Abstract
Understanding how glucose transporter isoform 4 (GLUT4) redistributes to the plasma membrane during insulin stimulation is a major goal of glucose transporter research. GLUT4 molecules normally reside in numerous intracellular compartments, including specialized storage vesicles and early/recycling endosomes. It is unclear how these diverse compartments respond to insulin stimulation to deliver GLUT4 molecules to the plasma membrane. For example, do they fuse with each other first or remain as separate compartments with different trafficking characteristics? Our recent live cell imaging studies are helping to clarify these issues. Using Rab proteins as specific markers to distinguish between storage vesicles and endosomes containing GLUT4, we demonstrate that it is primarily internal GLUT4 storage vesicles (GSVs) marked by Rab10 that approach and fuse at the plasma membrane and GSVs don’t interact with endosomes on their way to the plasma membrane. These new findings add strong support to the model that GSV release from intracellular retention plays a major role in supplying GLUT4 molecules onto the PM under insulin stimulation.
Keywords: GLUT4, IRAP, Rab10, Rab14, AS160, adipocytes, insulin, TIRF
Insulin stimulates glucose uptake into adipocytes and muscle tissues by recruiting GLUT4 molecules from intracellular sites to the plasma membrane (PM).1-3 In the absence of insulin stimulation, the majority of GLUT4 molecules are stored in small intracellular vesicles referred to as GLUT4 storage vesicles (GSVs).4-6 Following insulin secretion from the pancreas after a meal, insulin receptors on the surface of muscle cells and adipocytes are engaged by insulin. This sets off a signaling cascade involving PI3K, AKT/PKB, AS160,7-9 and Rab proteins10-13 that leads to GLUT4 redistribution from GSVs to the PM. Consequently, levels of GLUT4 molecules at the PM rise by ~30 fold.14,15
Understanding the precise membrane trafficking steps that underlie this dramatic buildup of GLUT4 proteins on the PM under insulin stimulation has been challenging. This is because GLUT4 molecules don’t only reside in GSVs.16-18 GLUT4 antibody uptake assays have shown that GLUT4 proteins continuously recycle through early and recycling endosomes.19 Because GLUT4 resides in both endosomes and GSVs, the pathway by which GLUT4 molecules redistribute from GSVs to the PM during insulin stimulation could be direct or indirect. That is, GLUT4 proteins could be delivered to the PM by direct fusion of GSVs with the PM, or by an indirect pathway involving initial fusion of GSVs with endosomes followed by later fusion of endosomes with the PM.2,20
Previous live cell imaging experiments attempting to distinguish between these models employed total internal reflection (TIRF) microscopy and a GLUT4-EGFP probe to visualize vesicles in close proximity to the PM. Hundreds of GLUT4-GFP-containing vesicles close to the PM were observed in both insulin-stimulated and non-stimulated cells.21-24 Indeed, the number of GLUT4-GFP vesicles visualized did not change before or during insulin treatment.21 Whenever a fraction of GLUT4-GFP vesicles fused with the PM, more vesicles moved into the TIRF zone to effectively replace them. Because the sizes of all the vesicles were below the diffraction limit of fluorescence microscopy, it was difficult to determine whether any vesicle that fused with the PM was a GSV or endosomal vesicle.25,26 Without probes to discriminate GSVs from endosomal compartments, therefore, addressing whether insulin-stimulated GLUT4 redistribution to the PM occurs by a direct or indirect route is unfeasible.
One group of markers capable of distinguishing GSVs from endosomes is the set of Rab proteins. These small GTPases function to modulate the surface characteristics of different subcellular organelles and help to define organelle identity.27,28 By determining which Rab proteins associate with GSVs and which with GLUT4-positive endosomal compartments, we reasoned it should be possible to distinguish between GSVs and endosomes in TIRF imaging experiments, and thereby address whether insulin-induced arrival of GLUT4 at the PM occurs by a direct or indirect route.
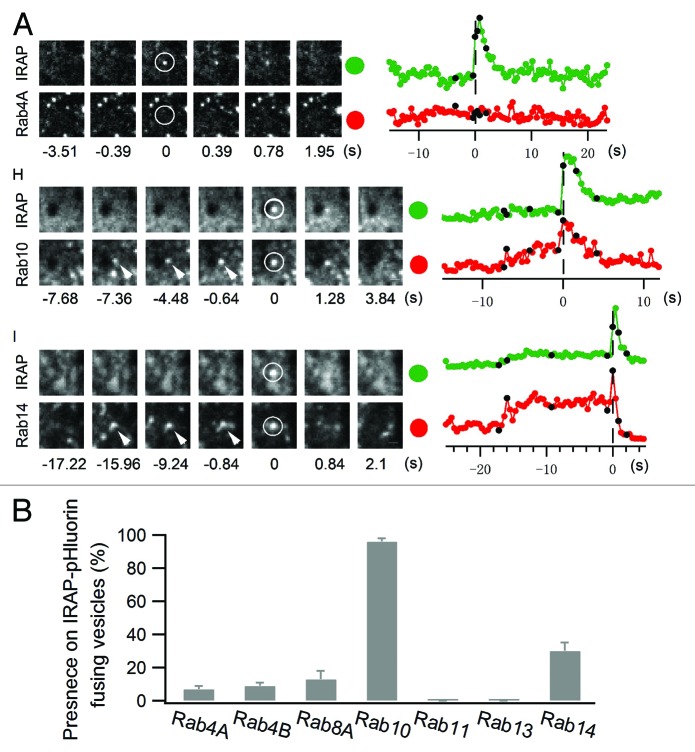
Toward this goal, 25 candidate Rab proteins were screened for their co-localization with GLUT4-containing vesicles close to the PM and their ability to fuse with the PM during insulin stimulation.29 To monitor GLUT4 vesicle fusion with the PM, we expressed the insulin responsive aminopeptidase (IRAP, which always co-localizes with GLUT4) tagged with pHluorin (IRAP-pHluorin).30 Because pHluorin produces a bright flash of light when it shifts from acidic to neutral pH,31 acidic intracellular vesicles containing IRAP-pHluorin could be visualized as they fused at the PM and became exposed to neutral pH. Screening 25 Rab protein family members using IRAP-pHluorin, we found that both Rab 10 and 14 were associated with IRAP-pHluorin vesicles that underwent fusion at the PM in response to insulin treatment (Fig. 1). Moreover, Rab10 vesicles showed little overlap with Rab14 vesicles and vice versa, suggesting each Rab protein was associated with a different subcellular compartment.29
Figure 1. Rab10 and Rab14 label exocytic GLUT4 vesicles. Rab proteins tagged with TagRFP were separately transfected into adipocytes along with IRAP-pHluorin. (A) IRAP-pHluorin fusion events were monitored using dual-color TIRF microscopy 3 min after insulin stimulation for the presence of a particular Rab protein on the fusing vesicles. Fusion site intensities were measured from both channels and plotted to the right. Black dots on the intensity traces indicate the time points at which image frames to the left are extracted. Scale bar: 0.5 µm. (B) Summary of the presence of different Rab proteins on IRAP-pHluorin fusing vesicles.
We next addressed whether Rab10 and Rab14 vesicles were positive for transferrin receptor (TfR, a receptor that constitutively cycles through the endosomal system). Nearly all Rab14 vesicles contained TfR, whereas virtually no Rab10-containing vesicles had TfR. These results indicated that Rab14 marked GLUT4-containing endosomes, while Rab10 marked GSVs.32 Because most of the GLUT4-containing vesicles that fused with the PM during insulin stimulation were Rab10 positive, we concluded that GSVs (marked by Rab10) were the primary vehicle by which GLUT4 was delivered to the PM during insulin stimulation. Moreover, since Rab10 vesicles did not fuse with other vesicles prior to PM fusion, the direct trafficking model for insulin-stimulated GLUT4 redistribution to the cell surface was supported.
To verify the role of Rab10 and Rab14 in delivery of GLUT4 to the PM under insulin stimulation, we knocked them down using siRNA technology. Rab10 knockdown significantly reduced insulin-stimulated GLUT4 translocation to the PM. This further confirmed that Rab10 activity on GSVs facilitates direct trafficking of GSVs to and fusion with the PM during insulin stimulation. Rab14 knockdown, by contrast, only modestly inhibited GLUT4 translocation. This provided evidence against the indirect model involving merging of GSVs and endosomes followed by endosome-PM fusion since Rab14 knockdown would be expected to have a major inhibitory effect in this scenario. When both Rab10 and Rab14 were knocked down, we observed an additive inhibitory effect on GLUT4 translocation. This raised the possibility that GSV-PM fusion (facilitated by Rab10) and endosome-PM fusion (facilitated by Rab14) independently contribute to insulin-stimulated delivery of GLUT4 to the PM.29
Identification of Rab10 as a GSV marker and Rab14 as a GLUT4-containing endosome marker has been a critical step in understanding insulin-stimulated trafficking pathway(s) for GLUT4 translocation to the PM. Previously, proteins used to study GLUT4 trafficking were either other cargo molecules that shared an identical trafficking itinerary with GLUT4 (e.g., IRAP)33 or didn’t solely mark GSVs (e.g., VAMP2 and sortilin).34,35 Given our finding that Rab10 activity is necessary for delivering GSVs to the PM after insulin stimulation, Rab10 would appear to be a unique marker among all other GLUT4 compartmental probes for highlighting GSVs poised to fuse with the PM.
Using Rab10 as a specific GSV marker, we explored in detail the identities and activities of GLUT4-containing compartments in the TIRF zone. In the absence of insulin stimulation, Rab10-labeled GSVs were barely observable in the TIRF zone. This suggested that GSVs are sequestrated internally, away from the cell periphery, in the basal state. Rab4A and Rab4B, 2 endocytic Rab proteins,28 co-localized extensively with GLUT4 in the TIRF zone under these conditions. This implied GLUT4 constitutively recycles through endocytic structures close to the PM in the basal state. After insulin stimulation, Rab10-labeled GSVs entered into the TIRF zone and fused at the PM. Fusion of Rab10-labeled GSVs at the PM was very efficient once GSVs moved into the TIRF zone. Indeed, GSV dwell time in the TIRF zone was extremely short compared with that of endosomal GLUT4-containing compartments. This helps explain why GSVs comprise only a small fraction of the total GLUT4 compartments in the TIRF zone both before and after insulin stimulation, and why GLUT4 vesicle density in the TIRF zone barely changes under these conditions.
While the majority of the GLUT4 molecules arriving at the PM under insulin stimulation did so as a result of GSV-PM fusion events, our data also suggested a contribution from endosome-PM fusion events, in particular, PM fusion of Rab14-positive, GLUT4-containing endosomal vesicles. This pathway might be important to maintain GLUT4 at the PM after GSV stores of GLUT4 are depleted during insulin stimulation,25 in addition to recycling GLUT4 back into GSVs as proposed by other groups.36,37 By being able to access GLUT4 molecules that recycle through endosomes, the cell would be able to maintain its GLUT4 PM pool during insulin stimulation even without GSVs.25
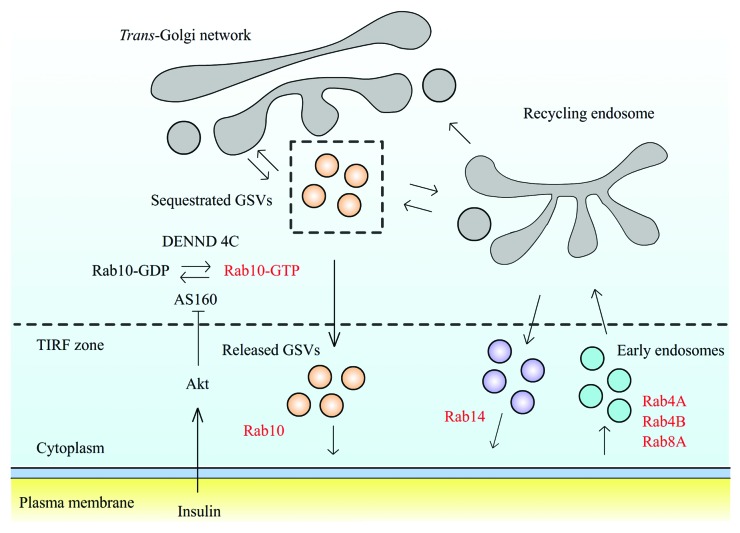
The above results suggest GLUT4 proteins during insulin stimulation are primarily delivered to the PM by direct fusion of GSVs with the PM. Rab14-containing endosomal vesicles also help redistribute GLUT4 onto the PM but the major pathway is through direct GSV-PM fusion. What seems to occur is that Rab10-containing GSVs are released from intracellular sequestration upon insulin stimulation and this enables them to move in a directed fashion to the cell periphery.29 The emerging consensus in favor of this direct trafficking model20 has led to interest in its molecular mechanisms (Fig. 2). The Rab10 GAP protein, AS160, has been suggested to negatively regulate GSV mobilization. In this view, AS160 functions in the absence of insulin stimulation as an active GAP causing Rab10 to be in its GDP-binding inactive state.8,38 Upon insulin stimulation, AS160 phosphorylation by Akt turns off AS160’s GAP domain, allowing GEF Dennd4c to convert Rab10 to its GTP-binding active state.10,39 Rab10 in the active state attaches to GSVs and recruits downstream effectors, including kinesin40,41 and myosin motors,14,42 to physically translocate GSVs close to the PM. This scenario is supported by our observations that Rab10 is already associated with GSVs before they move into the TIRF zone and that a constitutively active Rab10 mutant induces GLUT4 accumulation at the PM in the absence of insulin stimulation.29 An additional GSV sequestration mechanism may involve TUG (a putative tethering factor for GLUT4 that contains a ubiquitin-like UBX domain).43-45 TUG has been shown to directly interact with GLUT4 molecules and could retain GSVs deep in the cell in the absence of insulin.43 When TUG is cleaved upon insulin stimulation, GSVs are released and become ready to be mobilized.45 Activated Rab10 then attaches to the GSVs and facilitates their delivery to the PM.
Figure 2. Schematic model of GLUT4 trafficking in adipocytes. Insulin stimulation inhibits AS160 GAP activity, resulting in Rab10 shifting to GTP-bound active state. Rab10 activation releases intracellularly retained GLUT4 storage vesicles (GSVs) to the cell periphery, where they dock and fuse at the cell membrane. Meanwhile, insulin stimulation promotes Rab14-associated endosomal compartments to fuse at the cell membrane, which functions as a parallel pathway to deliver GLUT4 to the PM. At the cell membrane, GLUT4 molecules are endocytosed and travel through a variety of endosomal compartments labeled by multiple Rab proteins before being reloaded into GSVs.
In conclusion, live cell imaging studies using different Rab proteins have been successful at dissecting the complex trafficking routes followed by GLUT4 molecules under insulin stimulation. What is now needed is a better understanding of the specific roles these Rab proteins play in controlling GLUT4 trafficking. It is clear that Rab10 is necessary for GSV translocation to the PM, but is this GTPase also involved in GSV biogenesis and/or GSV fusion with the PM? Likewise, because Rab4 predominantly localizes at steady-state with GLUT4 vesicle adjacent to the PM, could its function be to retrieve GLUT4 off the PM and/or to help store GLUT4? These and other questions related to Rabs and GLUT4 trafficking now are ripe for addressing.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/smallgtpases/article/26471
References
- 1.Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3:267–77. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- 2.Foley K, Boguslavsky S, Klip A. Endocytosis, recycling, and regulated exocytosis of glucose transporter 4. Biochemistry. 2011;50:3048–61. doi: 10.1021/bi2000356. [DOI] [PubMed] [Google Scholar]
- 3.Watson RT, Kanzaki M, Pessin JE. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr Rev. 2004;25:177–204. doi: 10.1210/er.2003-0011. [DOI] [PubMed] [Google Scholar]
- 4.Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. 2012;13:383–96. doi: 10.1038/nrm3351. [DOI] [PubMed] [Google Scholar]
- 5.Stöckli J, Fazakerley DJ, James DE. GLUT4 exocytosis. J Cell Sci. 2011;124:4147–59. doi: 10.1242/jcs.097063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogan JS. Regulation of glucose transporter translocation in health and diabetes. Annu Rev Biochem. 2012;81:507–32. doi: 10.1146/annurev-biochem-060109-094246. [DOI] [PubMed] [Google Scholar]
- 7.Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, Lienhard GE. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem. 2002;277:22115–8. doi: 10.1074/jbc.C200198200. [DOI] [PubMed] [Google Scholar]
- 8.Sano H, Kane S, Sano E, Mîinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 9.Larance M, Ramm G, Stöckli J, van Dam EM, Winata S, Wasinger V, Simpson F, Graham M, Junutula JR, Guilhaus M, et al. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J Biol Chem. 2005;280:37803–13. doi: 10.1074/jbc.M503897200. [DOI] [PubMed] [Google Scholar]
- 10.Sano H, Eguez L, Teruel MN, Fukuda M, Chuang TD, Chavez JA, Lienhard GE, McGraw TE. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 2007;5:293–303. doi: 10.1016/j.cmet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Sano H, Roach WG, Peck GR, Fukuda M, Lienhard GE. Rab10 in insulin-stimulated GLUT4 translocation. Biochem J. 2008;411:89–95. doi: 10.1042/BJ20071318. [DOI] [PubMed] [Google Scholar]
- 12.Mîinea CP, Sano H, Kane S, Sano E, Fukuda M, Peränen J, Lane WS, Lienhard GE. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J. 2005;391:87–93. doi: 10.1042/BJ20050887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, Bilan PJ, Liu Z, Klip A. Rab8A and Rab13 are activated by insulin and regulate GLUT4 translocation in muscle cells. Proc Natl Acad Sci U S A. 2010;107:19909–14. doi: 10.1073/pnas.1009523107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen XW, Leto D, Chiang SH, Wang Q, Saltiel AR. Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev Cell. 2007;13:391–404. doi: 10.1016/j.devcel.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Muretta JM, Romenskaia I, Mastick CC. Insulin releases Glut4 from static storage compartments into cycling endosomes and increases the rate constant for Glut4 exocytosis. J Biol Chem. 2008;283:311–23. doi: 10.1074/jbc.M705756200. [DOI] [PubMed] [Google Scholar]
- 16.Livingstone C, James DE, Rice JE, Hanpeter D, Gould GW. Compartment ablation analysis of the insulin-responsive glucose transporter (GLUT4) in 3T3-L1 adipocytes. Biochem J. 1996;315:487–95. doi: 10.1042/bj3150487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karylowski O, Zeigerer A, Cohen A, McGraw TE. GLUT4 is retained by an intracellular cycle of vesicle formation and fusion with endosomes. Mol Biol Cell. 2004;15:870–82. doi: 10.1091/mbc.E03-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaddai V, Gonzalez T, Keslair F, Grémeaux T, Bonnafous S, Gugenheim J, Tran A, Gual P, Le Marchand-Brustel Y, Cormont M. Rab4b is a small GTPase involved in the control of the glucose transporter GLUT4 localization in adipocyte. PLoS One. 2009;4:e5257. doi: 10.1371/journal.pone.0005257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shewan AM, van Dam EM, Martin S, Luen TB, Hong W, Bryant NJ, James DE. GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in Syntaxins 6 and 16 but not TGN38: involvement of an acidic targeting motif. Mol Biol Cell. 2003;14:973–86. doi: 10.1091/mbc.E02-06-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dugani CB, Klip A. Glucose transporter 4: cycling, compartments and controversies. EMBO Rep. 2005;6:1137–42. doi: 10.1038/sj.embor.7400584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai L, Wang Y, Fan J, Chen Y, Ji W, Qu A, Xu P, James DE, Xu T. Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab. 2007;5:47–57. doi: 10.1016/j.cmet.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Lizunov VA, Matsumoto H, Zimmerberg J, Cushman SW, Frolov VA. Insulin stimulates the halting, tethering, and fusion of mobile GLUT4 vesicles in rat adipose cells. J Cell Biol. 2005;169:481–9. doi: 10.1083/jcb.200412069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang S, Lifshitz LM, Jones C, Bellve KD, Standley C, Fonseca S, Corvera S, Fogarty KE, Czech MP. Insulin stimulates membrane fusion and GLUT4 accumulation in clathrin coats on adipocyte plasma membranes. Mol Cell Biol. 2007;27:3456–69. doi: 10.1128/MCB.01719-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawicki-McKenna JM, Goldman YE, Ostap EM. Sites of glucose transporter-4 vesicle fusion with the plasma membrane correlate spatially with microtubules. PLoS One. 2012;7:e43662. doi: 10.1371/journal.pone.0043662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Y, Rubin BR, Orme CM, Karpikov A, Yu C, Bogan JS, Toomre DK. Dual-mode of insulin action controls GLUT4 vesicle exocytosis. J Cell Biol. 2011;193:643–53. doi: 10.1083/jcb.201008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Zhang J, Chen Y, Jiang L, Ji W, Xu T. Characterization of GLUT4-containing vesicles in 3T3-L1 adipocytes by total internal reflection fluorescence microscopy. Sci China C Life Sci. 2009;52:665–71. doi: 10.1007/s11427-009-0081-9. [DOI] [PubMed] [Google Scholar]
- 27.Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Annu Rev Biochem. 2012;81:637–59. doi: 10.1146/annurev-biochem-052810-093700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–25. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Wang Y, Zhang J, Deng Y, Jiang L, Song E, Wu XS, Hammer JA, Xu T, Lippincott-Schwartz J. Rab10 and myosin-Va mediate insulin-stimulated GLUT4 storage vesicle translocation in adipocytes. J Cell Biol. 2012;198:545–60. doi: 10.1083/jcb.201111091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang L, Fan J, Bai L, Wang Y, Chen Y, Yang L, Chen L, Xu T. Direct quantification of fusion rate reveals a distal role for AS160 in insulin-stimulated fusion of GLUT4 storage vesicles. J Biol Chem. 2008;283:8508–16. doi: 10.1074/jbc.M708688200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–5. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 32.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–32. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 33.Kandror KV, Pilch PF. gp160, a tissue-specific marker for insulin-activated glucose transport. Proc Natl Acad Sci U S A. 1994;91:8017–21. doi: 10.1073/pnas.91.17.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson AL, Knight JB, Pessin JE. Syntaxin 4, VAMP2, and/or VAMP3/cellubrevin are functional target membrane and vesicle SNAP receptors for insulin-stimulated GLUT4 translocation in adipocytes. Mol Cell Biol. 1997;17:2425–35. doi: 10.1128/mcb.17.5.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi J, Kandror KV. Sortilin is essential and sufficient for the formation of Glut4 storage vesicles in 3T3-L1 adipocytes. Dev Cell. 2005;9:99–108. doi: 10.1016/j.devcel.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Reed SE, Hodgson LR, Song S, May MT, Kelly EE, McCaffrey MW, Mastick CC, Verkade P, Tavaré JM. A role for Rab14 in the endocytic trafficking of GLUT4 in 3T3-L1 adipocytes. J Cell Sci. 2013;126:1931–41. doi: 10.1242/jcs.104307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadacca LA, Bruno J, Wen J, Xiong W, McGraw TE. Specialized sorting of GLUT4 and its recruitment to the cell surface are independently regulated by distinct Rabs. Mol Biol Cell. 2013;24:2544–57. doi: 10.1091/mbc.E13-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eguez L, Lee A, Chavez JA, Miinea CP, Kane S, Lienhard GE, McGraw TE. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab. 2005;2:263–72. doi: 10.1016/j.cmet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Sano H, Peck GR, Kettenbach AN, Gerber SA, Lienhard GE. Insulin-stimulated GLUT4 protein translocation in adipocytes requires the Rab10 guanine nucleotide exchange factor Dennd4C. J Biol Chem. 2011;286:16541–5. doi: 10.1074/jbc.C111.228908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semiz S, Park JG, Nicoloro SM, Furcinitti P, Zhang C, Chawla A, Leszyk J, Czech MP. Conventional kinesin KIF5B mediates insulin-stimulated GLUT4 movements on microtubules. EMBO J. 2003;22:2387–99. doi: 10.1093/emboj/cdg237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Wang Y, Ji W, Xu P, Xu T. A pre-docking role for microtubules in insulin-stimulated glucose transporter 4 translocation. FEBS J. 2008;275:705–12. doi: 10.1111/j.1742-4658.2007.06232.x. [DOI] [PubMed] [Google Scholar]
- 42.Boguslavsky S, Chiu T, Foley KP, Osorio-Fuentealba C, Antonescu CN, Bayer KU, Bilan PJ, Klip A. Myo1c binding to submembrane actin mediates insulin-induced tethering of GLUT4 vesicles. Mol Biol Cell. 2012;23:4065–78. doi: 10.1091/mbc.E12-04-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bogan JS, Hendon N, McKee AE, Tsao TS, Lodish HF. Functional cloning of TUG as a regulator of GLUT4 glucose transporter trafficking. Nature. 2003;425:727–33. doi: 10.1038/nature01989. [DOI] [PubMed] [Google Scholar]
- 44.Yu C, Cresswell J, Löffler MG, Bogan JS. The glucose transporter 4-regulating protein TUG is essential for highly insulin-responsive glucose uptake in 3T3-L1 adipocytes. J Biol Chem. 2007;282:7710–22. doi: 10.1074/jbc.M610824200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bogan JS, Rubin BR, Yu C, Löffler MG, Orme CM, Belman JP, McNally LJ, Hao M, Cresswell JA. Endoproteolytic cleavage of TUG protein regulates GLUT4 glucose transporter translocation. J Biol Chem. 2012;287:23932–47. doi: 10.1074/jbc.M112.339457. [DOI] [PMC free article] [PubMed] [Google Scholar]