Abstract
A monoclonal antibody (against 7A antigen), raised against a homogenate of embryonic rat forebrain, marks the distribution of region- and developmental stage-specific cell-surface antigens in the mammalian central nervous system. In the mouse, immunocytochemical staining revealed that 7A antigen is expressed almost exclusively in germinal layers of the cerebral cortex beginning as early as day 11 of gestation and becomes undetectable by birth. The typical staining is seen at embryonic days 13-15, in which only the ventricular and the subventricular zones of the cerebral cortex are intensely labeled, whereas most other areas of the brain are unlabeled. Tissue culture studies indicate that the 7A antigen is localized at the plasma membrane. Molecular species possessing the antigen are identified as neutral glycolipids that contain the X-determinant trisaccharide: (formula; see text) This glycoconjugate is expressed in embryonic brains of many mammalian species (mouse, rat, rabbit, calf, and human) and may be a cell-surface component important in normal development of the central nervous system.
Full text
PDF

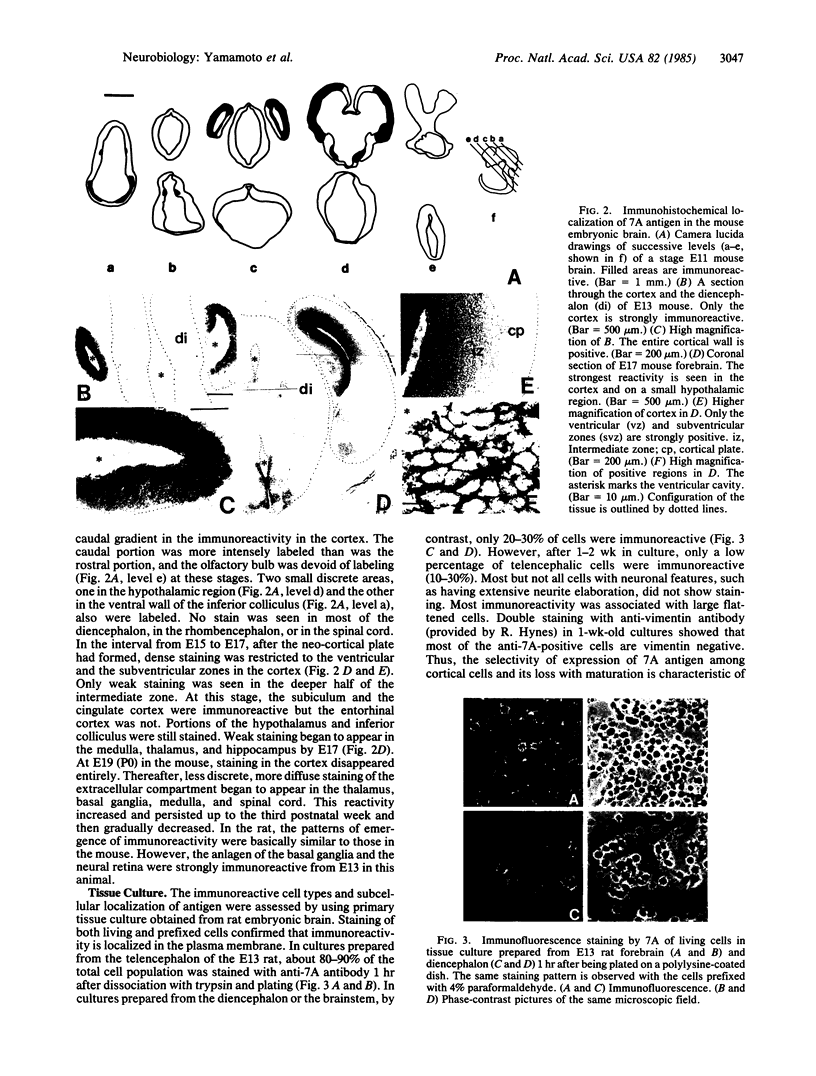
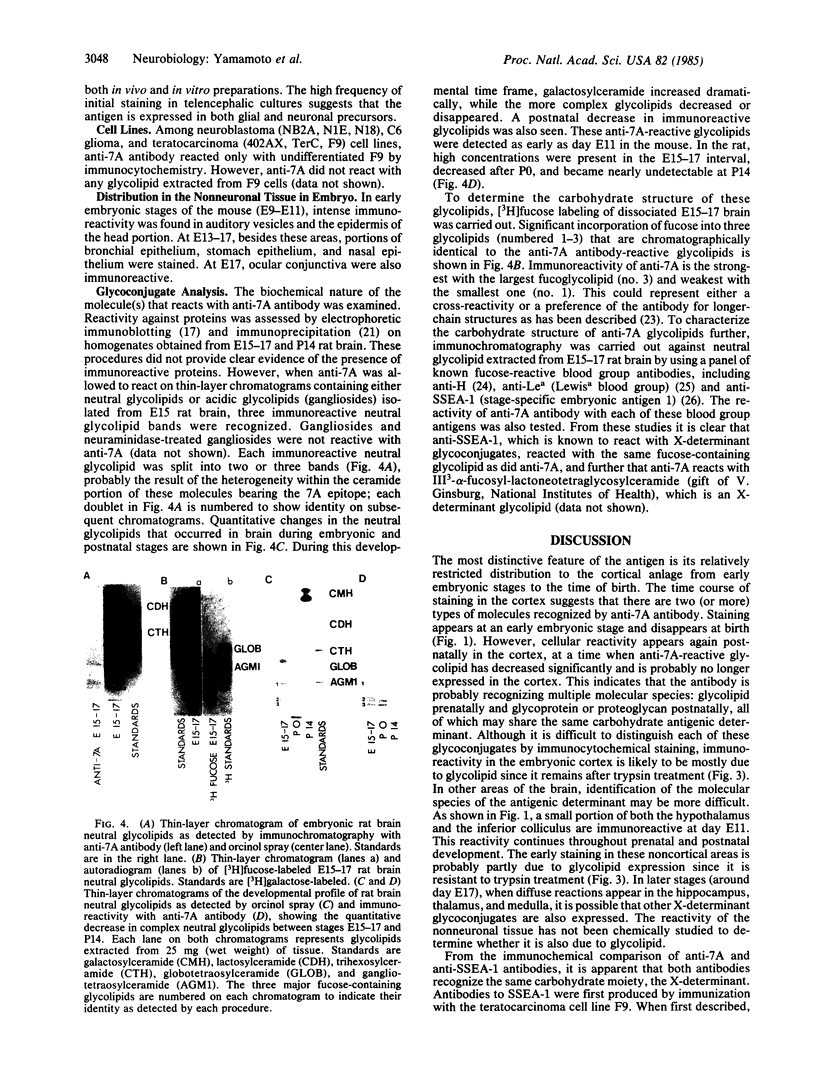

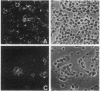
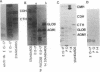
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brockhaus M., Magnani J. L., Blaszczyk M., Steplewski Z., Koprowski H., Karlsson K. A., Larson G., Ginsburg V. Monoclonal antibodies directed against the human Leb blood group antigen. J Biol Chem. 1981 Dec 25;256(24):13223–13225. [PubMed] [Google Scholar]
- Cheresh D. A., Varki A. P., Varki N. M., Stallcup W. B., Levine J., Reisfeld R. A. A monoclonal antibody recognizes an O-acylated sialic acid in a human melanoma-associated ganglioside. J Biol Chem. 1984 Jun 25;259(12):7453–7459. [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules. Science. 1983 Feb 4;219(4584):450–457. doi: 10.1126/science.6823544. [DOI] [PubMed] [Google Scholar]
- Fox N., Damjanov I., Knowles B. B., Solter D. Immunohistochemical localization of the mouse stage-specific embryonic antigen 1 in human tissues and tumors. Cancer Res. 1983 Feb;43(2):669–678. [PubMed] [Google Scholar]
- Fox N., Damjanov I., Martinez-Hernandez A., Knowles B. B., Solter D. Immunohistochemical localization of the early embryonic antigen (SSEA-1) in postimplantation mouse embryos and fetal and adult tissues. Dev Biol. 1981 Apr 30;83(2):391–398. doi: 10.1016/0012-1606(81)90487-5. [DOI] [PubMed] [Google Scholar]
- Fukushi Y., Hakomori S., Nudelman E., Cochran N. Novel fucolipids accumulating in human adenocarcinoma. II. Selective isolation of hybridoma antibodies that differentially recognize mono-, di-, and trifucosylated type 2 chain. J Biol Chem. 1984 Apr 10;259(7):4681–4685. [PubMed] [Google Scholar]
- Fukushi Y., Hakomori S., Shepard T. Localization and alteration of mono-, di-, and trifucosyl alpha 1----3 type 2 chain structures during human embryogenesis and in human cancer. J Exp Med. 1984 Aug 1;160(2):506–520. doi: 10.1084/jem.160.2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooi H. C., Feizi T., Kapadia A., Knowles B. B., Solter D., Evans M. J. Stage-specific embryonic antigen involves alpha 1 goes to 3 fucosylated type 2 blood group chains. Nature. 1981 Jul 9;292(5819):156–158. doi: 10.1038/292156a0. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Hakomori S., Nudelman E., Levery S., Solter D., Knowles B. B. The hapten structure of a developmentally regulated glycolipid antigen (SSEA-1) isolated from human erythrocytes and adenocarcinoma: a preliminary note. Biochem Biophys Res Commun. 1981 Jun;100(4):1578–1586. doi: 10.1016/0006-291x(81)90699-9. [DOI] [PubMed] [Google Scholar]
- Hatten M. E., Furie M. B., Rifkin D. B. Binding of developing mouse cerebellar cells to fibronectin: a possible mechanism for the formation of the external granular layer. J Neurosci. 1982 Sep;2(9):1195–1206. doi: 10.1523/JNEUROSCI.02-09-01195.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L. C., Civin C. I., Magnani J. L., Shaper J. H., Ginsburg V. My-1, the human myeloid-specific antigen detected by mouse monoclonal antibodies, is a sugar sequence found in lacto-N-fucopentaose III. Blood. 1983 May;61(5):1020–1023. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagenaur C., Schachner M., Solter D., Knowles B. Monoclonal antibody against SSEA-1 is specific for a subpopulation of astrocytes in mouse cerebellum. Neurosci Lett. 1982 Aug 16;31(2):181–184. doi: 10.1016/0304-3940(82)90113-6. [DOI] [PubMed] [Google Scholar]
- Ledeen R. W., Yu R. K., Eng L. F. Gangliosides of human myelin: sialosylgalactosylceramide (G7) as a major component. J Neurochem. 1973 Oct;21(4):829–839. doi: 10.1111/j.1471-4159.1973.tb07527.x. [DOI] [PubMed] [Google Scholar]
- Levine J. M., Beasley L., Stallcup W. B. The D1.1 antigen: a cell surface marker for germinal cells of the central nervous system. J Neurosci. 1984 Mar;4(3):820–831. doi: 10.1523/JNEUROSCI.04-03-00820.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchase R. B. Biochemical investigations of retinotectal adhesive specificity. J Cell Biol. 1977 Oct;75(1):237–257. doi: 10.1083/jcb.75.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgreen D. F., Gibbins I. L., Sauter J., Wallenfels B., Wütz R. Ultrastructural and tissue-culture studies on the role of fibronectin, collagen and glycosaminoglycans in the migration of neural crest cells in the fowl embryo. Cell Tissue Res. 1982;221(3):521–549. doi: 10.1007/BF00215700. [DOI] [PubMed] [Google Scholar]
- Newgreen D., Thiery J. P. Fibronectin in early avian embryos: synthesis and distribution along the migration pathways of neural crest cells. Cell Tissue Res. 1980;211(2):269–291. doi: 10.1007/BF00236449. [DOI] [PubMed] [Google Scholar]
- Rutishauser U. Developmental biology of a neural cell adhesion molecule. Nature. 1984 Aug 16;310(5978):549–554. doi: 10.1038/310549a0. [DOI] [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci U S A. 1978 Nov;75(11):5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery J. P., Duband J. L., Rutishauser U., Edelman G. M. Cell adhesion molecules in early chicken embryogenesis. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6737–6741. doi: 10.1073/pnas.79.21.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdal D. L., Brentnall T. A., Bernstein I. D., Hakomori S. I. A granulocyte reactive monoclonal antibody, 1G10, identifies the Gal beta 1-4 (Fuc alpha 1-3)GlcNAc (X determinant) expressed in HL-60 cells on both glycolipid and glycoprotein molecules. Blood. 1983 Nov;62(5):1022–1026. [PubMed] [Google Scholar]
- Williams M. A., McCluer R. H. The use of Sep-Pak C18 cartridges during the isolation of gangliosides. J Neurochem. 1980 Jul;35(1):266–269. doi: 10.1111/j.1471-4159.1980.tb12515.x. [DOI] [PubMed] [Google Scholar]
- Willinger M., Schachner M. GM1 ganglioside as a marker for neuronal differentiation in mouse cerebellum. Dev Biol. 1980 Jan;74(1):101–117. doi: 10.1016/0012-1606(80)90055-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Steinbusch H. W., Jessell T. M. Differentiated properties of identified serotonin neurons in dissociated cultures of embryonic rat brain stem. J Cell Biol. 1981 Oct;91(1):142–152. doi: 10.1083/jcb.91.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W. W., Jr, Johnson H. S., Tamura Y., Karlsson K. A., Larson G., Parker J. M., Khare D. P., Spohr U., Baker D. A., Hindsgaul O. Characterization of monoclonal antibodies specific for the Lewis a human blood group determinant. J Biol Chem. 1983 Apr 25;258(8):4890–4894. [PubMed] [Google Scholar]
- Young W. W., Jr, Portoukalian J., Hakomori S. Two monoclonal anticarbohydrate antibodies directed to glycosphingolipids with a lacto-N-glycosyl type II chain. J Biol Chem. 1981 Nov 10;256(21):10967–10972. [PubMed] [Google Scholar]