Abstract
We have recently demonstrated that a DNA vaccine targeting membrane-bound KIT ligand (KITL) inhibits tumor growth by interfering with vessel stabilization/permeability and by disrupting the recruitment of inflammatory cells and regulatory T cells, the latter being an essential mechanism by which tumors resist available treatments. Combining KITL-targeting vaccines with conventional chemotherapy might avert drug resistance and improve the efficacy of standard-of-care therapeutic interventions.
Keywords: DNA vaccination, membrane-bound KITL, non-functional angiogenesis
Solid tumors consist of neoplastic as well as of non-transformed cells, encompassing endothelial cells (ECs), pericytes, stromal fibroblasts, and tumor-infiltrating immune cells. These cells express the tyrosine kinase KIT as well as its ligand (KITL). KITL, which exists in both a soluble (sKITL) and a membrane-bound (mbKITL) form, delivers crucial pro-survival signals to KIT-expressing cells.1 mbKITL is expressed by proliferating ECs,2 tumor-associated ECs (TECs),3,4 as well as by malignant cells of different origin.4 This implies that the KIT/KITL system may be an ideal target for the development of novel anticancer regimens. As a matter of fact, small inhibitors of tyrosine kinase receptors have already been employed to treat breast carcinoma patients. However, mammary tumors that were initially responsive to such a therapeutic approach frequently acquired resistance over time.5 The limited rates of clinical responses induced by most targeted anticancer agents developed so far have driven the development of alternative treatment modalities, including immunotherapy.6 One advantage of vaccines over pharmacological inhibitors is that the former (but not the latter) are capable of eliciting a protective, memory immune response that is potentially able to control tumor recurrence for prolonged periods. This is particularly true when the target of such an immune response is expressed not only by cancer cells, but also by genetically stable cells of the tumor stroma, such as TECs. The therapeutic potential of vaccines targeting molecules that are expressed by the tumor-associated endothelium has already been demonstrated.7-9
Based on the these considerations, we have recently developed a DNA vaccine that targets human mbKITL and tested its efficacy in a per se non-immunogenic, transplantable model of mammary cancer.10 The choice of using a xenogeneic setting was taken based on the need to break the tolerance against a widely expressed, self antigen. We demonstrated that this DNA vaccine efficiently inhibit the growth of cancer cells administered afterwards in the majority of vaccinated mice. This protective effect became particularly evident in mice in which vaccination promoted a robust anti-mbKITL humoral response. The inability of our vaccine to break the immunological tolerance to mbKITL in some mice was not surprising in view of the crucial role that mbKITL plays in many biological processes.
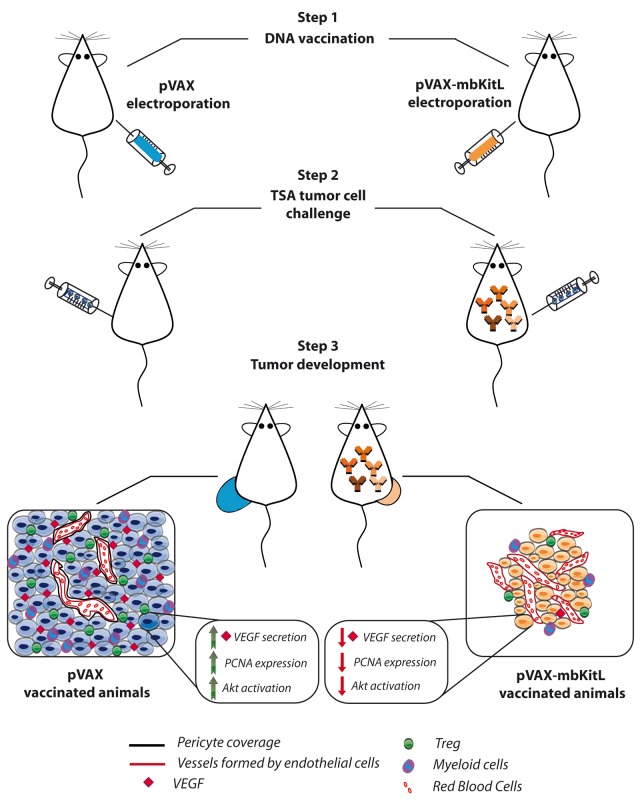
Impaired tumor growth upon vaccination was associated with a reduction in the number of functional blood vessels. This was mainly caused by a lack of proper pericyte coverage, in turn promoting vessel destabilization and altered vascular permeability. Vessel destabilization coupled to the hyper-dense immature vascular network observed in this setting resulted in a state of “non-functional angiogenesis” (Fig. 1). This was paralleled by an inadequate oxygen supply to malignant cells. Moreover, as in our model mbKITL is also expressed by cancer cells, a direct cytotoxic effect of vaccine-elicited antibodies on the malignant component of the tumor cannot be ruled out. In fact, cancer cells exhibit reduced proliferation rates in mice that produce anti-mbKITL antibodies, as shown by proliferating cell nuclear antigen (PCNA) staining.10
Figure 1. Non-functional angiogenesis and other consequences of a DNA-based vaccine targeting membrane-bound KITL. BALB/c mice were immunized every 2 weeks for a total of 3 applications by the intradermal injection of a plasmid encoding human membrane-bound KIT ligand (pVAX-mbKITL; vaccinated mice) or the empty vector (pVAX; control mice). Immediately after each injection, 2 25-ms low-voltage electric pulses were generated at the injection site, with a 300 ms interval between each pulse (Step 1). The presence of mbKITL-specific antibodies was assessed 1 wk after the last immunization. One week later control mice as well as mice generating polyclonal mbKITL-targeting antibodies received into the mammary fat pad a lethal dose of syngeneic TSA mammary cancer cells (Step 2). Tumor size was measured and immunohistochemical and biochemical analyses were performed. Only about 60% of vaccinated mice that exhibited a serological positive reaction developed tumors, and these lesions were smaller and displayed a lower proliferative rate when compared with those developing in control animals, as determined by proliferating cell nuclear antigen (PCNA) staining. In addition, these tumors were characterized by (1) hyper-dense non-functional vessels with scant pericyte coverage and altered permeability, (2) limited recruitment of myeloid cells and regulatory T cells (Tregs), (3) strongly decreased production of vascular endothelial growth factor (VEGF) and (4) inhibited AKT1 activation (Step 3).
Whereas the limited clinical efficacy of current anti-angiogenic drugs is mainly caused by the expression of hypoxia inducible factor 1 (HIF1) and/or HIF-related genes in response to vascular endothelial growth factor (VEGF) downregulation,5 our mbKITL-targeting vaccine inhibited VEGF production by ECs and malignant cells in the absence of signal transducer and activator of transcription 3 (STAT3) activation and HIF1 expression.10 This suggests that targeting mbKITL with vaccines might confer an additional therapeutic benefit as compared with anti-angiogenic drugs.
The tumor microenvironment is a crucial driver of immunosuppression and regulatory T cells (Tregs) robustly contribute to this phenotype by inhibiting effector cell functions.6 Moreover, the tumor microenvironment contains pro-inflammatory cells that contribute to tumor progression by altering the quality of the local vasculature. Interfering with mbKITL-delivered signals by vaccination limited the recruitment of Tregs and myeloid cells into the tumor microenvironment.10 Thus, mbKITL-targeting vaccines stand out as valuable strategy for overcoming tumor-mediated immunosuppression while hampering tumor-induced angiogenic responses. Moreover, the presence of mbKITL-specific antibodies failed to affect physiological wound healing and the long-term survival of bone marrow-derived progenitor cells in our pre-clinical model, ruling out major safety concerns about the induction of a long-lasting immune response against mbKITL. This is presumably due to the fact that vaccination is able to induce low-affinity antibodies specific for mbKITL, which is a self protein and hence normally subjected to immunological tolerance.6 It this therefore likely that these antibodies inhibit mbKITL functions to partial extents, especially in the bone marrow where mbKITL is abundantly expressed together with sKITL.1 Nevertheless, the interference that these low-affinity, polyclonal antibodies exert on mbKITL is sufficient to alter the tumor microenvironment and inhibit tumor growth.
Unlike targeted anticancer agents, immunotherapeutic strategies against tumor-associated antigens face numerous challenges. However, we provide evidence that, as compared with a strategy that only targets tumor-associated antigens, an approach that breaks the tolerance against a protein expressed by both the tumor vasculature and cancer cells might exert an improved therapeutic activity while being well tolerated. Moreover, as pericyte-covered vessels contribute to the resistance of some neoplastic lesions to current chemotherapeutics, targeting the tumor microenvironment in the course of standard-of-care therapeutic regimens might offer an alternative treatment modality that circumvent such a loss in chemosensitivity.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from the Italian Association for Cancer Research (AIRC) to M.F.B (IG 5649) and F.C. (IG 5377 and IG 11675).
Citation: Dentelli P, Cavallo F, Brizzi MF. Membrane-bound KIT ligand-targeting DNA vaccination inhibits mammary tumor growth. OncoImmunology 2013; 2:e27259; 10.4161/onci.27259
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27259
References
- 1.Miyazawa K, Williams DA, Gotoh A, Nishimaki J, Broxmeyer HE, Toyama K. Membrane-bound Steel factor induces more persistent tyrosine kinase activation and longer life span of c-kit gene-encoded protein than its soluble form. Blood. 1995;85:641–9. [PubMed] [Google Scholar]
- 2.Dentelli P, Rosso A, Balsamo A, Colmenares Benedetto S, Zeoli A, Pegoraro M, Camussi G, Pegoraro L, Brizzi MF. C-KIT, by interacting with the membrane-bound ligand, recruits endothelial progenitor cells to inflamed endothelium. Blood. 2007;109:4264–71. doi: 10.1182/blood-2006-06-029603. [DOI] [PubMed] [Google Scholar]
- 3.Defilippi P, Rosso A, Dentelli P, Calvi C, Garbarino G, Tarone G, Pegoraro L, Brizzi MF. beta1 Integrin and IL-3R coordinately regulate STAT5 activation and anchorage-dependent proliferation. J Cell Biol. 2005;168:1099–108. doi: 10.1083/jcb.200405116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dentelli P, Rosso A, Olgasi C, Camussi G, Brizzi MF. IL-3 is a novel target to interfere with tumor vasculature. Oncogene. 2011;30:4930–40. doi: 10.1038/onc.2011.204. [DOI] [PubMed] [Google Scholar]
- 5.Chew HK, Barlow WE, Albain K, Lew D, Gown A, Hayes DF, Gralow J, Hortobagyi GN, Livingston R. A phase II study of imatinib mesylate and capecitabine in metastatic breast cancer: Southwest Oncology Group Study 0338. Clin Breast Cancer. 2008;8:511–5. doi: 10.3816/CBC.2008.n.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini PL. 2011: the immune hallmarks of cancer. Cancer Immunol Immunother. 2011;60:319–26. doi: 10.1007/s00262-010-0968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmgren L, Ambrosino E, Birot O, Tullus C, Veitonmäki N, Levchenko T, Carlson LM, Musiani P, Iezzi M, Curcio C, et al. A DNA vaccine targeting angiomotin inhibits angiogenesis and suppresses tumor growth. Proc Natl Acad Sci U S A. 2006;103:9208–13. doi: 10.1073/pnas.0603110103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arigoni M, Barutello G, Lanzardo S, Longo D, Aime S, Curcio C, Iezzi M, Zheng Y, Barkefors I, Holmgren L, et al. A vaccine targeting angiomotin induces an antibody response which alters tumor vessel permeability and hampers the growth of established tumors. Angiogenesis. 2012;15:305–16. doi: 10.1007/s10456-012-9263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haller BK, Bråve A, Wallgard E, Roswall P, Sunkari VG, Mattson U, Hallengärd D, Catrina SB, Hellström M, Pietras K. Therapeutic efficacy of a DNA vaccine targeting the endothelial tip cell antigen delta-like ligand 4 in mammary carcinoma. Oncogene. 2010;29:4276–86. doi: 10.1038/onc.2010.176. [DOI] [PubMed] [Google Scholar]
- 10.Olgasi C, Dentelli P, Rosso A, Iavello A, Togliatto G, Toto V, Liberatore M, Barutello G, Musiani P, Cavallo F, et al. DNA vaccination against membrane-bound Kit ligand: A new approach to inhibiting tumour growth and angiogenesis. Eur J Cancer. 2013 doi: 10.1016/j.ejca.2013.09.016. Forthcoming. [DOI] [PubMed] [Google Scholar]