Abstract
The aim of this work was to develop a fast-dissolving film formulation containing EFdA for potential use as a topical vaginal microbicide for prevention of HIV sexual transmission. Solid state compatibility approaches were used to screen commonly used polymers for formulation development. Factorial design and desirability function were used to investigate the effect of two variables, the ratio of the polymers and the concentration of selected plasticizer on four mechanical responses including tensile strength, elongation at break, toughness and elastic modulus for optimization of the film formulation. Assessments of EFdA-loaded films included physicochemical characteristics, in vitro cytotoxicity, epithelia integrity, ex vivo permeability and bioactivity test. The optimal placebo film was composed of PVA, HPMC E5 and propylene glycol (7:3:3, w/w), and its mechanical characteristics were comparable to those of VCF® film (a commercial vaginal film product). Permeability studies using human ectocervical explants showed that there was no significant difference in cumulative permeated amount of EFdA between EFdA film and free EFdA. The results of in vitro cytotoxicity and bioactivity testing showed that 50% cytotoxic concentration (CC50) was several orders of magnitude higher than 50% effective concentration (EC50) of EFdA. Furthermore, epithelial integrity study showed that EFdA-loaded film had a much lower toxicity to HEC-1A cell monolayers as compared to VCF®. Therefore, EFdA-loaded vaginal film may be considered as a promising vaginal microbicide for HIV prevention.
Keywords: Polymeric film, EFdA, Microbicide, HIV prevention, Vaginal delivery
1. Introduction
Sexually transmitted infections (STIs), such as human immunodeficiency virus (HIV), herpes simplex virus (HSV) and gonorrhea are spread predominantly through unprotected heterosexual vaginal intercourse (UNAIDS, 2010). Women appear to be at higher risk for infection than men, with an estimated 30% to 40% of annual worldwide infections occurring through HIV invasion of the female genital tract via exposure to virus-containing semen, because of the inability to negotiate safe sex practices and biological vulnerability of female genital tract (Hladik and Hope, 2009). Although vaginal epithelial cells have limited permeability to particles greater than 30 nm (HIV virions are 80–100 nm), HIV seems to enter the superficial layers of the squamous epithelium by diffusing across a concentration gradient, and sequesters itself on the surface of epithelial cells until it can infect other cell types such as CD4+ cells and Langerhans cells (Cutler and Justman, 2008). Therefore, it is imperative to develop female-controlled strategies for the prevention of HIV transmission. Microbicides are an anti-infective medication formulated for topical self-administration before intercourse to protect against sexually transmitted pathogens (Stone, 2002), and are recognized as a promising method for the prevention of HIV transmission. Microbicides can be classified into two general categories based on the mechanism of microbicidal action, non-specific microbicides such as detergents and buffering agents, and highly specific anti-HIV agents including viral entry, fusion, integrase and reverse transcriptase inhibitors (Balzarini and Van Damme, 2007).
Nucleoside reverse transcriptase inhibitors (NRTIs) are crucial components of highly active antiretroviral therapy (HAART) for systemic treating HIV infection. All FDA-approved NRTIs are nucleoside analogs lacking a 3′-hydroxyl group. Thus upon incorporation of an NRTI into the growing viral DNA, further DNA chain extension is terminated (Sohl et al., 2012). We have been evaluating a novel experimental NRTI, 4′-ethynyl-2-fluoro-2′-deoxyadenosine (EFdA), which possesses a 3′-hydroxyl moiety yet functions as a chain terminator by a novel mechanism of action (Nakata et al., 2007). EFdA possesses highly potent activity against wild-type HIV-1 as well as diverse multidrug-resistant HIV strains, and has a substantially more favorable selectivity index (CC50/EC50) than any of the current clinically approved NRTIs (Kawamoto et al., 2008; Michailidis et al., 2009; Hattori et al., 2009). EFdA also showed potent activity in vivo in both HIV-1-infected hu-PBMC-NOJ mice model and in SIV-infected rhesus macaques, with no apparent toxicity noted in either study (Murphey-Corb et al., 2012; Rohan and Sassi, 2009). EFdA thus may be a very promising drug candidate for use both in HIV therapeutic and prevention modalities.
Currently, several dosage forms including gels, tablets, soft-gel capsules, polymeric films and intravaginal rings have been investigated as drug delivery systems for vaginal microbicide products ranging from liquid to solid dosages forms to provide users with options without the constrains of their social environment, personal choice and environmental conditions (Rohan and Sassi, 2009; Nel et al., 2011; Akil et al., 2011; Garg et al., 2010; Romano et al., 2008; Elias and Coggins, 2001; Raymond et al., 1999, 2005). Polymeric thin films are a vaginal drug delivery system which offers several advantages over traditionally established dosage forms (Rohan and Sassi, 2009; Akil et al., 2011). Polymeric films are thin strips of polymeric water-soluble excipients which dissolve when placed on the vaginal mucosal surface to release the active ingredient. Polymeric films offer accurate dose administration and can be applied without an applicator. Other advantages include good portability, easy storage, discreet use, no product leakage and reduced cost (Garg et al., 2010; Romano et al., 2008). Furthermore, vaginal polymeric thin films can be used to stabilize drugs susceptible to degradation in aqueous condition. Additionally, results of published acceptability studies showed that vaginal films were favorably accepted by women over other vaginal dosage forms such as gels, foams and tablets (Nel et al., 2011; Elias and Coggins, 2001; Raymond et al., 1999, 2005).
At present, there are only three commercially available vaginal films including vaginal contraceptive film (VCF), vaginal lubricating film and vaginal scented film. It is important to develop new drug-loaded vaginal film formulations as topical microbicide candidates for HIV prevention. The objective of this work was to develop a fast-dissolving vaginal film containing EFdA. The formulation of the film was optimized by factorial experimental design and desirability function. In vitro and ex vivo evaluations of EFdA-loaded films, including physicochemical properties, in vitro cytotoxicity, epithelial integrity, permeability and bioactivity testing, were performed. Our results suggest that EFdA can be readily formulated into vaginal films and that these films may have great potential to be an effective topical microbicide product for HIV prevention.
2. Materials and methods
2.1. Materials
EFdA was prepared by custom synthesis (Life Chemicals, Burlington, ON, Canada). BD Falcon ™ cell culture inserts, methanol (HPLC grade), 1-octanol, DMSO, HEPES, fetal bovine serum (FBS) potassium biphthalate, potassium phosphate monobasic, potassium chloride, sodium chloride, certified 0.2 M sodium hydroxide and hydrochloric acid solutions were obtained from Fisher Scientific (Pittsburgh, PA, US). Polyvinyl alcohol (PVA), carboxymethylcellulose sodium (CMC-Na), propylene glycol, glycerin, triacetin, PEG 400 and sorbitol were purchased from Spectrum (Gardena, CA, US). PEG 4000 and hydroxypropyl methyl cellulose E5 (HPMC E5) were obtained from Dow Chemical Company (Midland, MI, US). HPMC K4M was obtained from Colorcon (West point, US). HBSS was purchased from Lonza (Walkersville, MD, USA). MTT was purchased from Sigma-Aldrich (St. Louis, MO, USA). Methane-sulfonic acid was obtained from Acros (Morris Plains, NJ, US). PBS (pH 7.4), RPMI1640, Dulbecco’s modified Eagle medium (DMEM) and penicillin–streptomycin were purchased from Mediatech Inc (Manassas, VA, US). Boric acid was purchased from Professional Compounding Centers of American, Inc. (Houston, TX, US). All other chemicals were analytical grade. Ultrapure water was obtained in-house from a MilliQ water purification system.
2.2. HPLC analysis
An HPLC system (Waters Corporation, Milford, MA) equipped with an auto injector (model 717), a quaternary pump (model 600), and a Photodiode Array Detector (PDA, model 2996) was used for analytical method development. Empower Pro 2 software (Waters Corporation) was used to control the HPLC system. Separation of the compound of interest was achieved by using a Zorbax Eclipse XDB C18 column (3.5 μm, 100 × 4.6 mm). The mobile phase consisted of (A) 0.4% phosphoric acid in MilliQ water and (B) methanol using a gradient elution of 10–40% B at 0–5 min, 40–60% B at 5–10 min, 60% B at 5–6 min, 60–10% B at 10–13 min and 10% B at 13–20 min at a flow rate of 0.8 ml/min. Sample injection volume was 10 μl and EFdA was determined by UV detection at 260 nm. All experiments were performed at room temperature and the total peak area was used to quantify EFdA.
2.3. Solid state compatibility
Various polymer solutions with the concentration of 2% or 4% (w/w) were prepared by dissolving polymers in MilliQ water under stirring. Ethanol was used to dissolve EFdA. Approximate 1 ml polymer solution and 0.1 ml EFdA solution with the concentration of 10 or 20 mg/ml were mixed together. In cases where precipitation was observed, the samples were mixed vigorously prior to spreading using a vortex mixer. Approximately 0.5 ml of mixture was spread out over the slide using a coverslip. Following spreading, the samples were placed at room temperature overnight, and the slides were observed by a Zeiss Axioskop 40 inverted phase-contrast microscope (Thornwood, NY). In addition, polymer solution alone, and mixture of polymer solution and 0.1 ml ethanol were used as the control, respectively.
2.4. Film formulation
2.4.1. Preparation of placebo film
Based on the results of the compatibility study, PVA and HPMC E5 were selected as film-forming polymers. The placebo film was prepared by the solvent casting method. Briefly, PVA was dissolved in MilliQ water, and to expedite dissolution, the mixture was placed in a hot water bath (90 °C) until the PVA was completely hydrated. HPMC E5 was then slowly added into the PVA solution with moderate stirring using an overhead mixer. After the polymer solution became uniform and homogenous, the plasticizer dissolved in ethanol was added. The mixture was allowed to stir overnight in order to remove any entrapped air bubbles. The uniform polymer solution was cast onto a polyester substrate attached to the hot surface of an automatic film applicator (Elcometer® 4340) using an 8-inch doctor blade. The film sheet was allowed to dry for about 13 min before it was peeled off from the polyester substrate. Once the film sheet was obtained, it was cut into 1 in. × 2 in. pieces using a die press.
2.4.2. Optimization of the ratio of PVA and HPMC E5
In this study, the total amount of the polymers (PVA and HPMC E5) was kept constant (10%, w/w) while the composition was varied. To optimize the ratio of PVA and HPMC E5 in the film formulation, PEG 400 was employed as model plasticizer and a 32 randomized factorial design was used in this study (Table 1). Two independent variables, the percentage of PVA (%, X1) and the concentration of PEG 400 (%, X2), were set at three different levels. Low, medium and high levels of each factor were coded as −1, 0 and 1, respectively. Tensile strength, elongation at break, toughness and elastic modulus which were calculated by the following Eqs. (1–4), were selected as dependent responses since they were generally regarded as significant factors for assessing mechanical properties of the film.
Table 1.
Responses for 32 factorial design.
| Variables
|
Responses
|
Individual desirability
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Run | X1 | X2 | Tensile strength (Y1, g/mm2) | Elongation at break (Y2, %/mm2) | Toughness (Y3, g/mm) | Elastic modulus (Y4, g/mm2) | D1 | D2 | D3 | D4 | Overall desirability |
| 1 | −1 | −1 | 2113.02 | 125.75 | 23913.2 | 1073.64 | 1.00 | 0.00 | 0.02 | 0.00 | 0.000 |
| 2 | 0 | −1 | 2284.65 | 143.52 | 30931.22 | 917.99 | 0.00 | 0.05 | 0.18 | 0.18 | 0.000 |
| 3 | 1 | −1 | 2472.10 | 186.78 | 37317.73 | 787.92 | 0.00 | 0.18 | 0.32 | 0.33 | 0.000 |
| 4 | −1 | 0 | 1851.09 | 139.24 | 22790.08 | 852.65 | 1.00 | 0.04 | 0.00 | 0.25 | 0.000 |
| 5 | 0 | 0 | 1883.92 | 231.43 | 43510.24 | 352.59 | 1.00 | 0.32 | 0.45 | 0.83 | 0.588 |
| 6 | 1 | 0 | 2480.96 | 255.44 | 57488.59 | 457.13 | 0.00 | 0.39 | 0.76 | 0.71 | 0.000 |
| 7 | −1 | 1 | 1439.53 | 194.95 | 32262.19 | 344.74 | 1.00 | 0.21 | 0.21 | 0.84 | 0.436 |
| 8 | 0 | 1 | 1791.73 | 231.49 | 49124.28 | 303.54 | 1.00 | 0.32 | 0.58 | 0.88 | 0.635 |
| 9 | 1 | 1 | 1579.73 | 303.96 | 50043.04 | 202.17 | 1.00 | 0.54 | 0.60 | 1.00 | 0.753 |
| Independent variables | Levels | ||||||||||
| Low (−1) | Medium (0) | High (1) | |||||||||
| X1 | Percentage of PVA (%) | 6.5 | 7 | 7.5 | |||||||
| X2 | Concentration of PEG 400 (%) | 1 | 3 | 5 | |||||||
The total amount of film-forming polymers (PVA and HPMC E5) was kept constant at 10% (w/w) while the composition was varied.
| (1) |
| (2) |
| (3) |
| (4) |
An ideal film should have moderate tensile strength, high elongation at break and low elastic modulus. However, it was not possible to optimize all the conditions simultaneously because sometimes they did not coincide with each other, and conflict may also occur between these variables. Therefore, the multi-criteria problem can be treated as single criterion problem by utilizing the desirability function approach in order to find the best compromising formulation for all the responses (Zhang et al., 2009). At present, there is no optimal specification for tensile strength, so the range of tested values of a commercial film product (VCF®) was chosen as reference (1400–2200 g/mm2). As moderate tensile strength was expected, the formulation which had its value of tensile strength within this range would have a desirability value (d1) of 1, while the others which had values out of this range would have a desirability value (d1) of 0. The desirability function was described as below:
| (5) |
where d1 is the individual desirability of tensile strength; Ymin and Ymax represented the lowest and the highest value of VCF® during the measurement, respectively.
For a response to be maximized, the desirability function was defined as:
| (6) |
where d2 and d3 were the individual desirability functions of elongation at break and toughness, respectively; Ytarget indicated the characteristic value of VCF®.
For a response to be minimized, the desirability function was defined as:
| (7) |
where d4 was the individual desirability of elastic modulus.
The overall desirability value (D), a global desirability function, was calculated using the following expression:
| (8) |
2.4.3. Selection of plasticizer
Once the ratio of PVA and HPMC E5 was determined, another four plasticizers including PEG 4000, triacetin, propylene glycol and sorbitol were explored for optimization of the placebo films (Table 2).
Table 2.
Selection of different plasticizers.
| Variables No. | Variables
|
Responses
|
Individual desirability
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Category | Conc. (%) | Tensile strength (Y1, g/mm2) | Elongation at break (Y2, %/mm2) | Toughness (Y3, g/mm) | Elastic modulus (Y4, g/mm2) | D1 | D2 | D3 | D4 | Overall desirability | |
| 1 | Triacetin | 1 | 2472.36 | 179.87 | 39782.83 | 770.79 | 0.00 | 0.20 | 0.41 | 0.35 | 0.000 |
| 2 | 3a | – | – | – | – | – | – | – | – | – | |
| 3 | 5a | – | – | – | – | – | – | – | – | – | |
| 4 | PEG4000 | 1a | – | – | – | – | – | – | – | – | – |
| 5 | 3 | 1585.60 | 133.23 | 23204.01 | 569.08 | 1.00 | 0.07 | 0.06 | 0.58 | 0.224 | |
| 6 | 5b | – | – | – | – | – | – | – | – | – | |
| 7 | PEG400 | 1 | 2284.65 | 143.52 | 30931.22 | 917.99 | 0.00 | 0.05 | 0.18 | 0.18 | 0.000 |
| 8 | 3 | 1883.92 | 231.43 | 43510.24 | 352.59 | 1.00 | 0.32 | 0.45 | 0.83 | 0.588 | |
| 9 | 5 | 1791.73 | 231.49 | 49124.28 | 303.54 | 1.00 | 0.32 | 0.58 | 0.88 | 0.635 | |
| 10 | Propylene | 1 | 2110.02 | 109.57 | 20134.35 | 1040.99 | 1.00 | 0.00 | 0.00 | 0.04 | 0.000 |
| 11 | Glycol | 3 | 1728.70 | 567.49 | 73151.06 | 147.19 | 1.00 | 1.00 | 1.00 | 1.00 | 1.000 |
| 12 | 5 | 1018.52 | 554.03 | 45404.79 | 72.75 | 0.00 | 1.00 | 0.52 | 1.00 | 0.000 | |
| 13 | Sorbitol | 1 | 2311.99 | 109.91 | 24000.32 | 994.77 | 0.00 | 0.00 | 0.08 | 0.09 | 0.000 |
| 14 | 3 | 2205.40 | 216.28 | 46269.04 | 564.29 | 1.00 | 0.31 | 0.54 | 0.58 | 0.558 | |
| 15 | 5 | 2025.87 | 320.46 | 65897.26 | 325.86 | 1.00 | 0.61 | 0.95 | 0.86 | 0.838 | |
The films were too tough to measure, and the texture analyzer was overloaded during the measurement.
The film cannot be made using this polymer solution because of the phase separation.
2.4.4. Preparation of EFdA-loaded film
The solvent casting method was used to make the drug containing film as described above except that desired amounts of EFdA (0.1%, w/w) and propylene glycol were dissolved in ethanol.
2.5. Film characterization
2.5.1. Mechanical property
Mechanical characteristics of EFdA-loaded films including tensile strength, elongation at break, toughness and elastic modulus were measured using a texture analyzer (TA. XT. Plus®, New York, NY). Briefly, the film was placed between two clamps positioned at a distance of 4.85 mm in the same plane. During the measurement, the lower clamp was fixed and the film was pulled by the upper clamp at a rate of 3.00 mm/s. The force and elongation at the moment of break were recorded.
2.5.2. Moisture content
Residual water content of the films was measured using a Karl Fisher apparatus (Metrohm, 758 KFD Titrino) in accordance with the titration method specified by the manufacturer.
2.5.3. Drug content
For drug content determination, the EFdA-loaded film was dissolved in a 60% methanol solution and further diluted by MilliQ water. An aliquot was withdrawn from the diluted solution and analyzed using the previously described HPLC method. To ensure the uniform distribution of EFdA in the film, the drug content uniformity test was also conducted. Briefly, six 1 in. × 2 in. drug-loaded film pieces were collected from different locations of a whole film sheet. Each 1 in. × 2 in. film was further cut into six pieces, and each piece was processed and analyzed as described above.
2.5.4. Dissolution test
To determine the kinetics of drug release from EFdA-loaded films in different media, an in vitro release study was conducted using a class IV USP apparatus (SOTAX CP7, Horsham, US) using a 70 ml reservoir and a flow rate of 16 ml/min. Acetate buffer (pH 4.1), PBS (pH 5.2 and 7.4) and MilliQ water were used as the media. The test was carried out at 37 °C for 60 min. At appropriate time intervals, 0.5 ml aliquots were withdrawn and the samples were analyzed for EFdA by HPLC.
2.5.5. Stability assessment
To test physical stability, EFdA-loaded films were stored at 40 °C/75% RH for 3 months, and the films were monitored for time-dependent changes in weight, thickness, appearance, water content, drug content and in vitro release during the storage period.
2.5.6. Scanning electron microscopy (SEM)
Surface morphology of EFdA powder and EFdA-loaded film was analyzed by SEM (Philips XL30 FEG). Samples for SEM were mounted on aluminum holders by carbon conductive glue and coated with a platinum layer by platinum sputter coater before scanning.
2.5.7. X-ray diffraction (XRD)
XRD analysis of the films and EFdA powders was conducted by an X-ray diffractometer (Philips PW1830/00, Almelo, Netherlands) equipped with a Cu Kα radiation source (40 kV, 30 mA, λ = 0.15406 nm). EFdA powders were pressed onto the sample holder to form a thin EFdA layer, while the films were directly placed on the sample holder. The samples were measured from 2.5° to 45° at a rate of 0.04°/s.
2.6. In vitro cytotoxicity
CaSki, HEC-1A and A431 cell lines were obtained from ATCC and the cells were cultured at 37 °C with 5% CO2 under fully humidified conditions. A431 cells were cultured in DMEM medium, supplemented with 10% FBS, 100 IU/ml penicillin and 100 μg/ml streptomycin sulfate. CaSki cells were cultured in RPMI 1640 medium, supplemented with 10% FBS, 100 IU/ml penicillin and 100 μg/ml streptomycin sulfate, and HEC-1A cells were cultured in McCoy’s 5A modified medium, supplemented with 10% FBS, 100 IU/ml penicillin and 100 μg/ml streptomycin sulfate.
Cells were seeded at the density of 1 × 104 cells per well in 96-well plates. After 24 h incubation at 37 °C with 5% CO2, the growth medium was replaced with 200 μl medium containing either EFdA with the concentration ranging from 5 ng/ml to 50 μg/ml (EFdA equivalent dose for drug-loaded films) or placebo film at corresponding concentration. After 24 h incubation, cell survival was measured using MTT assay. A volume of 180 μl fresh growth medium and 20 μl of MTT (5 mg/ml) solution were added to each well. The plate was incubated for an additional 3 h at 37 °C and the media were removed, and then 200 μl of DMSO was added to each well to dissolve the purple formazan crystals formed. All the plates were vigorously shaken before measuring the intensity. The absorbance at 595 nm of each well was determined by a microplate reader (Beckman Coulter® DTX 880, USA).
2.7. Epithelial integrity study
For epithelial integrity assessment, HEC-1A cells were seeded at a density of 1 × 105 cells/well onto per polycarbonate insert (0.9 cm2, 0.4 μm, BD Falcon TM) in 12-well cell culture plates. The cells were cultured for 7 days and the cell growth medium was changed every other day. In order to determine the toxic effect of EFdA-loaded and placebo films on epithelial integrity, the transepithelial electrical resistance (TEER) values were determined. A drug-encapsulated film solution dissolved in complete cell culture medium with the concentration of 50 μg/ml EFdA and the corresponding placebo film solution were added into the apical chamber at t = 0 and resistance readings were measured at 30 min, 1, 2, 4, 8, and 24 h. In addition, a VCF® film (active ingredient: 28% Nonoxynol-9) solution with the same concentration as that of placebo (based on the weight of the film), and culture medium alone were used as positive and negative control, respectively. TEER values across each cell layer were measured with a MilliCell–ERS resistance system (Millipore, Billerica, MA). TEER values were corrected by subtracting the background TEER obtained from inserts containing the same volume of culture medium but in the absence of cells. The resulting TEER was corrected for membrane growth area and expressed in Ω cm2. The relative epithelia integrity (%) of each formulation was normalized with respect to the TEER value of negative control.
2.8. Tissue permeability assessment
2.8.1. Tissue procurement and processing
Freshly excised human ectocervical tissue was obtained from the Tissue Procurement Facility at Magee-Womens Hospital. Tissue samples were from four women with median age of 45–50 years undergoing hysterectomy for benign conditions. All tissue specimens were obtained within 2 h of surgical excision. Tissues were held at 4 °C in DMEM during transfer from surgery to the laboratory. Excessive stromal tissue was removed and the epithelial layer was isolated using a Thomas-Stadie Riggs tissue slicer (Thomas Scientific, Swedesboro, NJ). The thickness of each tissue was measured by placing the tissue between two slides and the thickness was measured using a micrometer.
2.8.2. Tissue permeability study
Human tissue permeability studies were conducted using a Franz cell system (PermeGear, Nazareth, PA). The Franz cell system is a two-compartment system consisting of donor compartment and receptor compartment. The system was water-jacketed and temperature was maintained at 37 °C throughout the experiment via a circulating water bath. The isolated epithelial sections of each tissue were placed between the donor and receptor compartments with the epithelial side of the tissue oriented toward the donor compartment which provided a diffusion area of 0.385 cm2. PBS (pH 5.2) solution was used as the medium. The receptor chamber was continuously stirred by a magnetic stir bar and the volume of the receptor compartment was 5.0 ml. The tissue was equilibrated with PBS in the donor compartment for about 5 min prior to the permeability study. After the equilibration period, PBS was removed from the donor chamber and replaced with 450 μl of EFdA in PBS (containing 100 μg drug). For the case of EFdA-loaded film, a piece of drug-loaded film to provide 100 μg EFdA was punched and carefully located onto the top of the epithelia, and then 450 μl PBS (pH 5.2) was added into the donor compartment. At various time intervals over a 6 h period, 200 μl aliquots were withdrawn from the receiver compartment. Receiver compartment medium was replenished with fresh medium after removal of each aliquot. EFdA amount in the receiver compartment was quantified by HPLC.
2.8.3. Histological evaluation
Tissues of pre-treatment and post-treatment were fixed in Clark’s solution (ethanol/acetic acid 75:25) for 24 h, transferred to ethanol for 24 h, and subsequently embedded in paraffin. Tissue sections of 5 μm thickness were cut and stained with hematoxylin and eosin (H&E) for histopathological analysis. The tissues were imaged on a Zeiss Axioskop 40 inverted phase-contrast microscope (Thornwood, NY).
2.9. Bioactivity study
Two types of bioactivity tests were carried out, (i) standard antiviral activity, in which cells were simultaneously exposed to varying concentrations of film-formulated drug and HIV, with drug being present throughout the infection process, and (ii) protective or memory effect, where cells were pretreated with varying concentrations of film-formulated drug for 4 h, then the drug-containing medium was removed by extensive washing. The cells were exposed to HIV in the absence of exogenous drug. HIV replication was evaluated in single replication cycle HIV assays, using P4R5 HIV infection indicator cells that express CD4, CXCR4 and CCR5 as well as a β-galactosidase reporter gene under the control of an HIV LTR promoter (from Dr. John Mellors, University of Pittsburgh). Cells were maintained in DMEM/10% FBS supplemented with puromycin (0.5 g/ml). Viral infectivity was assessed in 96-well microplate assays seeded with P4R5 cells at a density of 5 × 103 cells/well. Cells were inoculated with 25 ng HIV-1 p24/well and the extent of infection was evaluated 48 h post-infection using a fluorescence-based β-galactosidase detection assay as previously described (Abram and Parniak, 2005).
2.10. Statistical Analysis
Statistical analysis was performed using a two-tailed Student’s t-test. A P value < 0.05 was considered statistically significant.
3. Results
3.1. Solid state compatibility
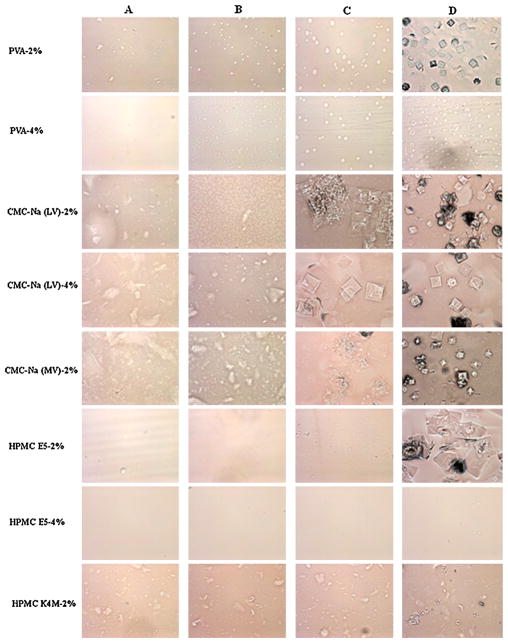
As shown in Fig. 1, CMC-Na with low or medium viscosity cannot be used as film-making polymer in the formulation because of its incompatibility with EFdA, evidenced by numerous EFdA crystals that appeared on the slides in all test groups. HPMC K4M was excluded from the polymer selection due to the high viscosity of polymer solution which can introduce a lot of air bubbles. Moreover, a number of small EFdA crystals were also observed in HPMC K4M tested group (Fig. 1, panel D). In contrast, PVA and HPMC E5 were found to be more compatible with the drug substance, and can be utilized for further formulation development.
Fig. 1.
Images for EFdA solid state compatibility studies. (A) Control 1: 1 ml polymer solution with different concentration (2% or 4%); (B) control 2: 1 ml polymer solution with different concentration (2% or 4%) + 0.1 ml ethanol; (C) 1 ml polymer solution with different concentration (2% or 4%) + 0.1 ml ethanol containing 1 mg EFdA; (D) 1 ml polymer solution with different concentration (2% or 4%) + 0.1 ml ethanol containing 2 mg EFdA.
3.2. Film formulation
The total amount of film-forming polymers (PVA and HPMC E5) was kept constant at 10% (w/w) while the composition was varied. PEG 400 was used as model plasticizer. As shown in Table 1, it indicated that run 8 (D: 0.635) and 9 (D: 0.753) had higher scores than the other formulations, but films in run 8 and 9 were found to be greasy, probably due to the excessive amount of PEG 400. Therefore, in terms of appearance and mechanical properties of the film, formulation 5 (D: 0.588) was selected and the optimal ratio of PVA and HPMC E5 was set at 7:3 (w/w). In the follow-up experiment, the effect of plasticizer concentration on mechanical property of films was evaluated (Table 2). Results showed that the highest value of D (1.0) could be obtained with formulation 11 which had the optimal appearance and physical property. Thus, propylene glycol (3%, w/w) was selected as optimal plasticizer in placebo film formulation. Furthermore, it was found that as the concentration of propylene glycol increased from 3% to 5% (w/w), the film became very soft and its tensile strength was much lower than the minimum of that of VCF®, probably due to the strong plasticization efficiency of propylene glycol. In addition, sorbitol (5%, w/w) was also a good plasticizer candidate.
3.3. Characterization of drug-loaded films
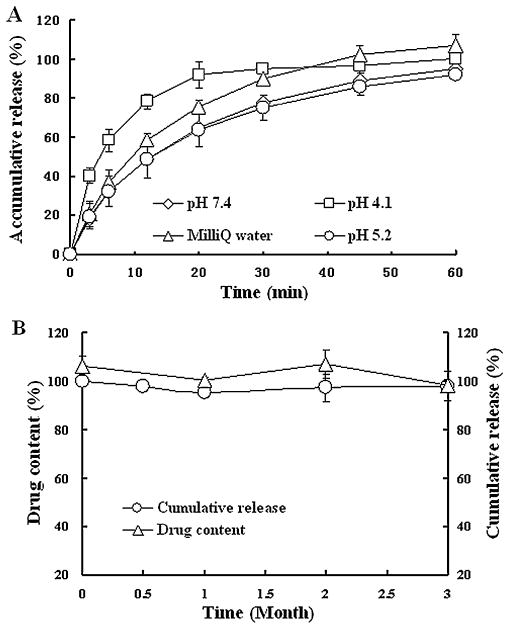
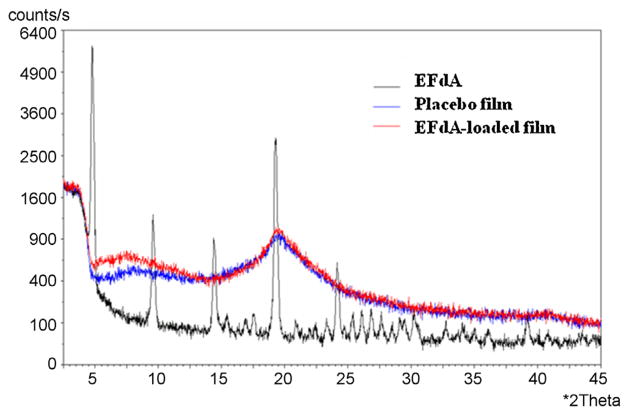
EFdA distributed uniformly throughout the whole sheet of films, and mechanical characteristics of drug-loaded films were comparable to those of the placebo films, indicating that the drug substance had little impact on the mechanical properties of EFdA-loaded films (Table 3). In vitro release studies of EFdA-loaded films showed that >95% of EFdA was released from film matrix in all the media after 60 min (Fig. 2A). Drug release from EFdA containing films was much faster in pH 4.1 acetate buffer than in MilliQ water or PBS (pH 5.2 and 7.4), which was in good agreement with the results of solubility studies (Zhang et al., 2013). However, there was no significant difference in the cumulative release of EFdA in acetate buffer (pH 4.1) compared to that in MilliQ water after 60 min (P > 0.05). Thus, MilliQ water was selected as the dissolution medium for quality control. Additionally, as presented in Fig. 2B, drug content and cumulative release of EFdA-loaded film did not change over a 3 month period when films were maintained under accelerated conditions (40 °C/75% RH), showing excellent stability of this film formulation. In addition, there was no significant change in phys-icochemical properties of EFdA containing films including weight, thickness, water content and appearance during the storage period. Fig. 3 shows representative SEM images of the surface morphologies of EFdA powder and drug-loaded film. EFdA crystals exist in planar or flaky shape of a non-uniform particle size. The surface of EFdA-loaded films was relatively smooth and no EFdA crystals were observed on the surface of the film. XRD analysis was also performed to elucidate the crystalline state of EFdA in drug-loaded film. EFdA powder presented high crystallinity by sharp and intense diffractive peaks at 4.8°, 9.5°, 14.4°, 19.3° and 24.2° (Fig. 4). However, the XRD pattern of the EFdA-loaded film was almost the same as that of placebo film, further suggesting that the incorporated EFdA was in an amorphous state.
Table 3.
Physicochemical properties of EFdA-loaded film.
| Parameter | |
|---|---|
| Size (in.2) | 2 × 1 |
| Drug content (mg EFdA/Film) (n = 6) | 1.24 ± 0.05 |
| Drug content uniformity (RSD %) (n = 36) | 2.76 |
| Appearance | Translucent, smooth and flexible |
| Weight (mg) (n = 28) | 162.0 ± 7.8 |
| Thickness (μm) (n = 28) | 95.0 ± 7.5 |
| Moisture content (% w/w) (n = 3) | 4.51 ± 0.29 |
| Tensile strength (g/mm2) (n = 3) | 2043.59 ± 141.96 |
| Elongation at break (%/mm2) (n = 3) | 506.67 ± 39.09 |
| Toughness (g/mm) (n = 3) | 83,168.56 ± 9247.50 |
| Elastic modulus (g/mm2) (n = 3) | 177.02 ± 17.15 |
Fig. 2.
(A) In vitro release profiles of drug-loaded film as a function of time in different media; (B) EFdA amount and cumulative release of drug-loaded film stored at 40 °C/75% RH for 3 months. Each point represents mean ± SD (n = 3).
Fig. 3.
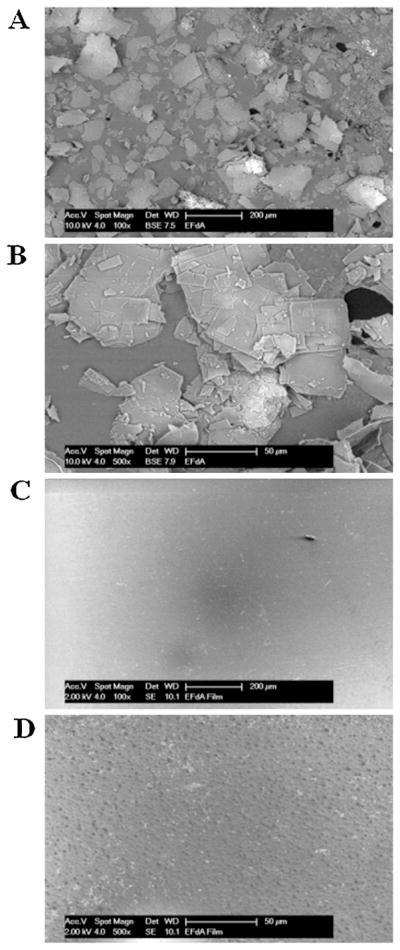
SEM images of EFdA powder (A and B) and EFdA-loaded film (C and D) with different scale bar.
Fig. 4.
X-ray diffractograms of EFdA, placebo and EFdA-loaded films.
3.4. In vitro cytotoxicity
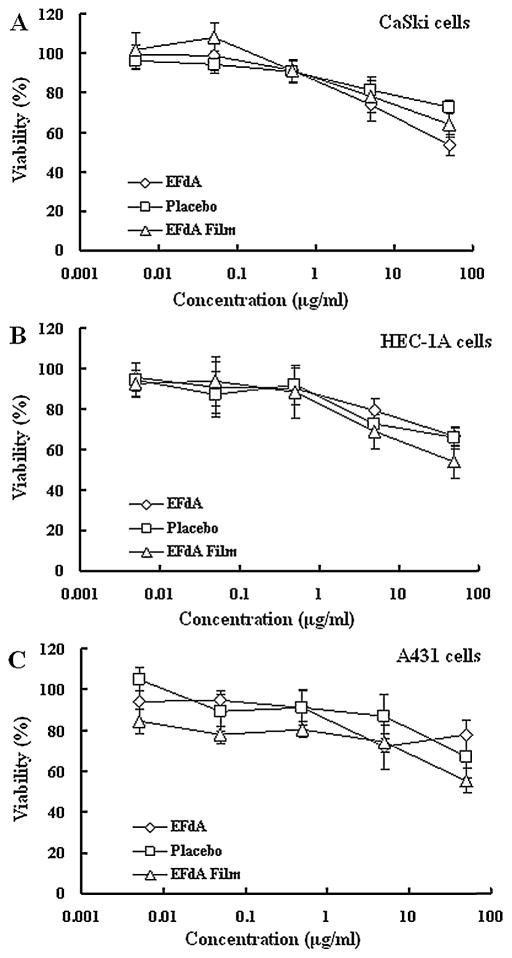
Topical formulations intended for prevention of HIV infection must be safe for patients. Various dilutions of EFdA, EFdA-loaded films and placebo films were cultured with three different epithelial cell lines for 24 h and the viability was measured as compared to the untreated control using MTT assay. As illustrated in Fig. 5, the 50% cytotoxic concentration (CC50) of free EFdA and EFdA-loaded film in CaSki, HEC-1A and A431 cell lines was greater than 50 μg/ml (approximately 170 μM), which is over 100,000 times the level that provides anti-HIV activity. No cytotoxicity was seen with placebo films, indicating that both EFdA and the film components are likely to be safe for topical use.
Fig. 5.
In vitro cytotoxicy of EFdA, drug-loaded film and placebo film at various concentrations against CaSki (A), HEC-1A (B) and A 431 (C) epithelia cells after 24 h incubation. Each point represents mean ± SD (n = 8).
3.5. Epithelial integrity study
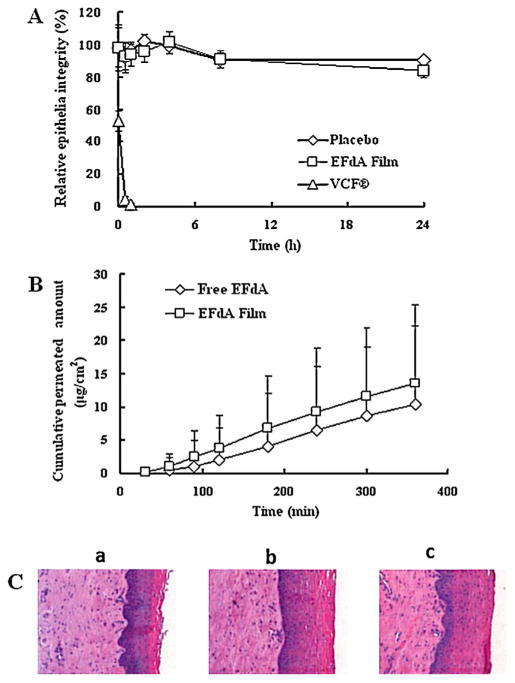
The VCF® film solution (positive toxicity control) reduced the TEER value of HEC-1A cell monolayers by ~95% at 0.5 h compared to that of negative control group; the TEER value did not return to normal levels after 24 h (Fig. 6A), suggesting that the high cytotoxicity and disruption of TEER caused by Nonoxynol-9 was irreversible. In contrast, placebo and EFdA-loaded film-treated HEC-1A monolayers varied by no more than 15% in TEER values over the incubation period, indicating low toxicity of the films to the cell monolayers, which was in good agreement with the in vitro cytotoxicity tests.
Fig. 6.
(A) Relative HEC-1A epithelial monolayer integrity of placebo and EFdA-loaded films as a function of time; (B) the cumulative amount of EFdA permeated through the human ectocervical tissues versus time. (C) Comparison of morphology of human ectocervical tissues: (a) EFdA film post-exposure; (b) Free EFdA post-exposure; (c) Pre-exposure. Each point represents mean ± SD (n = 4).
3.6. Tissue permeability
To determine whether the drug-loaded film has some impact on the EFdA permeation profile, we carried out tissue permeability studies using excised human ectocervical explants. EFdA permeation through the ectocervical tissue was determined by quantitating the amount of EFdA found in the receptor compartment at predetermined time intervals using the HPLC method as described above. As shown in Fig. 6B, both free EFdA and film formulated EFdA were able to permeate through the epithelia of excised human ectocervical explants, and it was found that there was no significant difference between free drug and drug-loaded film groups in the permeated amount of EFdA at each time point (P > 0.05), suggesting that incorporation of EFdA into the polymeric film matrix had no impact on the permeability of drug substance. Additionally, H&E staining showed that no significant morphological change in the human ectocervical tissues was observed (Fig. 6C).
3.7. Bioactivity study
In single replication cycle cell-based HIV assays, film-formulated EFdA showed potent antiviral activity (Fig. 7) similar to that provided by non-formulated drug (data not shown). Furthermore, a potent barrier to subsequent infection was established when uninfected cells were exposed to the film formulated material for 4 h prior to removal of the drug and infection with HIV (Fig. 7). Thus the polymeric film matrix had no apparent impact on cell accessibility to the formulated EFdA.
Fig. 7.
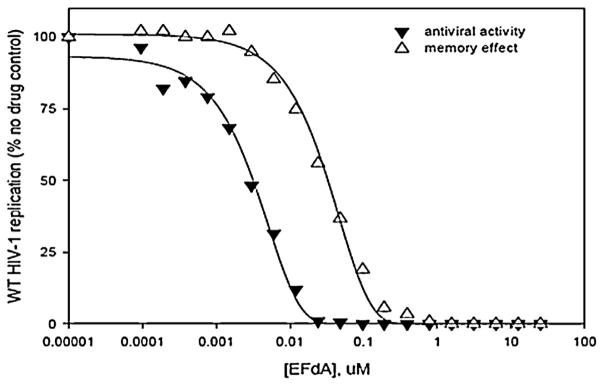
Bioactivity analysis of film-formulated EFdA. Antiviral activity: P4R5 cells were simultaneously exposed to HIV-1 and the indicated concentrations of the drug. Drug was maintained in the culture throughout the 48 h infection period; Protective activity or ‘memory’ effect: uninfected P4R5 cells were preincubated with the indicated concentration of drug for 4 h. The cells were washed free of exogenous drug and then exposed to infectious HIV. No exogenous drug was present throughout the 48 h infection period.
4. Discussion
Over the last 20 years of microbicide research, none of the 11 effectiveness trials of six candidate products have demonstrated meaningful protection against HIV infection. The results of the CAPRISA 004 clinical trial in 2010 showed that a pericoitally dosed 1% tenofovir (an NRTI) vaginal gel had potential to be formulated as a safe, effective, and acceptable product (Abdool Karim et al., 2010). Unfortunately, these promising results were not validated in the recently discontinued VOICE study which indicated that a once-daily regimen of 1% tenofovir vaginal gel was not effective in preventing HIV transmission. The differences in dosing regimens may account in part for these discrepant results, and the currently launched FACTS clinical trial in South Africa is designed to address the reproducibility of pericoital dosing (Ham et al., 2012).
Many factors in addition to the active pharmaceutical ingredient (API) can impact on pharmaceutical product development and efficacy, including dosing regimen, patient compliance, dosage form, formulation composition, etc. Film formulations usually comprise API, water soluble polymers, plasticizers, fillers, colorants and flavoring agents. Film-making polymers should be non-toxic; non-irritant; possess good wetting, spreadability and peelability; exhibit moderate mechanical properties; and inexpensive to manufacture and package (Garg et al., 2010). Additionally, thin film formulations usually include plasticizer to provide flexibility and pliability. The fast-dissolving vaginal film developed in this study was composed of PVA, HPMC E5 and propylene glycol. It is well known that PVA is used in the commercially available vaginal film product (VCF®) as the main film-making polymer. HPMC E5 and propylene glycol were also listed in the FDA Inactive Ingredients Database as accepted excipients in vaginal products. Therefore, the excipients selected in our formulation were FDA approved and found to be safe, as evidenced by our in vitro cytotoxicity and epithelial integrity tests.
The NRTI EFdA is as highly potent antiretroviral agent, inhibiting HIV in single replication cycle assays with an EC50 of about 1 nM (Michailidis et al., 2009). Similar antiviral efficacy was noted in studies using the film-formulated EFdA, indicating that film components had no negative impact on drug accessibility or activity. It was well known that a central source of toxicity in clinical study stemmed from the interaction of NRTIs with human mitochondrial DNA polymerase γ, the only human polymerase capable of using these NRTIs as substrates (Koczor and Lewis, 2010; Lewis et al., 2003). Fortunately, it indicated that EFdA was a poor polymerase γ substrate, suggesting the minimum toxicity mediated by polymerase γ (Sohl et al., 2012). Additionally, results showed that mice treated with EFdA at doses of 5 to 50 mg/kg for 14 days did not show any body weight loss, and no acute and subacute whole-body effects were observed (Hattori et al., 2009), demonstrating the low cytotoxicity of EFdA in vivo. Taken together, EFdA was a very promising anti-HIV agent for HIV prevention.
In this work, an overall desirability function was used for the optimization of placebo film formulation. The responses comprising tensile strength (Y1), elongation at break (Y2), toughness (Y3) and elastic modulus (Y4) were transformed into the individual desirability d1, d2, d3 and d4, respectively. Among them, Y1 should be moderate, and Y2 and Y3 had to be maximized while Y4 had to be minimized. The desirable ranges were from 0 to 1 (least to most desirable). The overall desirability function was calculated by Eqs. (5–8), and the result was shown in Table 1. The results showed that an increase in PVA content can result in a higher tensile strength, elongation and toughness, while an increase in PEG 400 concentration reflected a decrease in tensile strength and an increase in elongation and toughness, which suggested that: (i) PVA was not only a film-making polymer but also a kind of “weak plasticizer”, because addition of higher amount of PVA in the formulation resulted in an increase in elongation and toughness (Table 2); (ii) higher percentage of PVA (7.5%) can increase the tensile strength as well and make the film much tougher and more rigid, as evidenced by run 3 and 6; (iii) the plasticizer (PEG 400) can modify mechanical properties of the film by weakening intermolecular interactions between entangled polymer chains through hydrogen-bonding effect. From a molecular perspective, the plasticizer can penetrate into the polymer and increase the free space between the polymer chains by decreasing the cumulative intermolecular forces along the polymer chains (Tarvainen et al., 2001). In our study, it was found that propylene glycol had better plasticization effect than those of PEG 400 and 4000. The possible reason was that at equal concentration (3%, w/w), the total number of propylene glycol molecules in the film formulation was greater than that of PEG 400. Therefore, propylene glycol had more functional groups (–OH) than PEG 400, which can promote the plasticizer–polymer interaction by hydrogen-bonding effect (Heng et al., 2003). Moreover, as the molecular weight (MW) of PEG increased from 400 to 4000, a significant decrease of elongation at break and toughness of the film was observed as shown in Table 2, probably due to the lack of functional groups (–OH) and steric hindrance effect of PEG 4000 which can cause some difficulty in hydrogen bond formation and insertion between the polymer network (Luangtana-anan et al., 2010).
Microbicides are promising strategies to prevent sexual HIV transmission. During the preclinical screening of microbicide candidates, the drug substances and delivery systems should be assessed in suitable in vitro systems to determine the toxicity of each candidate before being further evaluated in animal models and clinical settings. In this study, in vitro cytotoxicity and epithelial integrity studies were performed to evaluate the toxicity of drug-loaded vaginal films. Three different epithelial cell lines were used for cytotoxicity study. The uterine HEC-1A cell line originates from a human endometrial adenocarcinoma. Caski is a uterine cervical carcinoma cell line. Apart from these two female genital epithelial cell lines, another human epidermoid carcinoma cell line A431 was also utilized to evaluate in vitro cytotoxicity. All the CC50 values of EFdA and drug-loaded films upon these three cell lines were found to be above 50 μg/ml. As reported, EFdA showed potent antiviral activity (EC50 = 4 nM, which was several orders of magnitude lower than CC50 of EFdA) against NRTI-resistant HIV strains (Kawamoto et al., 2008), suggesting that EFdA had a low cytotoxicity profile, and a low dose of EFdA might be required for formulation development because of its high potency. VCF® film containing 28% Nonoxynol-9 was used as positive control in epithelial integrity assessment and it was found that Nonoxynol-9 can cause severe damage to the integrity of HEC-1A cell monolayers compared to that of EFdA containing films. Nonoxynol-9 has been used as the active component of spermicides for more than 20 years, and the epithelial disruption and induced serious inflammation were observed in clinical setting (Gali et al., 2010; Hillier et al., 2005). Therefore, it is quite essential to screen out microbicide candidates that are active but do not disrupt epithelial integrity and function. Since EFdA is a novel NRTI, and works by interfering with the viral replication cycle, it is important for EFdA to permeate the stratified multilayer vaginal or cervical epithelium and reach the underlying target cells to take action. Thus, in order to understand the permeation profiles of free EFdA and EFdA-loaded film, four different fresh excised human cervical tissues were used for tissue permeability studies. Results showed that no significant difference was observed in permeated amount of drug substance in the receptor compartment between free EFdA and EFdA-loaded film groups (P > 0.05), suggesting that EFdA containing films can dissolve quickly and simultaneously release the active drug substance. Similarly, the potent antiviral activity noted with the film formulated EFdA (Fig. 7) is entirely consistent with rapid film dissolution and release of the active drug substance.
5. Conclusion
The fast-dissolving vaginal film containing EFdA was made by solvent casting method and optimized by using 32 factorial design and desirability function. The drug-loaded film with desired physicochemical property was found to be stable, non-toxic and highly potent against HIV infection. Thus, EFdA-loaded film could be considered as a promising solid dosage form for vaginal drug delivery.
Acknowledgments
We gratefully acknowledge the staff associated with the Materials Micro-Characterization Laboratory, Department of Mechanical Engineering and Materials Science, University of Pittsburgh, for assistance with the SEM and XRD conducted in this study. Research reported in this publication was supported in part by grants AI076119 and AI079801 from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
References
- Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany ABM, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abram ME, Parniak MA. Virion instability of human immunodeficiency virus type 1 reverse transcriptase (RT) mutated in the protease cleavage site between RT p51 and the RT ribonuclease H domain. J Virol. 2005;79:11952–11961. doi: 10.1128/JVI.79.18.11952-11961.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil A, Parniak MA, Dezzutti CS, Moncla BJ, Cost MR, Li M, Rohan LC. Development and characterization of a vaginal film containing dapivirine, a non-nucleoside reverse transcriptase inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Delivery Transl Res. 2011;1:511–517. doi: 10.1007/s13346-011-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarini J, Van Damme L. Microbicide drug candidates to prevent HIV infection. Lancet. 2007;369:787–797. doi: 10.1016/S0140-6736(07)60202-5. [DOI] [PubMed] [Google Scholar]
- Cutler B, Justman J. Vaginal microbicides and the prevention of HIV transmission. Lancet Infect Dis. 2008;8:685–697. doi: 10.1016/S1473-3099(08)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias C, Coggins C. Acceptability research on female-controlled barrier methods to prevent heterosexual transmission of HIV: where have we been? Where are we going? J Womens Health Gender Based Med. 2001;10:163–173. doi: 10.1089/152460901300039502. [DOI] [PubMed] [Google Scholar]
- Gali Y, Ariën KK, Praet M, Van de Bergh R, Temmerman M, Delezay O, Vanham G. Development of an in vitro dual-chamber model of the female genital tract as a screening tool for epithelial toxicity. J Virol Methods. 2010;165:186–197. doi: 10.1016/j.jviromet.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Garg S, Goldman D, Krumme M, Rohan LC, Smoot S, Friend DR. Advances in development, scale-up and manufacturing of microbicide gels, films and tablets. Antiviral Res. 2010;88S:19–29. doi: 10.1016/j.antiviral.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Ham AS, Rohan LC, Boczar A, Yang L, Buckheit KW, Buckheit RW., Jr Vaginal film drug delivery of the pyrimidinedione IQP-0528 for the prevention of HIV infection. Pharm Res. 2012;29:1897–1907. doi: 10.1007/s11095-012-0715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori S, Ide K, Nakata H, Harade H, Suzu S, Ashida N, Kohgo S, Hayakawa H, Mitsuya H, Okada S. Potent activity of a nucleoside reverse transcriptase inhibitor, 4′-ethynyl-2-fluoro-2′-deoxyadenosine, against human immunodeficiency virus type 1 infection in a model using human peripheral blood mononuclear cell-transplanted Nod/SCID janus kinase 3 knockout mice. Antimicrob Agents Chemother. 2009;53:3887–3893. doi: 10.1128/AAC.00270-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng PW, Chan LW, Ong KT. Influence of storage conditions and type of plasticizers on ethyl cellulose and acrylate films formed from aqueous dispersions. J Pharm Pharm Sci. 2003;6:334–344. [PubMed] [Google Scholar]
- Hillier SL, Moench T, Shatock R, Black R, Reichelderfer P, Veronese F. In vitro and in vivo: the story of nonoxynol 9. J Acquired Immune Defic Syndr. 2005;39:1–8. doi: 10.1097/01.qai.0000159671.25950.74. [DOI] [PubMed] [Google Scholar]
- Hladik F, Hope TJ. HIV infection of the genital mucosa in women. Curr HIV/AIDS Rep. 2009;6:20–28. doi: 10.1007/s11904-009-0004-1. [DOI] [PubMed] [Google Scholar]
- Kawamoto A, Kodama E, Sarafianos SG, Sakagami Y, Kohgo S, Kitano K, Ashida N, Iwai Y, Hayakawa H, Nakata H, Mitsuya H, Arnold E, Matsuoka M. 2′-Deoxy-4′-C-ethynyl-2-halo-adenosines active against drug-resistant human immunodeficiency virus type 1 variants. Int J Biochem Cell Biol. 2008;40:2410–2420. doi: 10.1016/j.biocel.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Koczor CA, Lewis W. Nucleoside reverse transcriptase inhibitor toxicity and mitochondrial DNA. Expert Opin Drug Metab Toxicol. 2010;6:1493–1504. doi: 10.1517/17425255.2010.526602. [DOI] [PubMed] [Google Scholar]
- Lewis W, Day BJ, Copeland WC. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nat Rev Drug Discovery. 2003;2:812–822. doi: 10.1038/nrd1201. [DOI] [PubMed] [Google Scholar]
- Luangtana-anan M, Nunthanid J, Limmatvapirat S. Effect of molecular weight and concentration of polyethylene glycol on physicochemical properties and stability of shellac film. J Agric Food Chem. 2010;58:12934–12940. doi: 10.1021/jf1031026. [DOI] [PubMed] [Google Scholar]
- Michailidis E, Marchand B, Kodama EN, Singh K, Matsuoka M, Kirby KA, Ryan EM, Sawani AM, Nagy E, Mitsuya H, Parniak MA, Sarafianos SG. Mechanism of inhibition of HIV-1 reverse transcriptase by 4′-ethynyl-2-fluoro-2′-deoxyadenosine triphosphate, a translocation defective reverse transcriptase inhibitor. J Biol Chem. 2009;284:35681–35691. doi: 10.1074/jbc.M109.036616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey-Corb M, Rajakimar P, Michael H, Nyaundi J, Didier P, Reeve AB, Mitsuya H, Sarafianos SG, Parniak MA. Efficacy of the novel nucleoside reverse transcriptase inhibitor 4′-ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) in controlling virus burden and treating AIDS-like disease in SIV-infected macaques. Antimicrob Agents Chemother. 2012;56:4707–4712. doi: 10.1128/AAC.00723-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata H, Amano M, Koh Y, Kodama E, Yang G, Bailey CM, Kohgo S, Hayakawa H, Matsuoka M, Anderson KS, Cheng YC, Mitsuya H. Activity against human immunodeficiency virus type 1, intracellular metabolism, and effects on human DNA polymerases of 4′-ethynyl-2-fluoro-2′-deoxyadenosine. Antimicrob Agents Chemother. 2007;51:2701–2708. doi: 10.1128/AAC.00277-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel AM, Mitchnick LB, Risha P, Makoye Muungo LT, Norick PM. Acceptability of vaginal film, soft-gel capsule, and tablet as potential microbicide delivery methods among African women. J Women’s Health. 2011;20:1207–1214. doi: 10.1089/jwh.2010.2476. [DOI] [PubMed] [Google Scholar]
- Raymond EG, Alvarado G, Ledesma L, Diaz S, Bassol S, Morales E, Fernandez V, Carlos G. Acceptability of two spermicides in five countries. Contraception. 1999;60:45–50. doi: 10.1016/s0010-7824(99)00060-8. [DOI] [PubMed] [Google Scholar]
- Raymond EG, Chen PL, Condon S, Luoto J, Barnhart KT, Creinin MD, Poindexter A, Wan L, Martens M, Scheken R, Blackwell R. Acceptability of five nonoxynol-9 spermicides. Contraception. 2005;71:438–442. doi: 10.1016/j.contraception.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan LC, Sassi AB. Vaginal drug delivery systems for HIV prevention. AAPS J. 2009;11:78–87. doi: 10.1208/s12248-009-9082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano J, Malcolm RK, Garg S, Rohan LC, Kaptur PE. Microbicide delivery: formulation technologies and strategies. Curr Opin HIV AIDS. 2008;3:558–566. doi: 10.1097/COH.0b013e328305b96e. [DOI] [PubMed] [Google Scholar]
- Sohl CD, Singh K, Kasiviswanathan R, Copeland WC, Mitsuya H, Sarafianos SG, Anderson KS. Mechanism of interaction of human mitochondrial DNA polymerase γ with the novel nucleoside reverse transcriptase inhibitor 4′-ethynyl-2-fluoro-2′-deoxyadenosine indicates a low potential for host toxicity. Antimicrob Agents Chemother. 2012;56:1630–1634. doi: 10.1128/AAC.05729-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone A. Microbicides: a new approach to preventing HIV and other sexually transmitted infections. Nat Rev Drug Discov. 2002;1:977–985. doi: 10.1038/nrd959. [DOI] [PubMed] [Google Scholar]
- Tarvainen M, Sutinen R, Somppi M, Paronen P, Poso A. Predicting plasticization efficiency from three-dimensional molecular structure of a polymer plasticizer. Pharm Res. 2001;18:1760–1766. doi: 10.1023/a:1013386900232. [DOI] [PubMed] [Google Scholar]
- UNAIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic 2010. UNAIDS; 2010. [Google Scholar]
- Zhang W, Hao JG, Shi Y, Li YJ, Wu J, Sha XY, Fang XL. Paclitaxel-loaded Pluronic P123/F127 mixed polymeric micelles: formulation, optimization and in vitro characterization. Int J Pharm. 2009;376:176–185. doi: 10.1016/j.ijpharm.2009.04.030. [DOI] [PubMed] [Google Scholar]
- Zhang W, Parniak MA, Mitsuya H, Sarafianos SG, Graebing PW, Rohan LC. Preformulation studies of EFdA, a novel nucleoside reverse transcriptase inhibitor for HIV prevention. Drug Dev Ind Pharm. 2013 doi: 10.3109/03639045.2013.809535. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]