Development of a self-tolerant T-cell receptor (TCR) repertoire with the potential to recognize the universe of infectious agents depends on proper regulation of TCR signaling. The repertoire is whittled down during T-cell development in the thymus by the ability of quasi-randomly generated TCRs to interact with self-peptides presented by major histocompatibility complex proteins (MHCp). Low-affinity TCR interactions with self-MHCp generate weak signals that initiate “positive selection”, causing maturation of CD4 or CD8αβ-expressing “single positive” (SP) thymocytes from CD4+CD8αβ+ “double positive” (DP) precursors1. These develop into mature naïve T-cells of the secondary lymphoid organs. TCR interaction with high-affinity agonist self-ligands results in “negative selection” by activation-induced apoptosis or “agonist selection” of functionally differentiated self-antigen-experienced T-cells2,3. Here we show that positive selection is enabled by the ability of the T-cell specific protein Themis4–9 to specifically attenuate TCR signal strength via SHP1 recruitment and activation in response to low but not high-affinity TCR engagement. Themis acts as an analog-to-digital converter translating graded TCR affinity into clear-cut selection outcome – by dampening mild TCR signals Themis increases the affinity threshold for activation, enabling positive selection of T-cells with a naïve phenotype in response to low-affinity self-antigens.
Themis-deficient mice have severely reduced numbers of SP thymocytes and peripheral T-cells4–8, but the mechanism by which Themis controls T-cell development or function remains obscure. Its rapid phosphorylation after TCR stimulation4,9,10 and subtle signaling defects in Themis−/− DP thymocytes suggested a role in proximal TCR signaling4,9,11, although others failed to find any alteration in TCR signaling5–7,10. Such mild or undetected signaling defects seemed incompatible with the strong positive selection defect in Themis-deficient mice.
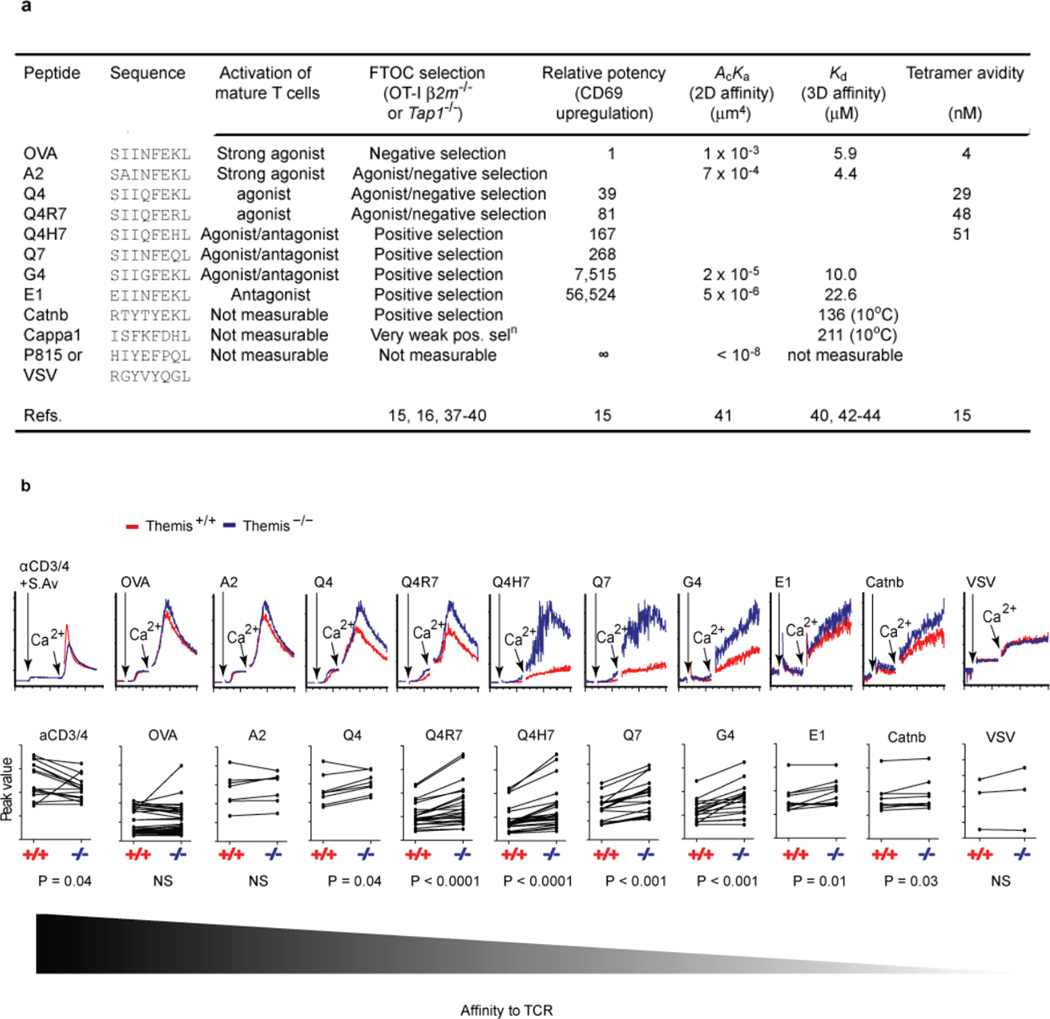
We suspected that activation by antibody-mediated TCR crosslinking may have masked genuine signaling defects that would be revealed with more physiological stimulation. Calcium flux is a hallmark of early TCR signaling and is very sensitive to differences in signal strength12. We titrated streptavidin crosslinking for anti-CD3/CD4 antibodies to better mimic graded signal strengths TCRs might generate in vivo. Over a broad concentration range, we found only minor reduction in calcium signal in Themis-deficient thymocytes, consistent with our previous observations4, but of dubious biological relevance (Extended Data Fig. 1). Therefore, we used the well-characterized OT-I TCR-transgenic model13 to enable thymocyte activation by more physiological MHCp ligands. Natural positively-selecting and antigen-variant peptides eliciting a full spectrum of signal strengths are well-documented (Extended Data Fig. 2a). OT-I-Tap1−/− mice express low cell-surface MHC-I, so thymocyte development is arrested at the pre-selection DP stage. Therefore OT-I-Tap1−/−Themis−/− or Themis+/+ thymocytes are an excellent system to study TCR signaling after stimulation with appropriate Kb-tetramers. As expected, Themis-sufficient DP thymocytes gave very low but sustained Ca2+ responses to positively-selecting peptides Q4H7, Q7, and G412. Remarkably, Themis-deficient cells responded much stronger than Themis-sufficient cells to these ligands, also giving increased responses to lower-affinity E1 and the natural positive-selector Catnb. Responses to strong agonists A2 and OVA were similar between genotypes (Fig. 1a–c, Extended Data Fig. 2b). Similar results were obtained by stimulation on supported lipid bilayers (Fig. 1d). These results were confirmed by imaging Ca2+ in thymocytes recognizing RMA-S cells presenting representative high (OVA) or low (G4) affinity peptides. Themis-deficient thymocytes responded more strongly than Themis-sufficient to G4, but similarly to OVA (Fig. 1e,f; Extended Data Fig. 3). The strong, transient Ca2+ response to negative-selecting ligands versus low, sustained signal to positively-selecting ligands12, are reflected in differential nuclear translocation of NFAT14. Themis-sufficient thymocytes induced strong, transient NFAT translocation in response to Kb-OVA tetramer, and delayed but more sustained translocation in response to Kb-G4 (Fig. 1g, Extended Data Fig. 4). In contrast, in Themis-deficient thymocytes, both Kb-OVA and Kb-G4 tetramers induced nearly identical NFAT translocation kinetics, similar to the Kb-OVA response of wild-type thymocytes. These data demonstrate that the role of Themis in thymocyte signaling is manifest in the response to weak ligands, but masked in responses to strong (agonist) ligands. These differences were widest with ligands below the previously-described positive-negative selection border12. Differences were noticeable for ligands slightly above this threshold (weak agonists), but were completely masked by antibody cross-linking.
Extended Data Figure 1.
Ca2+ flux in Themis-deficient thymocytes stimulated by TCR crosslinking. Thymocytes from wild-type or Themis-deficient mice were first stained with saturated amount of anti-CD3/CD4 antibodies and subsequently cross-linked with titrated amount of Streptavidin (S.Av). Two independent experiments are shown here.
Extended Data Figure 2.
OT-I TCR system and Comparison of Ca2+ flux between Themis-sufficient and –deficient pre-selection thymocytes. (a) Summary of responses of OT-I T cells and thymocytes to different peptides. Data from references cited in table. (b) Representative FACS plots from Fig.1a are shown here again for illustration (top panel), statistical analysis of Ca2+ flux between Themis-sufficient (+/+) and Themis-deficient (−/−) thymocytes were calculated using Wilcoxon signed rank test (P value listed), each line links cells from a pair of mice being compared in the same tube as described (Ref. 30) (bottom panel). Results are pooled from multiple experiments.
Figure 1. Differentially regulated Ca2+ flux in Themis−/− thymocytes.
(a,b) Themis+/+ or Themis−/− OT-I pre-selection thymocytes were incubated with indicated stimuli. Results of representative experiment shown in (a), same-genotype comparisons in (b). (c) Summary of Ca2+ flux differences. Each dot represents one Themis−/− sample normalized to its same-tube Themis+/+ control30 (sex and age-matched), thus blinding and randomization was unnecessary. Each paired sample represents a biological and technical replicate30. Data obtained from 8 litters of each genotype over 10 months. Peak-height (upper) and area-under-the-curve (AUC, lower) shown. Statistical significance determined by 1-way ANOVA Dunnett’s Test, with OVA as comparison control. *P<0.05, **P<0.01, ***P<0.001. Minimal sample size needed to obtain P=0.05, 90% power, estimated based on 1.2-fold difference. (d) Ca2+ flux imaging on supported lipid bilayers: cells settled on Kb-monomer or antibody-coated lipid bilayer were imaged before and after adding CaCl2. Graphs show mean Fluo-4 intensity (WT: n=11, 27, 17, 29; KO: n=20, 14, 24, 23, for cells stimulated by Ab, OVA, G4 and Catnb, respectively), representative of two experiments. (e) Live Ca2+ imaging of stimulation by RMA-S cells pre-loaded with OVA (n=5) or G4 (n=8) peptide, measured as in (d). Representative of three experiments. (f) Representative images of OVA-stimulation of cells analyzed in (e). Themis−/− cells identified by Cy5 staining (red on transmitted light image), Ca2+ scale on right. (g) NFAT1 translocation in thymocytes responding to Kb-OVA and Kb-G4 tetramers. Nuclear:cytosolic NFAT1 protein ratios (Extended Data Fig. 4) calculated using CellProfiler, n=70 cells per data-point. Mean±s.e.m. shown. *P<0.05, unpaired t-test. Representative of two experiments.
Extended Data Figure 3.
Comparison of Ca2+ flux induced by different methods. Thymocytes from indicated mice were either stimulated with peptide presented on thymocytes themselves (left) or with Kb-peptide tetramers (right). As shown, similar results were obtained by both stimulation methods.
Extended Data Figure 4.
Quantitation of NFAT1 nuclear translocation. Thymocytes were stimulated with Ionomycin/PMA (Iono+PMA) to obtain maximal extent of NFAT1 nuclear translocation as a positive control for image analysis. NFAT1 translocation in non-stimulated cells (CONTROL) is used as negative control for image analysis. DAPI and NFAT staining are color-coded as indicated.
Appropriate ERK signaling kinetics are critical for positive selection15–17. As shown previously, antibody-crosslinking induced slightly weaker ERK phosphorylation in Themis-deficient compared to -sufficient cells4 (Extended Data Fig. 5). Further analysis of phospho-(p)-ERK by flow cytometry showed roughly equal p-ERK responses induced with Kb-OVA tetramer in both genotypes. However, ERK signaling was faster and stronger in Themis-deficient DP in response to ligands weaker than Kb-OVA (Fig. 2a,b; Extended Data Fig. 6), and confirmed by immunoblot (Extended Data Fig. 5). These data further support the notion that Themis−/− cells receive signals induced by classical positive-selecting ligands as activating signals, similar to negative/agonist ligand-induced signals.
Extended Data Figure 5.
Biochemical analyses of Erk phosphorylation in Themis deficient thymocytes. Erk1 and 2 phosphorylation of indicated thymocytes in response to different stimuli, normalized to Vav. Representative of 4 experiments.
Figure 2. Themis-deficiency allows low-affinity ligands to elicit negative selection-like characteristics of ERK activation.
Themis-sufficient or deficient pre-selection thymocytes were stimulated with Kb-tetramers. (a,b) ERK phosphorylation detected by flow cytometry following intracellular staining. Representative FACS plots shown in (a) and Extended Data Fig. 6. (b) Summarized data compiled from multiple experiments (n=5 for OVA, Q4R7, Q4H7, n=8 for G4), presented as mean±s.e.m. of percentage p-ERK+ cells (left), MFI (right). Sample sizes estimated as Fig. 1. *P<0.05, **P<0.01, and ***P<0.001, paired t-test. (c,d) Localization of p-ERK determined by staining stimulated thymocytes (2 min) with DAPI and anti-p-ERK12. (c) Representative images, (d) Percentage of cells with membrane-proximal p-ERK, n=20 for each condition, mean±s.e.m. Representative of four experiments for each tetramer, two different operators, not blinded. Unpaired t-test on two identical experiments, *P<0.05.
Extended Data Figure 6.
Flow-cytometric analysis of Erk phosphorylation. Thymocytes were prepared and stimulated with Kb-peptide tetramers. Representative FACS plots are shown in (a). Data compiled from several experiments. PMA and ionomycin (P+I) treatment was used as a positive control for stimulation to obtain maximal Erk phosphorylation. b, Same p-Erk data as in Fig.2b, presented as responses to the different ligands overlaid within the same mouse genotype.
The subcellular localization of p-ERK in thymocytes differs with ligand affinity, with negative/agonist selecting stimuli inducing p-ERK more proximal to the plasma membrane than positive selection stimuli, which induced p-ERK deeper within the cell12. To determine if Themis controls the topology of TCR-induced ERK activation, we compared p-ERK localization in Themis-deficient or -sufficient pre-selection− DP thymocytes responding to different ligands (Fig. 2c,d; Extended Data Fig. 7). Weak ligands that normally induce intracellular p-ERK in Themis-expressing cells instead gave a relatively high proportion of membrane-proximal p-ERK in Themis−/− DPs. In aggregate, these data suggest that, without Themis, the ERK cascade was both mis-localized and hyperactivated, so that positive-selecting ligands were mistakenly interpreted as negative/agonist-selectors. Thus, Themis−/− DP cells appear unable to precisely distinguish low from high-affinity TCR signaling.
Extended Data Figure 7.
3-dimensional reconstruction images of negative-selection-like Erk signaling in Themis−/− thymocytes in response to positive selecting ligands. Themis+/+ or Themis−/− OT-I Tap−/− pre-selection thymocytes were stimulated with Kb-OVA (a), Kb-G4 (b) or Kb-VSV (c). Localization of p-Erk was determined by specific staining with anti-p-Erk antibody. Nuclei were counterstained with Hoesch 33342, and plasma membrane was labeled with Cy3.5. Top panels represent each separate channel of a single centered plane. Bottom panels represent two different 3D reconstructions of 30 planes (step=0.2 μm), surface rendering (left) and volume rendering (right). d, Fluorescence line profile analysis of representative cells in a, b and c, green line (p-Erk) blue line (Hoesch) and red line (Cy3.5).
Both the Ca2+ response and ERK signaling cascade occur distal to TCR stimulation. We therefore examined more proximal signaling events to determine where these dramatic changes in signal transduction to low-affinity ligands were initiated. We found substantially elevated phosphorylation of LAT and PLCγ1 (Fig. 3a), though not SLP-76 (Extended Data Fig. 8) in Themis-deficient versus –sufficient cells upon stimulation with low-affinity ligands. This point of action is consistent with previous reports that Themis interacts with PLCγ1 and is part of the LAT signalosome4,9,11. These data demonstrate that Themis−/− cells mount an augmented response to low-affinity ligands, similar to a wild-type response to high-affinity ligands, suggesting that Themis restricts TCR signaling in DP cells stimulated by low-affinity ligands. SHP1, a cytoplasmic protein tyrosine phosphatase, is an important negative regulator in TCR signaling (reviewed in ref.18). It is activated by tyrosine phosphorylation, and reported to control the thresholds for positive and negative selection18–21. To test if Themis might limit TCR signaling by controlling SHP1, we analyzed SHP1 phosphorylation in Themis+/+ and Themis−/− DP cells. Remarkably, p-SHP1 (but not total protein) was decreased dramatically in Themis-deficient cells, clearly opposite to enhanced p-ERK. Indeed p-SHP1 was barely induced in the Themis-deficient cells (Fig. 3b, Extended Data Fig. 9). Moreover, Themis interacted constitutively with SHP1 but p-SHP1 was induced in Themis-SHP1 complexes in response to TCR stimulation (Fig. 3c). Themis-GRB2 binding was constitutive (Fig. 3d)5–7,9. Because activated LCK is a SHP1 substrate18,22, we tested LCK phosphorylation, finding that the activated pY394 form was indeed increased in Themis-deficient thymocytes (Fig. 3e).
Figure 3. Proximal TCR signaling in Themis-deficient thymocytes responding to positive selecting ligands.
Themis KO or WT pre-selection thymocytes were stimulated with indicated tetramers and analyzed by immunoblot. (a) LAT and PLCγ1 phosphorylation, (b) SHP1 and ERK1/2 phosphorylation, (c) Themis interaction with SHP1 and p-SHP1, (d) Themis interaction with GRB2. (e) Phosphorylation of LCK in response to different stimuli. The lower-molecular mass band in blots probed with anti-p-Y394 is LCK (p56), the higher is FYN (p59). Representative of 2 (b), 3 (a, c, e), and 6 (d) experiments respectively.
Extended Data Figure 8.
SLP-76 phosphorylation is not affected in Themis-deficient thymocytes. Representative of 2 experiments.
Extended Data Figure 9. Decreased SHP1 phosphorylation in Themis−/− DP cells.
(a) Phosphorylation of SHP1 was determined in cell lysates. In this separate experiment, cell lysates from the same time point after stimulation (0.5, 2, and 5 min, respectively) were grouped together and directly compared on the same gel. (b) Quantitation: the intensity ratio of pSHP1 to total Erk was determined by LiCor Odyssey software.
These results suggest that Themis “caps” the signal strength by controlling SHP1 activation, moderating strength and kinetics of responses to relatively low-affinity ligands. This allows these self-antigen-experienced cells to mature into naïve T-cells. Without Themis, thymocytes receive strong agonist/negative selection-like signals. To test whether these redirect low-affinity stimulation to induce negative selection, we stimulated pre-selection DP thymocytes with Kb-tetramers, and assayed caspase-3 activation, a readout for apoptosis23. Indeed, caspase-3 activation was significantly increased in Themis−/− versus Themis+/+ cells in response to ligands that normally induce positive selection, but was similar for ligands that normally induce negative selection (Fig. 4a left). Reduced activated caspase-3+ cells in OVA-stimulated populations versus weaker ligands is likely due to phagocytosis of dead cells, as OVA signals were clearly stronger based on TCR downmodulation (Fig. 4a right). Deficiency of the pro-apoptotic protein Bim rescues thymocytes from negative selection24. Intriguingly, Bim co-disruption rescued the previously reported4–8 impaired SP thymocyte development in Themis−/− mice and defective CD8SP thymocyte development in OT-I Themis−/− mice (Fig. 4b,c). This indicated that the defect in thymocyte development in Themis−/− mice is caused at least partially by misplaced negative selection (agonist selection would not be affected by Bim-deficiency). This suggested the possibility that, while conventional naïve T-cell development requiring weak sustained signaling is severely impaired, agonist-selection of non-conventional T-cells by intermediate affinity ligands might be spared or compensated in Themis-deficient mice. To test this, we simultaneously examined development of wild-type and Themis-deficient T-cells in mixed bone marrow chimeras, avoiding potential homeostatic proliferation artifacts (Fig. 4d). Themis+/+ vastly outnumbered Themis−/− cells amongst conventionally-selected naïve peripheral T-cells, but TCRγδ+ IELs, TCRαβ+ CD8αα IELs and liver iNKT-cells, which all are selected from DP by strong TCR signals2,3,25, showed roughly equal reconstitution by both genotypes. Thus, development of agonist-selected T-cells is not, or at most very mildly, affected by the absence of Themis, in contrast to generation of peripheral naïve T-cells, supporting our notion that the role of Themis differs in relation to ligand strength.
Figure 4. Themis-deficient mice show enhanced negative selection that can be rescued by Bim-deficiency, but normal agonist-selection.
(a) Pre-selection thymocytes were stimulated with tetramers for 17h, then analyzed for activated caspase-323. Triplicate measurements for two mice per genotype, showing mean±s.e.m. Right panel shows TCR downmodulation. Representative of 3 experiments. (b) Thymocytes were analyzed for CD4 and CD8 expression. 4–6 week-old mice of both sex included, no randomization nor blinding necessary for genetic experiment read by flow cytometry. Sample sizes chosen to obtain P=0.01, 90% power for 5-fold difference. Left: representative plots showing mean±s.e.m percentage of each subset labeled. Right: summarized data of absolute numbers (#) for each subset. (c) Mice of indicated genotypes as (b) but expressing OT-I TCR-transgene, analyzed for Vα2hiCD8+ subset. Summarized data of absolute cell numbers (#) shown. In b and c, each symbol represents a single mouse. Statistics: unpaired t-test (a) and Mann-Whitney test (b,c). Fold increases (×) and P values between indicated samples shown in each scheme. (d) Phenotypic analysis of indicated subsets in competitive bone marrow-reconstituted mice. Data pooled from multiple experiments, shown as mean±s.e.m after normalizing Themis+/+ derived donor cells to Themis−/− donor cells (n=4 pairs mice per timepoint except 2 months, 8 pairs). Grey zone indicates ratio between 0.5 and 2. Group sizes based on prior experience.
This study shows that Themis acts early in the TCR signaling cascade, reducing signal strength in response to low-affinity but not high-affinity MHCp ligands. Without Themis, TCR signaling in response to low-affinity MHCp ligands mimics normal signaling responses to negative or agonist-selecting ligands, generating stronger signals that redirect the selection outcome. Themis performs this function through controlling recruitment and activation of the phosphatase SHP1, which limits TCR signaling and reportedly affects the thresholds for positive and negative selection19–21, as well as helping discriminate between agonist and lower-affinity antagonist ligands22. A recent study using a conditional knockout of SHP1, where deletion occurred at the immature DP stage of thymocyte development, showed that SHP1 was not required for normal thymocyte development26. It is possible that incomplete deletion of SHP1 mediated by transgenic CD4-Cre may mask the requirement of SHP1 in thymocyte development. Alternatively other phosphatases can act redundantly in selection26. The dominant negative SHP1 transgenes that appeared to show SHP1’s involvement in development19,20 would likely have blocked other phosphatases in addition to SHP1. We therefore tested whether Themis is able to interact with SHP2. We found that Themis indeed interacts constitutively with SHP2 (Extended Data Fig. 10), indicating that the lack of a Themis−/−-like phenotype in the conditional SHP1 knockout is likely due to redundancy between phosphatases, raising the possibility that different phosphatases may be important in TCR signaling in different situations.
Extended Data Figure 10.
THEMIS forms complexes with SHP1 and SHP2. HEK293 cells were transiently transfected with the indicated expression vectors. Pull-down assays using Streptactin beads were performed 2 days after transfection and the precipitate subjected to SDS-PAGE and immunoblotting with anti-HA tag (a) and anti-SHP2 (b) antibodies, respectively. Representative of (a) 3 and (b) 2 similar experiments. Note that HEK293 cells express SHP2 but little SHP1. Cells originally from ATCC, tested negative for mycoplasma within previous 3 months, not STR profiled. Constitutive binding of GRB2 to THEMIS has been reported previously.
Themis acts to enforce the threshold between positive and negative/agonist selection, which occurs over a very narrow range of TCR-MHCp affinities12. Themis causes an analog continuum of TCR affinities to elicit a digital selection outcome – positive versus negative/agonist selection. We speculate that altered Themis expression shifts the selection window, changing the mature TCR repertoire, and therefore affecting disease susceptibility. Indeed, single nucleotide polymorphisms in the noncoding region between human THEMIS and PTPRK genes have been associated with susceptibility to celiac disease and multiple sclerosis27–29. More work is needed to understand the connection between these polymorphisms, Themis expression, and the etiology of disease.
METHODS SUMMARY
Animal experiments were performed in accordance with the TSRI Animal Care and Use Committee. Thymocytes from Themis+/+ or Themis−/− OT-I-Tap1−/− 4–6 week old mice, both sexes, were used. For ex vivo experiments, thymocytes were rested 3hr at 37°C before assay. For Ca2+ assays, knockout cells were pre-labeled with Cy5, mixed with wild-type cells, loaded with Ca2+-sensitive dye Fluo-4, then washed in Ca2+/Mg2+ free medium, 1mM EGTA30. Cells were stimulated with tetramers for flow cytometry30, or with antigen-presenting lipid bilayers or peptide-loaded RMA-S cells for imaging: after allowing cells to settle, 1v/v pre-warmed medium containing 2.5mM CaCl2 and MgCl2 was added (t=0). For biochemistry, thymocytes were stimulated with tetramers or antibodies, and analyzed by western blot as described4,12. Rabbit anti-Themis antibody (Millipore #06-1328) was used in immunoprecipitation and blotting.
METHODS
Mice
Themis−/− mice (B6.129S-Themistm1Gasc) were produced at TSRI and are available from Jackson Laboratory (Stock# 010919). C57BL/6 (Thy1.2+CD45.2+) mice were bred at TSRI. OT-I and OT-I-Tap1−/− animals were obtained from S. Jameson and K. Hogquist (University of Minnesota, Minneapolis, MN) and Bim knockout mice from L. Sherman (TSRI). All experiments were performed in accordance with the guidelines of the Animal Care and Use Committee of TSRI.
Antibodies
Anti-CD3 (145-2C11), anti-CD4 (RM4.4, RM4.5 and GK1.5), anti-CD8α (53-6.7), anti-CD8β, and anti-Vα2 (B20.1) were from eBioscience or Biolegend. Antibodies against phosphorylated extracellular signal-regulated kinase 1 and 2 (ERK1/2) (T202/Y204, Cat# 9101), phosphorylated PLC-γ1 (Cat# 2821), phosphorylated LAT (Cat# 3584), phosphorylated SHP1 (Cat# 8849), VAV (Cat# 2502), and NFAT1 (Cat# 5861) were from Cell Signaling Technology. Anti-phosphorylated tyrosine (4G10, Cat# 05-1050) and rabbit anti-Themis antibody were from Millipore (Cat# 06-1328). Anti-SHP1 antibody was from Santa Cruz Biotechnology (sc-287). Phospho-Src family (Tyr416) antibody (Cell signaling Cat#2101) cross-reacts with other Src-family kinases phosphorylated at the activating site. As a result probing thymocyte lysates with this mAb shows phosphorylated forms of both p56 Lck and p59 Fyn.
Ca2+ flux
Ca2+ flux measurement by flow cytometry was performed as previously described30. Briefly, thymocyte suspensions were prepared and put in separate tubes. One of the two cell types was loaded for 5 min at room temperature with indodicarbocyanine (Cy5; 1 mg/ml) or was mock treated, followed by washing. Equal numbers of Cy5-labeled cell populations were mixed with the unlabeled sample; for example, OT-I-Tap1−/−Themis+/+ (mock) plus OT-I-Tap1−/−Themis−/− (Cy5). Cells were suspended at a density of 2–6 × 106 cells per ml in cRPMI (RPMI medium supplemented with 10% (vol/vol) FCS, 100 U/ml of penicillin, 10 mg/ml of streptomycin, 292 mg/ml of glutamine, 50 mM 2-mercaptoethanol and 25 mM HEPES, pH 7.3) and were incubated for 30 min at 37°C in 5% CO2 with the calcium indicator Indo-1-AM (2 mM; Molecular Probes). Cells were washed twice with cRPMI. For antibody stimulation, cells were stained for 20 min on ice with biotin-conjugated anti-CD3 and anti-CD4 (RM4.4), as well as PerCP-Cy5.5–conjugated anti-CD8α and PE–conjugated anti-CD4 (GK1.5) in cRPPM. Cells were washed once with cRPMI and once with cHBSS (Ca2+-free and Mg2+-free Hank’s balanced-salt solution supplemented with 1% (vol/vol) FCS, 1 mM MgCl2, 1 mM EGTA and 10 mM HEPES, pH 7.3) and were resuspended in cHBSS. Cells were prewarmed to 37°C before analysis and were kept at 37°C during event collection on an LSR II (Becton-Dickinson). For cell stimulation, streptavidin (10 mg/ml; Jackson ImmunoResearch) was added to crosslink biotinylated antibodies; alternatively, cells were stimulated with tetramers (NIH tetramer core facility). CaCl2 (2mM) was added during analysis. Mean fluorescence ratio was calculated with FlowJo (TreeStar).
Imaging of Ca2+ flux
Knockout cells were pre-labeled with Cy5 as above30 then both wild-type and knockout cells were mixed and loaded with 2µM Ca2+-sensitive dye Fluo-4. After washing in Ca2+/Mg2+ free medium with 1mM EGTA, cells were mixed and added to the 37°C imaging chamber containing either the antigen-presenting lipid bilayer or peptide-loaded RMA-S cells. After allowing cells to settle and starting imaging, 1 volume of pre-warmed medium containing 2.5mM CaCl2 and 2.5mM MgCl2 was added so that Ca2+ influx started at t=0.
p-ERK analysis
For intracellular staining of phosphorylated ERK, stimulated cells were fixed with 4% paraformaldehyde for 12 min at room temperature and then plated in microtiter tubes (for phospho-flow analysis) or in poly-L-Lysine coated LabTek II chambers (for imaging). Cells were permeabilized with 0.3% Triton X-100 for 3 min at room temperature. Cells were then blocked with 5% normal goat serum and incubated with rabbit monoclonal antibody against phospho-ERK, after which they were incubated with Alexa Fluor 488–conjugated Fab fragments against rabbit antibodies (Molecular Probes) for secondary detection (either phospho-flow or microscopy). For flow cytometry analysis, one of the two cell types were pre-labeled with dye Cy5 and the other one was mock labeled. Then the two cell types were mixed in 1:1 ratio and processed for stimulation and staining. Phospho-flow analysis of p-ERK was done on a BD LSR-II digital flow cytometer and the two cell types were distinguished by Cy5 labeling. p-ERK imaging was done as described previously with modification31. Briefly, after intracellular staining of p-ERK, nuclei were counterstained with the DNA binding dye 4’,6-diamidino-2-phenylindole (DAPI, Invitrogen). All images were captured with a Zeiss Axiovert 200 M inverted microscope. SlideBook software (Intelligent Imaging Innovations) was used for the capture as well as for deconvolution and image analysis, and ImageJ was used for image presentation.
NFAT1 imaging
Nuclear translocation of NFAT1 was imaged as described above regarding p-ERK imaging31, and analyzed using CellProfiler software (Broad Institute).
Immunoprecipitation and western blotting analysis
Briefly, cells were stained with antibodies against CD3 and CD4 or tetramers on ice for 15 min and stimulated by rapidly warming to 37°C with pre-warmed PBS for indicated times. Cells were then lysed and processed for immunoprecipitation or immunoblot analysis as described in detail in Ref.32. In some cases, after primary antibody incubation, membranes were incubated with goat anti-rabbit IgG (H+L), DyLight 800 conjugated (Pierce, Cat#35571) or goat anti-mouse IgG (H+L), DyLight 680 conjugated (Pierce, Cat#35571), and detected and quantified with LiCor Odyssey infrared imaging system. Human embryonic kidney epithelial cells (HEK293), which express SHP2 but little SHP133,34, were transfected by calcium phosphate precipitation and lysates prepared as described11. Streptactin-Sepharose beads (IBA BioTAGnology) were used for THEMIS-Strep pull-downs, after which beads were washed three times with lysis buffer. Bound proteins were eluted with 5 mM biotin and subjected to SDS-PAGE11.
In vitro apoptosis assay
Experiments were performed as described previously23. Briefly, pre-selection thymocytes were stimulated with the various tetramers for 17 hours. Apoptosis was determined by staining active Caspase-3 with FITC-conjugated specific antibody following manufacturer’s protocol (APO ACTIVE 3 detection kit, Cell Technology Inc, Cat# FAB200-1), and analyzed by FACS.
Phenotyping of agonist-selected T-cells
Agonist-selected T-cells were analyzed in bone marrow reconstituted mice. Briefly, Themis+/+ (CD45.1+/CD45.2+) and Themis−/− bone marrow cells (CD45.2+) were mixed 1:1 and injected into lethally irradiated (2 × 5.5 Gy, separated by 3–4 hrs) B6 recipient mice (CD45.1+). At various times post bone marrow reconstitution, tissues and organs of recipient mice were harvested and relevant T-cell subsets were analyzed. Splenocytes, IELs and liver mononuclear cells were prepared as previously described35,36. Cells were suspended in PBS + 5% FCS + 0.16% azide and anti-CD16/CD32 (2.4G2) Fc-receptor antibody (BD Biosciences, San Diego, CA, USA) was used to block Fc-antibody binding. The following antibodies were purchased from BD Biosciences (San Diego, CA, USA): TCRγδ-FITC (GL3), TCRβ- APC-ef780 (H57-597), NK1.1-FITC (PK136), CD8α-PE (53-6.7), CD8β-FITC (53-5.8), CD19-APC (1D3), CD45.2-PercP-Cy5.5 (104). The following antibodies were purchased from eBioscience (San Diego, CA, USA): CD4-eF450 (RM4-5), CD5-ef450 (53-7.3), CD45.1-PE-Cy7 (A20), CD69-eF450 (H1.2F3), CD90.2-APC (53-2.1). The following antibodies were purchased from Invitrogen CD8α-PO (5H10). The following antibodies were purchased from Caltag: CD4-PO (RM4-5). All antibodies were directly conjugated to fluorophores. An LSR II cell analyzer (BD Biosciences, San Jose, CA, USA) was used for the flow cytometry, and FlowJo software (TreeStar, Ashland, OR, USA) was used for analysis.
Statistical testing
Statistics were performed as described in Figure legends using Excel, GraphPad Prism, or Igor Pro (Wavemetrics, Inc).
Acknowledgements
We thank Xiao Lei Chen and Yan Xing for valuable technical advice. We thank Jeanette Ampudia, Sebastién Vallée, Jianfang Hu, and Stephanie Feldstein for help, and the NIH Tetramer Core Facility for production of MHC-I tetramers. Supported by NIH grants AI073870, DK094173, and GM065230 to NRJG, DP1OD006433 to H.C., GM100785 and AI070845 to K.S., Wellcome Trust Grant GR076558MA to O.A., and by National University of Singapore. J.C. was supported by a fellowship from the Spanish Ministerio de Ciencia e Innovacion (MICIIN), J.A.H.H. by the Irving S. Sigal Fellowship of the American Chemical Society and NIH training grant T32AI07244, and K.S. by The Leukemia & Lymphoma Society Scholar Award 1440-11. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, or other funding agencies. This is manuscript number 21592 from The Scripps Research Institute.
Footnotes
Extended data are linked to the online version of the paper.
Author Contributions G.F., J.C., S.R., V.R., F.L., J.B., K.S., and J.A.H.H. performed experiments and analyzed data; W.P. and O.A. performed initial experiments on Themis-SHP1/2 interaction, G.F. and N.R.J.G. designed the project with help and insight from W.P., O.A., H.C., and K.S.; G.F. and N.R.J.G wrote the manuscript with help from the others.
The authors declare no competing financial interests.
REFERENCES
- 1.Morris GP, Allen PM. How the TCR balances sensitivity and specificity for the recognition of self and pathogens. Nat Immunol. 2012;13:121–128. doi: 10.1038/ni.2190. [DOI] [PubMed] [Google Scholar]
- 2.Cheroutre H, Lambolez F. The thymus chapter in the life of gut-specific intra epithelial lymphocytes. Curr Opin Immunol. 2008;20:185–191. doi: 10.1016/j.coi.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stritesky GL, Jameson SC, Hogquist KA. Selection of Self-Reactive T Cells in the Thymus. Annu Rev Immunol. 2012;30:95–114. doi: 10.1146/annurev-immunol-020711-075035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu G, et al. Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling. Nat Immunol. 2009;10:848–856. doi: 10.1038/ni.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson AL, et al. Themis is a member of a new metazoan gene family and is required for the completion of thymocyte positive selection. Nat Immunol. 2009;10:831–839. doi: 10.1038/ni.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesourne R, et al. Themis, a T cell-specific protein important for late thymocyte development. Nat Immunol. 2009;10:840–847. doi: 10.1038/ni.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patrick MS, et al. Gasp, a Grb2-associating protein, is critical for positive selection of thymocytes. Proc Natl Acad Sci U S A. 2009;106:16345–16350. doi: 10.1073/pnas.0908593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kakugawa K, et al. A novel gene essential for the development of single positive thymocytes. Mol Cell Biol. 2009;29:5128–5135. doi: 10.1128/MCB.00793-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brockmeyer C, et al. T cell receptor (TCR)-induced tyrosine phosphorylation dynamics identifies THEMIS as a new TCR signalosome component. J Biol Chem. 2011;286:7535–7547. doi: 10.1074/jbc.M110.201236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lesourne R, et al. Interchangeability of Themis1 and Themis2 in thymocyte development reveals two related proteins with conserved molecular function. J Immunol. 2012;189:1154–1161. doi: 10.4049/jimmunol.1200123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paster W, et al. GRB2-mediated recruitment of THEMIS to LAT is essential for thymocyte development. J Immunol. 2013;190:3749–3756. doi: 10.4049/jimmunol.1203389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniels MA, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 13.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 14.Oh-Hora M, et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mariathasan S, et al. Duration and strength of extracellular signal-regulated kinase signals are altered during positive versus negative thymocyte selection. J Immunol. 2001;167:4966–4973. doi: 10.4049/jimmunol.167.9.4966. [DOI] [PubMed] [Google Scholar]
- 16.Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 17.McNeil LK, Starr TK, Hogquist KA. A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc Natl Acad Sci U S A. 2005;102:13574–13579. doi: 10.1073/pnas.0505110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol Rev. 2009;228:342–359. doi: 10.1111/j.1600-065X.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, et al. Involvement of the SHP-1 tyrosine phosphatase in regulation of T cell selection. J Immunol. 1999;163:3012–3021. [PubMed] [Google Scholar]
- 20.Plas DR, et al. Cutting edge: the tyrosine phosphatase SHP-1 regulates thymocyte positive selection. J Immunol. 1999;162:5680–5684. [PubMed] [Google Scholar]
- 21.Carter JD, Neel BG, Lorenz U. The tyrosine phosphatase SHP-1 influences thymocyte selection by setting TCR signaling thresholds. Int Immunol. 1999;11:1999–2014. doi: 10.1093/intimm/11.12.1999. [DOI] [PubMed] [Google Scholar]
- 22.Stefanova I, et al. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 23.Rybakin V, Gascoigne NRJ. Negative selection assay based on stimulation of T cell receptor transgenic thymocytes with peptide-MHC tetramers. PLoS One. 2012;7:e43191. doi: 10.1371/journal.pone.0043191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouillet P, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 25.Moran AE, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson DJ, et al. Shp1 regulates T cell homeostasis by limiting IL-4 signals. J Exp Med. 2013;210:1419–1431. doi: 10.1084/jem.20122239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubois PC, et al. Multiple common variants for celiac disease influencing immune gene expression. Nature Genetics. 2010;42:295–302. doi: 10.1038/ng.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trynka G, et al. Dense genotyping identifies and localizes multiple common and rare variant association signals in celiac disease. Nature Genetics. 2011;43:1193–1201. doi: 10.1038/ng.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawcer S, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu G, Gascoigne NRJ. Multiplexed labeling of samples with cell tracking dyes facilitates rapid and accurate internally controlled calcium flux measurement by flow cytometry. J Immunol Methods. 2009;350:194–199. doi: 10.1016/j.jim.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu G, et al. Protein kinase C η is required for T cell activation and homeostatic proliferation. Sci Signal. 2011;4:ra84. doi: 10.1126/scisignal.2002058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang YH, et al. Positive regulation of Itk PH domain function by soluble IP4. Science. 2007;316:886–889. doi: 10.1126/science.1138684. [DOI] [PubMed] [Google Scholar]
- 33.Minoo P, Zadeh MM, Rottapel R, Lebrun JJ, Ali S. A novel SHP-1/Grb2-dependent mechanism of negative regulation of cytokine-receptor signaling: contribution of SHP-1 C-terminal tyrosines in cytokine signaling. Blood. 2004;103:1398–1407. doi: 10.1182/blood-2003-07-2617. [DOI] [PubMed] [Google Scholar]
- 34.Simoneau M, et al. Activation of Cdk2 stimulates proteasome-dependent truncation of tyrosine phosphatase SHP-1 in human proliferating intestinal epithelial cells. J Biol Chem. 2008;283:25544–25556. doi: 10.1074/jbc.M804177200. [DOI] [PubMed] [Google Scholar]
- 35.Hammond KJ, et al. CD1d-restricted NKT cells: an interstrain comparison. J Immunol. 2001;167:1164–1173. doi: 10.4049/jimmunol.167.3.1164. [DOI] [PubMed] [Google Scholar]
- 36.Gangadharan D, et al. Identification of pre- and postselection TCRαβ+ intraepithelial lymphocyte precursors in the thymus. Immunity. 2006;25:631–641. doi: 10.1016/j.immuni.2006.08.018. [DOI] [PubMed] [Google Scholar]