Abstract
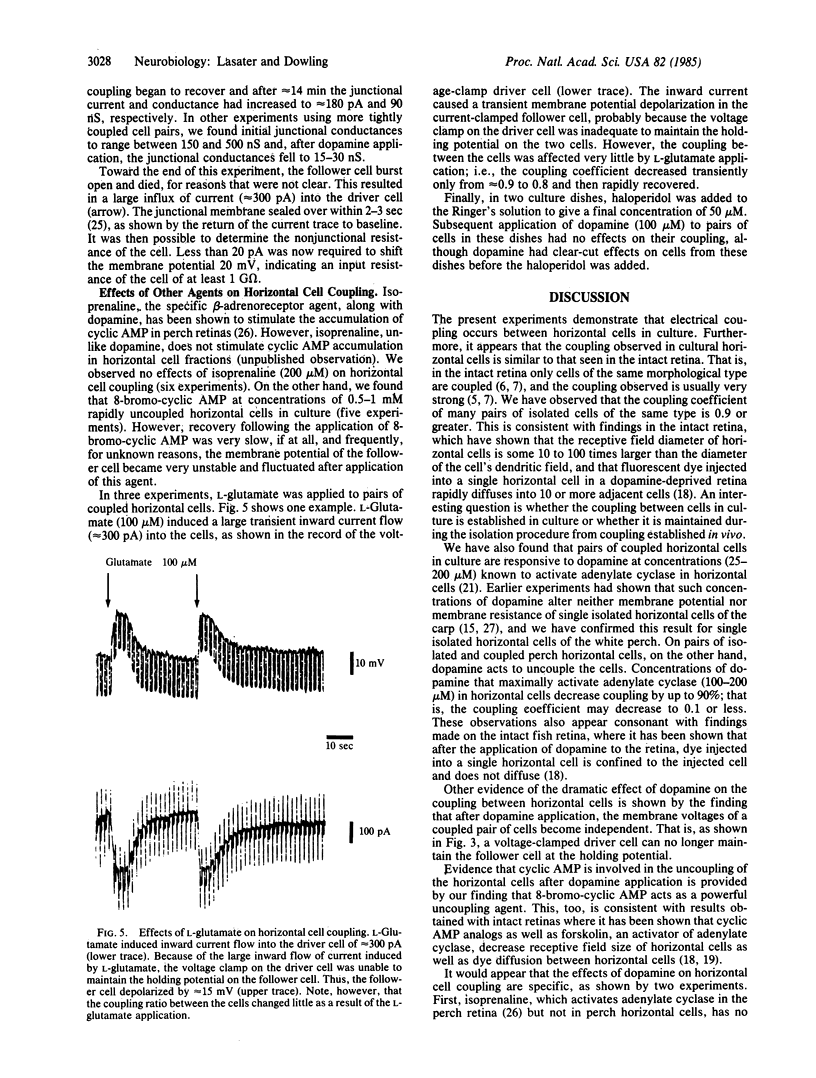
Horizontal cells from the white perch were isolated by enzymatic treatment and trituration of the retina and were maintained in culture for 1-5 days. Overlapping pairs of horizontal cells were identified, and the two cells were recorded from simultaneously, using whole-cell patch clamp techniques. Electrical coupling between cells was determined by passing current pulses into one cell, the driver cell, while (i) recording voltage changes in the other, follower cell, or (ii) measuring current flow into the follower cell. Most cell pairs of the same morphological type were coupled electrically, with coupling coefficients often greater than 0.9. Junctional resistance was typically found to be between 20 and 60 M omega and junctional conductance was between 150 and 500 nS. After application of 1-microliter pulses of dopamine (200 microM) to coupled pairs of cells, the coupling coefficient fell to approximately equal to 0.1, junctional resistance increased to 300-700 M omega, and junctional conductance decreased to 15-30 nS. Recovery of coupling took, for most cell pairs tested, 8-15 min after dopamine application. The exogenous application of 8-bromo-cyclic AMP (0.5-1 mM) also caused uncoupling of horizontal cell pairs; however, neither isoprenaline nor L-glutamate altered coupling significantly.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. L., Dowling J. E. The role of the retinal interplexiform cell: effects of 6-hydroxydopamine on the spatial properties of carp horizontal cells. Brain Res. 1983 Apr 4;264(2):307–310. doi: 10.1016/0006-8993(83)90830-2. [DOI] [PubMed] [Google Scholar]
- Davis G. W., Naka K. Spatial organization of catfish retinal neurons. I. Single- and random-bar stimulation. J Neurophysiol. 1980 Mar;43(3):807–831. doi: 10.1152/jn.1980.43.3.807. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Ehinger B. The interplexiform cell system. I. Synapses of the dopaminergic neurons of the goldfish retina. Proc R Soc Lond B Biol Sci. 1978 Apr 13;201(1142):7–26. doi: 10.1098/rspb.1978.0030. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Lasater E. M., Van Buskirk R., Watling K. J. Pharmacological properties of isolated fish horizontal cells. Vision Res. 1983;23(4):421–432. doi: 10.1016/0042-6989(83)90089-5. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. S-potentials in the skate retina. Intracellular recordings during light and dark adaptation. J Gen Physiol. 1971 Aug;58(2):163–189. doi: 10.1085/jgp.58.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harris A. L., Spray D. C., Bennett M. V. Control of intercellular communication by voltage dependence of gap junctional conductance. J Neurosci. 1983 Jan;3(1):79–100. doi: 10.1523/JNEUROSCI.03-01-00079.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassin G. Pikeperch horizontal cells identified by intracellular staining. J Comp Neurol. 1979 Aug 15;186(4):529–540. doi: 10.1002/cne.901860403. [DOI] [PubMed] [Google Scholar]
- Hedden W. L., Jr, Dowling J. E. The interplexiform cell system. II. Effects of dopamine on goldfish retinal neurones. Proc R Soc Lond B Biol Sci. 1978 Apr 13;201(1142):27–55. doi: 10.1098/rspb.1978.0031. [DOI] [PubMed] [Google Scholar]
- Ishida A. T., Fain G. L. D-aspartate potentiates the effects of L-glutamate on horizontal cells in goldfish retina. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5890–5894. doi: 10.1073/pnas.78.9.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A. Electrical connexions between horizontal cells in the dogfish retina. J Physiol. 1971 Feb;213(1):95–105. doi: 10.1113/jphysiol.1971.sp009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M., Dowling J. E. Carp horizontal cells in culture respond selectively to L-glutamate and its agonists. Proc Natl Acad Sci U S A. 1982 Feb;79(3):936–940. doi: 10.1073/pnas.79.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein W. R., Nakas M., Socolar S. J. Junctional membrane uncoupling. Permeability transformations at a cell membrane junction. J Gen Physiol. 1967 Aug;50(7):1865–1891. doi: 10.1085/jgp.50.7.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. The generation and spread of S-potentials in fish (Cyprinidae). J Physiol. 1967 Sep;192(2):437–461. doi: 10.1113/jphysiol.1967.sp008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I. The horizontal cells. Vision Res. 1972 Apr;12(4):573–588. doi: 10.1016/0042-6989(72)90153-8. [DOI] [PubMed] [Google Scholar]
- Negishi K., Drujan B. D. Reciprocal changes in center and surrounding S potentials of fish retina in response to dopamine. Neurochem Res. 1979 Jun;4(3):313–318. doi: 10.1007/BF00963801. [DOI] [PubMed] [Google Scholar]
- Norton A. L., Spekreijse H., Wolbarsht M. L., Wagner H. G. Receptive field organization of the S-potential. Science. 1968 May 31;160(3831):1021–1022. doi: 10.1126/science.160.3831.1021. [DOI] [PubMed] [Google Scholar]
- Piccolino M., Neyton J., Gerschenfeld H. M. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5'-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984 Oct;4(10):2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Permeability of a cell junction and the local cytoplasmic free ionized calcium concentration: a study with aequorin. J Membr Biol. 1976 Aug 27;28(1):87–119. doi: 10.1007/BF01869692. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981 Feb 13;211(4483):712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- Spray D. C., White R. L., de Carvalho A. C., Harris A. L., Bennett M. V. Gating of gap junction channels. Biophys J. 1984 Jan;45(1):219–230. doi: 10.1016/S0006-3495(84)84150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell W. K., Lightfoot D. O. Color-specific interconnections of cones and horizontal cells in the retina of the goldfish. J Comp Neurol. 1975 Feb 15;159(4):473–502. doi: 10.1002/cne.901590404. [DOI] [PubMed] [Google Scholar]
- TOMITA T., TOSAKA T., WATANABE K., SATO Y. The fish EIRG in response to different types of illumination. Jpn J Physiol. 1958 Mar 30;8(1):41–50. doi: 10.2170/jjphysiol.8.41. [DOI] [PubMed] [Google Scholar]
- Teranishi T., Negishi K., Kato S. Dopamine modulates S-potential amplitude and dye-coupling between external horizontal cells in carp retina. Nature. 1983 Jan 20;301(5897):243–246. doi: 10.1038/301243a0. [DOI] [PubMed] [Google Scholar]
- Turin L., Warner A. E. Intracellular pH in early Xenopus embryos: its effect on current flow between blastomeres. J Physiol. 1980 Mar;300:489–504. doi: 10.1113/jphysiol.1980.sp013174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buskirk R., Dowling J. E. Isolated horizontal cells from carp retina demonstrate dopamine-dependent accumulation of cyclic AMP. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7825–7829. doi: 10.1073/pnas.78.12.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Dowling J. E. Synaptic relationships in the plexiform layers of carp retina. Z Zellforsch Mikrosk Anat. 1969;100(1):60–82. doi: 10.1007/BF00343821. [DOI] [PubMed] [Google Scholar]
- Yamada E., Ishikawa T. The fine structure of the horizontal cells in some vertebrate retinae. Cold Spring Harb Symp Quant Biol. 1965;30:383–392. doi: 10.1101/sqb.1965.030.01.038. [DOI] [PubMed] [Google Scholar]