Abstract
A new hexaurea receptor has been synthesized, which absorbs atmospheric CO2 to produce an air-stable solid carbonate complex under normal conditions. Structural analysis of the carbonate complex with this receptor suggests that the carbonate is fully encapsulated within its highly organized intramolecular cavity via twelve strong NH···O bonds in the range of 2.703(3) – 2.989(3) Å from six urea units, with each anionic oxygen coordinated via four NH···O bonds with two urea groups.
Continuous increase of carbon dioxide (CO2) concentration in the atmosphere resulting from various human activities poses a threat to global warming.1 CO2 is also a major source of water acidification by dissolving in water to form carbonic acid (H2CO3).2 Therefore, there is a current need to develop new chemical devices for the recycling of carbon dioxide to produce chemical commodities.3 Recently epoxide-based molecules have been used to react with CO2 in the presence of a catalyst to produce new polycarbonates and cyclic carbonates.4 Gale et al. reported that simple mono-functional urea-based neutral compounds are capable of absorbing CO2 in the presence of primary aliphatic amines to form carbamates [>N(CO2)−].5 Increasing the functional groups, tren-based tris-ureas/thioureas,6 such as pentafluorophenyl substituted tris-urea,6a m-nitrophenyl substituted tris-urea,6b phenyl substituted tris-thiourea,6c and p-cyanophenyl substituted tris-thiourea6d have recently been shown to bind carbonate anions, forming 2:1 (host:guest) complexes. Although the carbonate is coordinated via 12 hydrogen bonds (NH···O < 3.2 Å) in most cases, two receptors are required to provide complementary binding sites for the anion. Therefore, the complete coordination for a carbonate anion can ideally be achieved by a receptor possessing 12 complementary binding sites around a single cavity.
It is well-documented that increasing the effective binding sites in a host, leads to the enhancement of its binding ability for a guest due to the chelate effect.7 From this viewpoint, we have been interested in synthesizing polyurea-functionalized receptors based on the commercially available ‘tren’ as a core. Herein, we report a highly organized hexaurea receptor possessing 12 H-bond donors, which absorbs atmospheric CO2 in the form of carbonate encapsulated in a single cavity surrounded by perfectly arranged six urea units. Within this self-generated intramolecular cavity, the unique orientation of 12 binding sites provides an ideal complementarity for the trigonal planar carbonate anion.
The new hexaurea receptor 1 was synthesized by a three-step strategy (Scheme 1). The tris(2-aminoethyl) amine (tren) and o-nitrophenyl isocyanate were reacted to give a nitro-functionalized tris-urea 2 which was reduced with hydrazine hydrate and Pd/C (10%) to produce an amino-functionalized tris-urea 3.8 The final coupling was achieved by reacting 3 with pentafluorophenyl isocyanate 4 to form an o-xylyl bridged hexaurea 1.
Scheme 1.
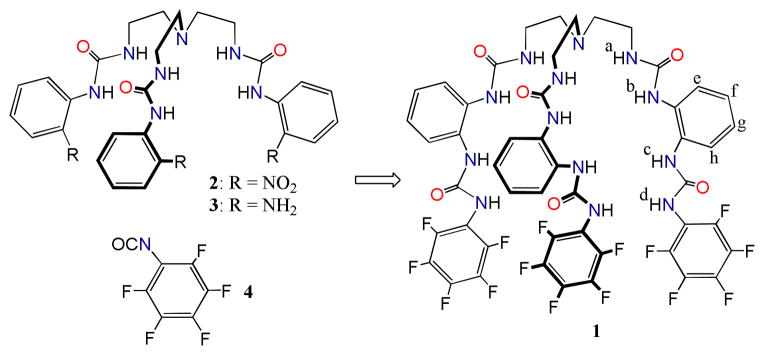
Synthesis of the hexaurea 1.
Single crystals of carbonate complex [1(CO3)](n-Bu4N)2 were obtained from slow evaporation of a DMSO solution of 1 with an excess amount of n-tetrabutyl ammonium fluoride (TBAF) in a vial at room temperature in 2 days. The needle-like crystals were isolated by simple decantation technique, and were found to be air stable. Although, the primary goal was to obtain a fluoride complex, crystallographic analysis revealed that, instead of fluoride, one carbonate was encapsulated within the cavity of 1. The bound carbonate was originated from atmospheric CO2 absorbed by the basic TBAF–receptor solution which was left open to the atmosphere. The formation of carbonate in the presence of TBAF is well documented in the literature.6
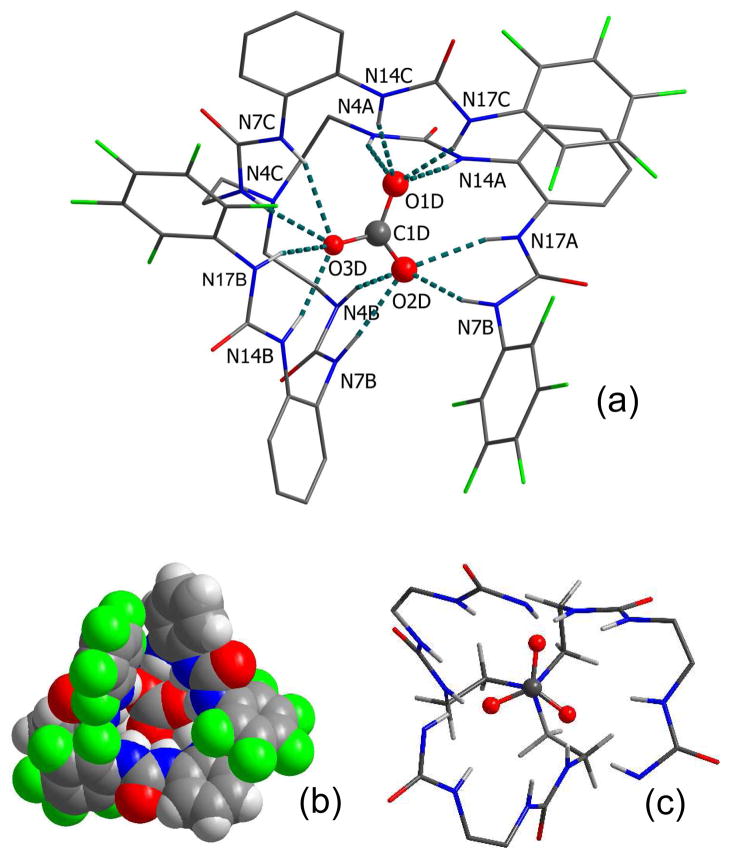
Structural analysis from single crystal X-ray diffraction of the carbonate complex reveals that the complex crystallizes in an orthorhombic Pna21 space group to give a molecular formula, [1(CO3)](n-Bu4N)2 in which one divalent carbonate is fully encapsulated.9 Two tetrabutyl ammonium ions remain outside the cavity to balance the anionic charges. In the receptor unit, all protons on NH groups are pointed towards the cavity, and serve as H-bond donors for the bound anion.
As shown in Figure 1, the encapsulated carbonate is held tightly inside the cavity by strong 12 NH···O bonds (dNH···O = 2.703(3) – 2.989(3) Å) from 12 NH groups of urea units (Table 1). Each oxygen of the carbonate is coordinated to four NH’s from two different arms of the hexaurea receptor, with the two inner NH’s from one arm and two outer NH’s from other arm. Within a single arm of the receptor, the pentafluorophenyl and o-xylyl rings linked with an outer urea group, are almost perpendicular to each other, while these two aromatic rings from two different arms are stacked together via π···π or C—H···π interactions (πc···πc = 3.602 Å, C8E—H···π = 3.841 Å; and C5E—H···π = 3.855 Å). Such arrangements of the aromatic rings make the receptor preorganized for the complete participation of all six ureas in coordinating the internal anion. The space-filling view of the complex (Figure 1b), illustrates the encapsulated carbonate inside the cavity of 1, showing the stacking of the aromatic groups. The trigonal planar carbonate is almost perpendicular to the axis of the tertiary nitrogen (N4C) of 1 and the carbon (C1D) of CO32−, forming a pseudo C3-symmetric complex (Figure 1c).10
Figure 1.
(a) Single crystal X-ray structure of the carbonate complex of 1 (counter cations and hydrogen atoms on carbons are omitted for clarity), (b) space filling model of encapsulated carbonate complex, (c) partial view of pseudo C3-symmetric arrangement of tripodal cavity with the encapsulated carbonate.
Table 1.
H-bonding parameters (Å, °) for [1(CO3)]2−.
| D—H···O | H···O | D···O | ∠DHO |
|---|---|---|---|
| N4A — H4A···O1D | 2.20(3) | 2.966(3) | 152(3) |
| N7A — H7A···O1D | 2.01(3) | 2.852(2) | 165(3) |
| N14A — H14A ··· O2D | 2.16(3) | 2.935(3) | 155(2) |
| N17A — H17A ··· O2D | 1.84(3) | 2.703(3) | 161(3) |
| N7B — H7B ··· O2D | 1.99(3) | 2.869(3) | 162(2) |
| N4B — H4B ··· O2D | 2.19(3) | 2.949(3) | 154(3) |
| N14B — H14B ··· O3D | 1.93(3) | 2.801(3) | 157(2) |
| N17B — H17B ··· O3D | 2.00(3) | 2.788(3) | 154(3) |
| N4C — H4C ··· O3D | 2.03(3) | 2.822(3) | 162(3) |
| N7C — H7C ··· O3D | 2.21(3) | 2.989(3) | 153(3) |
| N14C — H14C ··· O1D | 1.97(3) | 2.783(2) | 160(3) |
| N17C — H17C ··· O1D | 1.92(3) | 2.746(3) | 156(3) |
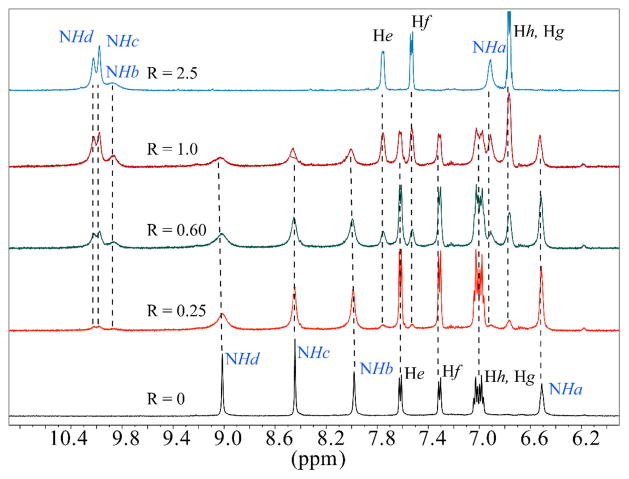
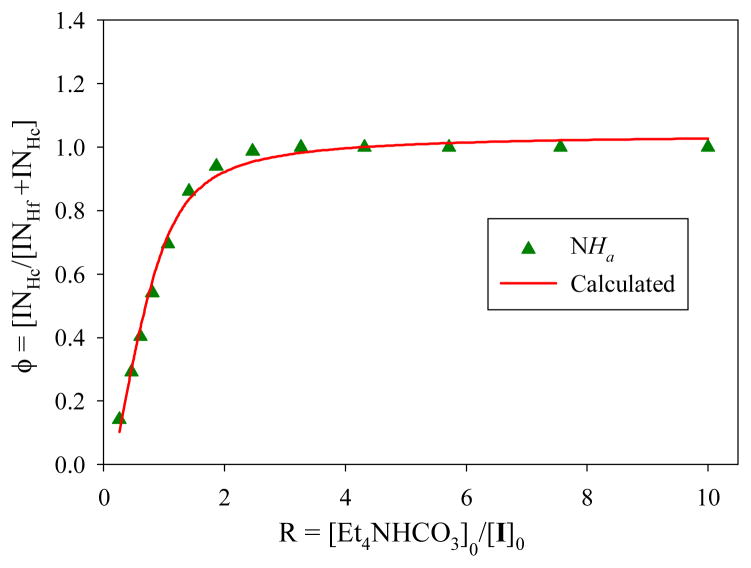
Proton NMR titrations were performed to examine the stability and stoichiometry of the receptor-carbonate complex in DMSO-d6 using Et4NHCO3. Upon the addition of Et4NHCO3 (20 mM) to the receptor (2 mM), a new set of NMR spectrum appeared due to slow exchange on the NMR time scale (Figure 2).11 All four NH signals were shifted significantly to downfield (ΔδNHa = 0.41ppm, ΔδNHb = 1.88 ppm, ΔδNHc = 1.53 ppm and ΔδNHd = 1.08 ppm), indicating the interactions of all NH groups with the anion. Presumably, the chelation of the bound anion, as also observed in the solid state structure of the complex of 1, results into the formation of a kinetically stable complex on the NMR time scale.8 The relative change in the integration intensity NH resonaces of the 1-bicarbonate complex and the free 1, allowed us to determine the binding constant (Figure 3).11 The experimental data provided the best fit to a 1:1 (host: guest) binding model,12 yielding a binding constant K = 1780 M−1. The 1:1 binding in solution was further supported by a Job’s plot analysis (Figure S17). It is noted that, because of the unavailability of a suitable DMSO soluble CO32− salt, the HCO3− as a tetraethyl ammonium (Et4N+) salt was used in the NMR titration studies, as previously used by other groups6 for tren-based ligands. Thus, the determined binding constant (K = 1780 M−1) is the result of the interactions of 1 with singly charged HCO3− as opposed to CO32− observed in the crystal. The time dependent NMR spectra of 1 and Et4NHCO3 in DMSO-d6 showed no change in the NMR signals, suggesting that HCO3− was not deprotonated to form CO32− during the titration process (Figure S18).
Figure 2.
Partial 1H NMR spectra of 1 with an increasing amount of Et4NHCO3 (R = [Et4NHCO3]0/[1]0) in DMSO-d6.
Figure 3.
1H NMR titration curve of 1 (2 mM) with an increasing amount of Et4NHCO3 (R = [Et4NHCO3]0/[1]0) in DMSO-d6. The titration plot was obtained from the relative change in the integration intensity Δϕ(Δϕ = INHc/[INHf +INHc], where INHc is the intensity of NHa signal in the carbonate-1 complex and INHf is the intensity of the corresponding NH signal in the free 1).
The binding affinity of 1 for Et4NHCO3 was compared with its precursor tris-urea receptor 2 (Scheme 1) under similar conditions in DMSO-d6. The results show that this receptor binds HCO3− with K = 226 M−1 (Figure S24 in ESI), which is much weaker than 1780 M−1 observed for 1. An n-butyl-functionalized tris-thiourea reported previously was shown to bind bicarbonate with K = 564 M−1 in DMSO-d6.6c This observation further demonstrates that the unique orientation of the binding sites in the hexaurea 1, makes it an excellent binder for the carbonate anions due to the possible chelate effect observed in traditional transition metal complexes.7b We also performed 1H NMR titrations of 1 for other anions, showing strong affinity for HSO4− (2250 M−1). The association constants of 1 with Cl− and CH3COO− were 256 and 200 M−1, respectively. However, the receptor showed no significant binding for I−, ClO4− and NO3−. Determination of binding constants for F− and H2PO4− was hampered due to the broadening of NMR peaks.
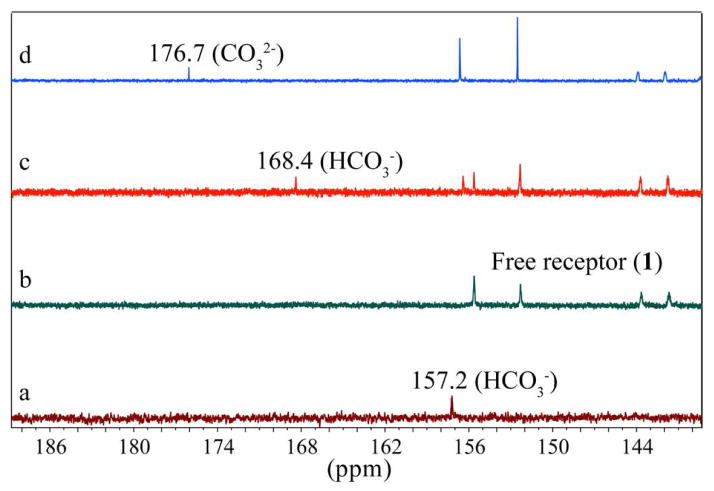
The formation of the complex in DMSO-d6 was further evaluated by a series of 13C NMR spectra (Figure 4). Partial 13C NMR of Et4NHCO3 and free 1 are shown in Figure 4a and Figure 4b, respectively. The sharp signal at 157.19 ppm in the free Et4NHCO3 shifted to 168.38 ppm (Δδ = 11.19 ppm) after the addition of one equivalent of the ligand (Figure 4c), indicating the encapsulation of HCO3− in the receptor’s cavity.6a Figure 4d displays the 13C NMR of [1(CO3)](n-Bu4N)2] used for single crystal diffraction, showing a peak on more downfield at 176.7 ppm for CO32−. This difference could be due to the charges (HCO3− vs CO32−) and the counter cations (Et4N+ vs n-Bu4N+) present in the two different samples.
Figure 4.
Partial 13C NMR spectra (125 MHz, DMSO-d6) of (a) Et4NHCO3, (b) free receptor 1, (c) 1 in the presence of one equivalent of Et4NHCO3, and (d) solid carbonate complex of the receptor ([1(CO3)](n-Bu4N)2).
The solid state FT-IR analysis was also performed to examine the interactions of the receptor with carbonate. The free receptor shows two peaks at 1650 cm−1 and 1600 cm−1 for two carbonyl groups (-C=O),13 which shift to higher wave numbers at 1696 cm−1 (ΔνCO =46 cm−1) and 1630 cm−1 (ΔνCO = 30 cm−1), respectively (Figure S15). Further, the significant upward shift (ΔνN-H=74 cm−1) of broad NH’s stretching frequency from 3330 cm−1 (free 1) to 3404 cm−1 (complex) was observed, suggesting the strong N—H···O interactions between NH groups and the carbonate.
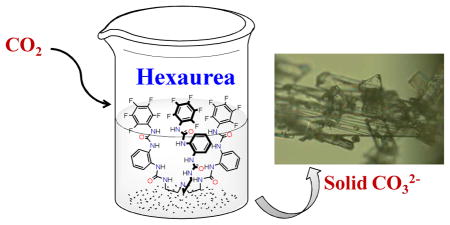
The ability of 1 to uptake of the atmospheric CO2 was examined by dissolving the receptor with TBAF in DMSO and keeping the mixture in open air (Figure 5). The solid products deposited at the interface of the liquid and air, were collected to give almost 99% carbonate complex. The complex was characterized by 1H NMR and also verified from X-ray diffraction analysis (CCDC 969272), giving an identical structure described in the preceding section. Treating the complex with water and methanol (1:1, v/v) gave over 95% recovery of 1. In previous reports, a pentafluorophenyl substituted tris-urea, 6a and a m-nitrophenyl substituted tris-urea6b were shown to fix atmospheric CO2, forming 98% and 90% complexes, respectively.
Figure 5.

The uptake of CO2 and the recovery of 1. Magnified 1.carbonate crystals are shown at the right side.
In conclusion, we have developed a novel receptor with six urea groups possessing an intramolecular cavity that is suitable to absorb atmospheric CO2 as a CO32− under mild basic conditions. The highly functionalized receptor molecule utilizes all twelve NH-binding sites to interact with the internal carbonate anion, forming an air-stable complex. The solid state structure described herein represents an exceptional example of the encapsulated carbonate in a single molecule providing an ideal complementarity of binding sites for the anion. Given the significant role of CO2 in environmental, chemical and biological systems,3,14 the present findings hold promise for developing novel chemical devices for the recycling of atmospheric carbon dioxide.
Supplementary Material
Acknowledgments
The National Science Foundation is acknowledged for a CAREER award (CHE-1056927) to MAH. NMR core facility at JSU was supported by the National Institutes of Health (G12RR013459).
Footnotes
Supporting Information available: Crystallographic file in CIF format, synthetic procedures and NMR titration spectra in pdf format. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Hansen J, Nazarenko L, Ruedy R, Sato M, Willis J, Genio AD, Koch D, Lacis A, Lo K, Menon S, Novakov T, Perlwitz J, Russell G, Schmidt GA, Tausnev N. Science. 2005;308:1431. doi: 10.1126/science.1110252. [DOI] [PubMed] [Google Scholar]; (b) Climate Change 2007: Synthesis Report, International Panel on Climate Change. Cambridge University Press; Cambridge, UK: 2007. [Google Scholar]
- 2.National Research Council. Ocean Acidification: A National Strategy to Meet the Challenges of a Changing Ocean. Washington, D.C., USA: The National Academies Press; 2010. p. 1. [Google Scholar]
- 3.Das C, Gomes N, Jacquet O, Villiers C, Thuéry P, Ephritikhine M, Cantat T. Angew Chem, Int Ed. 2012;51:187. doi: 10.1002/anie.201105516. [DOI] [PubMed] [Google Scholar]
- 4.(a) Darensbourg DJ. Chem Rev. 2007;107:2388. doi: 10.1021/cr068363q. [DOI] [PubMed] [Google Scholar]; (b) Sakakura T, Kohno K. Chem Commun. 2009:1312. doi: 10.1039/b819997c. [DOI] [PubMed] [Google Scholar]
- 5.(a) Edwards PR, Hiscock JR, Gale PA. Tetrahedron Lett. 2009;50:4922. [Google Scholar]; (b) Edwards PR, Hiscock JR, Gale PA, Light ME. Org Biomol Chem. 2010;8:100. doi: 10.1039/b917140a. [DOI] [PubMed] [Google Scholar]
- 6.(a) Ravikumar I, Ghosh P. Chem Commun. 2010;46:1082. doi: 10.1039/b915661e. [DOI] [PubMed] [Google Scholar]; (b) Dey SK, Chutia R, Das G. Inorg Chem. 2012;51:1727. doi: 10.1021/ic2020379. [DOI] [PubMed] [Google Scholar]; (c) Busschaert N, Gale PA, Haynes CJE, Light ME, Moore SJ, Tong CC, Davis JT, Harrell JWA. Chem Commun. 2010;46:6252. doi: 10.1039/c0cc01684e. [DOI] [PubMed] [Google Scholar]; (d) Bose P, Dutta R, Santra S, Chowdhury B, Ghosh P. Eur J Inorg Chem. 2012:5791. [Google Scholar]
- 7.(a) Anslyn EV, Dougherty DA. Modern Physical Organic Chemistry. University Science Books; California: 2006. [Google Scholar]; (b) Smith RM, Martell AE. Critical Stability Constants. Plenum; New York: 1975. [Google Scholar]
- 8.Jia C, Wu B, Li S, Huang X, Zhao Q, Li QS, Yang XJ. Angew Chem, Int Ed. 2011;50:486. doi: 10.1002/anie.201004461. [DOI] [PubMed] [Google Scholar]
- 9.Crystal data for [1(CO3)][n-Bu4N]2: M = 1720.82, a = 41.2339(11) Å, b = 13.6303(4) Å, c = 15.0005(4) Å, α = 90°, β = 90°, γ = 90°, V = 8430.7(4) Å3, T = 100(2) K, space group Pna21, Z = 4, μ(MoKα) = 0.112 mm−1, 85226 reflections measured, 19877 independent reflections (Rint = 0.0294). The final R1 values were 0.0360 (I > 2σ(I)). Flack parameter = 0.06(10). CCDC 963688.
- 10.Isiklan M, Saeed MA, Pramanik A, Wong BM, Fronczek FR, Hossain MA. Cryst Growth Des. 2011;11:959. doi: 10.1021/cg2001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hossain MA, Kang SO, Llinares JM, Powell DR, Bowman-James K. Inorg Chem. 2003;42:5043. doi: 10.1021/ic034735r. [DOI] [PubMed] [Google Scholar]
- 12.(a) Schneider HJ, Kramer R, Simova S, Schneider U. J Am Chem Soc. 1988;110:6442. [Google Scholar]; (b) Mendy JS, Pilate ML, Horne T, Day VW, Hossain MA. Chem Commun. 2010;46:6084. doi: 10.1039/c0cc01699c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Custelcean R, Remy P, Bonnesen PV, Jiang DE, Moyer BA. Angew Chem Int Ed. 2008;47:1866. doi: 10.1002/anie.200704937. [DOI] [PubMed] [Google Scholar]
- 14.Aresta M, Dibenedetto A. Dalton Trans. 2007:2975. doi: 10.1039/b700658f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.