with RhoA-ROCK pathway that facilitates F-actin polymerization. However, Rac and Cdc42 both signal through either WAVE or WASP proteins to stimulate the Arp2/3 complex, a process not recognized as a Rho function, which may be necessary for actin branching during lamellae formation. Alternatively, Rac and Cdc42 have been suggested to recruit mDia to Rho,9 thus facilitating lamellae formation.
The studies to date on Rho in membrane ruffling and lamellae formation have implicated RhoA. However, is it possible that RhoA and RhoC could share functions in these processes or potentially swap roles under select conditions? RhoA and RhoC share high homology and activate many of the same effectors, including ROCK, mDia and Rhotekin.22,34 While the absolute affinities of each of these shared effectors for the individual GTPases has not been systematically assessed, it is likely that subtle differences in affinities could affect effector choice. Alternatively, GTPase localization through its hypervariable region or select activation by specific GEFs could ultimately govern the individual function of the two GTPases. Considering the evidence for RhoA at the leading edge, it is possible that RhoA functions in membrane ruffling and lamellae formation while RhoC functions in the cell body to mediate actin cytoskeletal contraction and trailing edge retraction. However, in a recent study by Anne Ridley’s group, RhoC was found to specifically bind FMNL3, which may help to define differences between RhoA and RhoC functions during carcinoma cell migration. In that study, they suggest that RhoA functions at the leading edge to mediate membrane ruffling while RhoC contracts the base of the lamellae to prevent lamellae broadening and loss of orientation.63 This study is an important example of the cooperation of RhoA and RhoC in cell migration. How RhoA and RhoC parcel out their duties during tumor invasion and how these functions change in a 3D environment will require further investigation.
Concluding Remarks
Tumor invasive growth is a complex, multistep program involved in the interplay of tumor cells and the microenvironment, and in turn tumor cells acquire the propensity for migration, invasion and proliferation.64 Rho signaling is engaged in at least two distinct types of motility in three-dimensional matrix, amoeboid motility and mesenchymal motility.65 Interestingly, these two types of migration are interchangeable. Beyond this versatility, we highlight new pathways involving the Rho effector Rhotekin and the metastasis associated S100A4 that direct Rho signaling from migration-inhibiting stress fibers to migration- and invasion-promoting lamellipodial ruffles and lamellae. These observations highlight the amazing dynamics of RhoA signaling, which dramatically impacts how we view RhoA signaling during cancer invasion and ultimately the metastatic process. Furthermore, these studies demonstrate how contractility functions of RhoA can be tempered to favor actin polymerization; and that the protrusive functions of RhoA are as critical for tumor progression as its impact on cellular traction, matrix tension, and actin contractility.
Acknowledgments
This work was funded by NIH R01-CA109136.
Glossary
Abbreviations:
- 2D
two-dimensional
- 3D
three-dimensional
- FRET
fluorescence resonance energy transfer
- GAP
GTPase activating proteins
- GDIs
guanine nucleotide-dissociation inhibitors
- GEFs
guanine nucleotide-exchange factors
- MHC-IIA
heavy chain of non-muscle myosin IIA
- RBD
Rho binding domain
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/smallgtpases/article/25131
References
- 1.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 2.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–14. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 3.Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093–101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 4.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–14. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 5.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–10. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 6.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–99. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 7.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–42. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 8.Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta 2009; 1796:91-8. [DOI] [PubMed]
- 9.Kurokawa K, Matsuda M. Localized RhoA activation as a requirement for the induction of membrane ruffling. Mol Biol Cell. 2005;16:4294–303. doi: 10.1091/mbc.E04-12-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–72. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 11.Fukata Y, Oshiro N, Kinoshita N, Kawano Y, Matsuoka Y, Bennett V, et al. Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility. J Cell Biol. 1999;145:347–61. doi: 10.1083/jcb.145.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishiyama T, Sasaki T, Takaishi K, Kato M, Yaku H, Araki K, et al. rac p21 is involved in insulin-induced membrane ruffling and rho p21 is involved in hepatocyte growth factor- and 12-0-tetradecanoylphorbol-13-acetate (TPA)-induced membrane ruffling in KB cells. Mol Cell Biol. 1994;14:2247–456. doi: 10.1128/MCB.14.4.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connor KL, Nguyen B-K, Mercurio AM. RhoA function in lamellae formation and migration is regulated by the α6β4 integrin and cAMP metabolism. J Cell Biol. 2000;148:253–8. doi: 10.1083/jcb.148.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charras G, Paluch E. Blebs lead the way: how to migrate without lamellipodia. Nat Rev Mol Cell Biol. 2008;9:730–6. doi: 10.1038/nrm2453. [DOI] [PubMed] [Google Scholar]
- 15.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–79. doi: 10.1016/S0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 16.Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–22. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zondag GCM, Evers EE, ten Klooster JP, Janssen L, van der Kammen RA, Collard JG. Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity and epithelial-mesenchymal transition. J Cell Biol. 2000;149:775–82. doi: 10.1083/jcb.149.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arthur WT, Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol Biol Cell. 2001;12:2711–20. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spiering D, Hodgson L. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adh Migr. 2011;5:170–80. doi: 10.4161/cam.5.2.14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, et al. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Sibai M, Pertz O, Pang H, Yip SC, Lorenz M, Symons M, et al. RhoA/ROCK-mediated switching between Cdc42- and Rac1-dependent protrusion in MTLn3 carcinoma cells. Exp Cell Res. 2008;314:1540–52. doi: 10.1016/j.yexcr.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 23.O’Connor KL, Chen M, Towers LN. Integrin α6β4 cooperates with LPA signaling to stimulate Rac through AKAP-Lbc-mediated RhoA activation. Am J Physiol Cell Physiol. 2012;302:C605–14. doi: 10.1152/ajpcell.00095.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 25.Bustelo XR, Sauzeau V, Berenjeno IM. GTP-binding proteins of the Rho/Rac family: regulation, effectors and functions in vivo. Bioessays. 2007;29:356–70. doi: 10.1002/bies.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–15. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren XD, Bokoch GM, Traynor-Kaplan A, Jenkins GH, Anderson RA, Schwartz MA. Physical association of the small GTPase Rho with a 68-kDa phosphatidylinositol 4-phosphate 5-kinase in Swiss 3T3 cells. Mol Biol Cell. 1996;7:435–42. doi: 10.1091/mbc.7.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilmore AP, Burridge K. Regulation of vinculin binding to talin and actin by phosphatidyl-inositol-4-5-bisphosphate. Nature. 1996;381:531–5. doi: 10.1038/381531a0. [DOI] [PubMed] [Google Scholar]
- 29.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348:241–55. doi: 10.1042/0264-6021:3480241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004;18:862–76. doi: 10.1101/gad.1185504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahai E, Olson MF, Marshall CJ. Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J. 2001;20:755–66. doi: 10.1093/emboj/20.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Connor KL, Shaw LM, Mercurio AM. Release of cAMP gating by the α6β4 integrin stimulates lamellae formation and the chemotactic migration of invasive carcinoma cells. J Cell Biol. 1998;143:1749–60. doi: 10.1083/jcb.143.6.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen M, Sinha M, Luxon BA, Bresnick AR, O’Connor KL. Integrin α6β4 controls the expression of genes associated with cell motility, invasion, and metastasis, including S100A4/metastasin. J Biol Chem. 2009;284:1484–94. doi: 10.1074/jbc.M803997200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reid T, Furuyashiki T, Ishizaki T, Watanabe G, Watanabe N, Fujisawa K, et al. Rhotekin, a new putative target for Rho bearing homology to a serine/threonine kinase, PKN, and rhophilin in the rho-binding domain. J Biol Chem. 1996;271:13556–60. doi: 10.1074/jbc.271.23.13556. [DOI] [PubMed] [Google Scholar]
- 35.Nagata K, Ito H, Iwamoto I, Morishita R, Asano T. Interaction of a multi-domain adaptor protein, vinexin, with a Rho-effector, Rhotekin. Med Mol Morphol. 2009;42:9–15. doi: 10.1007/s00795-008-0433-8. [DOI] [PubMed] [Google Scholar]
- 36.Sudo K, Ito H, Iwamoto I, Morishita R, Asano T, Nagata K. Identification of a cell polarity-related protein, Lin-7B, as a binding partner for a Rho effector, Rhotekin, and their possible interaction in neurons. Neurosci Res. 2006;56:347–55. doi: 10.1016/j.neures.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Ito H, Iwamoto I, Morishita R, Nozawa Y, Asano T, Nagata K. Identification of a PDZ protein, PIST, as a binding partner for Rho effector Rhotekin: biochemical and cell-biological characterization of Rhotekin-PIST interaction. Biochem J. 2006;397:389–98. doi: 10.1042/BJ20052015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ying-Tao Z, Yi-Ping G, Lu-Sheng S, Yi-Li W. Proteomic analysis of differentially expressed proteins between metastatic and non-metastatic human colorectal carcinoma cell lines. Eur J Gastroenterol Hepatol. 2005;17:725–32. doi: 10.1097/00042737-200507000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Liu CA, Wang MJ, Chi CW, Wu CW, Chen JY. Rho/Rhotekin-mediated NF-kappaB activation confers resistance to apoptosis. Oncogene. 2004;23:8731–42. doi: 10.1038/sj.onc.1208106. [DOI] [PubMed] [Google Scholar]
- 40.Pusapati GV, Eiseler T, Rykx A, Vandoninck S, Derua R, Waelkens E, et al. Protein Kinase D regulates RhoA activity via rhotekin phosphorylation. J Biol Chem. 2012;287:9473–83. doi: 10.1074/jbc.M112.339564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garrett SC, Varney KM, Weber DJ, Bresnick AR. S100A4, a mediator of metastasis. J Biol Chem. 2006;281:677–80. doi: 10.1074/jbc.R500017200. [DOI] [PubMed] [Google Scholar]
- 42.Boye K, Maelandsmo GM. S100A4 and metastasis: a small actor playing many roles. Am J Pathol. 2010;176:528–35. doi: 10.2353/ajpath.2010.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helfman DM, Kim EJ, Lukanidin E, Grigorian M. The metastasis associated protein S100A4: role in tumour progression and metastasis. Br J Cancer. 2005;92:1955–8. doi: 10.1038/sj.bjc.6602613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saleem M, Adhami VM, Ahmad N, Gupta S, Mukhtar H. Prognostic significance of metastasis-associated protein S100A4 (Mts1) in prostate cancer progression and chemoprevention regimens in an autochthonous mouse model. Clin Cancer Res. 2005;11:147–53. [PubMed] [Google Scholar]
- 45.Rudland PS, Platt-Higgins A, Renshaw C, West CR, Winstanley JH, Robertson L, et al. Prognostic significance of the metastasis-inducing protein S100A4 (p9Ka) in human breast cancer. Cancer Res. 2000;60:1595–603. [PubMed] [Google Scholar]
- 46.Gongoll S, Peters G, Mengel M, Piso P, Klempnauer J, Kreipe H, et al. Prognostic significance of calcium-binding protein S100A4 in colorectal cancer. Gastroenterology. 2002;123:1478–84. doi: 10.1053/gast.2002.36606. [DOI] [PubMed] [Google Scholar]
- 47.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, et al. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Semov A, Moreno MJ, Onichtchenko A, Abulrob A, Ball M, Ekiel I, et al. Metastasis-associated protein S100A4 induces angiogenesis through interaction with Annexin II and accelerated plasmin formation. J Biol Chem. 2005;280:20833–41. doi: 10.1074/jbc.M412653200. [DOI] [PubMed] [Google Scholar]
- 49.Li ZH, Bresnick AR. The S100A4 metastasis factor regulates cellular motility via a direct interaction with myosin-IIA. Cancer Res. 2006;66:5173–80. doi: 10.1158/0008-5472.CAN-05-3087. [DOI] [PubMed] [Google Scholar]
- 50.Takenaga K, Nakamura Y, Sakiyama S, Hasegawa Y, Sato K, Endo H. Binding of pEL98 protein, an S100-related calcium-binding protein, to nonmuscle tropomyosin. J Cell Biol. 1994;124:757–68. doi: 10.1083/jcb.124.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kriajevska M, Fischer-Larsen M, Moertz E, Vorm O, Tulchinsky E, Grigorian M, et al. Liprin beta 1, a member of the family of LAR transmembrane tyrosine phosphatase-interacting proteins, is a new target for the metastasis-associated protein S100A4 (Mts1) J Biol Chem. 2002;277:5229–35. doi: 10.1074/jbc.M110976200. [DOI] [PubMed] [Google Scholar]
- 52.Dulyaninova NG, Malashkevich VN, Almo SC, Bresnick AR. Regulation of myosin-IIA assembly and Mts1 binding by heavy chain phosphorylation. Biochemistry. 2005;44:6867–76. doi: 10.1021/bi0500776. [DOI] [PubMed] [Google Scholar]
- 53.Tarabykina S, Griffiths TR, Tulchinsky E, Mellon JK, Bronstein IB, Kriajevska M. Metastasis-associated protein S100A4: spotlight on its role in cell migration. Curr Cancer Drug Targets. 2007;7:217–28. doi: 10.2174/156800907780618329. [DOI] [PubMed] [Google Scholar]
- 54.Chen M, Bresnick AR, O'Connor KL. Coupling S100A4 to rhotekin alters Rho signaling output in breast cancer cells. Oncogene. 2012 doi: 10.1038/onc.2012.383. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shih W, Yamada S. Myosin IIA dependent retrograde flow drives 3D cell migration. Biophys J. 2010;98:L29–31. doi: 10.1016/j.bpj.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim JI, Sabouri-Ghomi M, Machacek M, Waterman CM, Danuser G. Protrusion and actin assembly are coupled to the organization of lamellar contractile structures. Exp Cell Res. 2010;316:2027–41. doi: 10.1016/j.yexcr.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chew T-L, Wolf WA, Gallagher PJ, Matsumura F, Chisholm RL. A fluorescent resonant energy transfer-based biosensor reveals transient and regional myosin light chain kinase activation in lamella and cleavage furrows. J Cell Biol. 2002;156:543–53. doi: 10.1083/jcb.200110161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am J Pathol. 2011;178:1221–32. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat Rev Cancer. 2003;3:921–30. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 60.Provenzano PP, Keely PJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J Cell Sci. 2011;124:1195–205. doi: 10.1242/jcs.067009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Provenzano PP, Inman DR, Eliceiri KW, Trier SM, Keely PJ. Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophys J. 2008;95:5374–84. doi: 10.1529/biophysj.108.133116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Provenzano PP, Eliceiri KW, Keely PJ. Shining new light on 3D cell motility and the metastatic process. Trends Cell Biol. 2009;19:638–48. doi: 10.1016/j.tcb.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vega FM, Fruhwirth G, Ng T, Ridley AJ. RhoA and RhoC have distinct roles in migration and invasion by acting through different targets. J Cell Biol. 2011;193:655–65. doi: 10.1083/jcb.201011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trusolino L, Comoglio PM. Scatter-factor and semaphorin receptors: cell signalling for invasive growth. Nat Rev Cancer. 2002;2:289–300. doi: 10.1038/nrc779. [DOI] [PubMed] [Google Scholar]
- 65.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–9. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]