Abstract
Background
Little is known about the long-term effects of dietary energy density (ED) on weight gain.
Methods
We conducted a prospective study of 50,026 women (mean age: 36.5; SD: 4.6) in the Nurses' Health Study II followed from 1991 to 1999. Dietary ED and body weight were ascertained in 1991, 1995, and 1999. Total dietary ED was calculated by dividing each subject's daily energy intake (kcal) by the reported weight (g) of all foods consumed.
Results
Dietary ED was positively correlated with saturated fat (r=0.16), trans fat (r=0.15), and the glycemic index (r=0.16), but inversely correlated with vegetable protein (r=−0.30), vegetables (r=−0.27), and fruits (r=−0.17). ED was not significantly correlated with total fat intake (r=0.08). Women who increased their dietary ED during follow-up the most (5th quintile) had a statistical significant greater multivariate-adjusted weight gain as compared with those who decreased their dietary ED most (1st quintile) (in the 8-y time period: 6.42 kg versus 4.57 kg; p for trend < 0.001). However, the amount of weight change over time varied considerably according to the ED of individual foods and beverages.
Conclusion
Increases in total dietary ED were associated with long-term weight gain among younger and middle-aged women. However, public health recommendations cannot be made simply based on ED values of individual foods and beverages only. Reducing consumption of foods high in saturated and trans fats and refined carbohydrates and increasing consumption of fruits and vegetables may help to reduce dietary ED and prevent weight gain.
INTRODUCTION
Currently, obesity is one of the most important public health problems worldwide (1, 2). According to the latest estimates from the World Health Organization (WHO), approximately 1.6 billion adults were overweight and at least 400 million obese in 2005 (3).
Due to the magnitude of the problem, it is important to develop effective practical strategies to prevent age-related weight gain (4). Obesity is the result of a long-term and sustained energy imbalance (energy intake must exceed energy expenditure). Therefore, there has been an increasing interest in understanding the factors that contribute to energy imbalance. One has been the energy density (ED) of the diet, defined as the amount of energy in a given weight of food (5). A diet with a low ED provides less energy per gram than do diets with a high ED (5). In short-term laboratory studies, people tended to consume a similar weight of food rather than a constant amount of energy (6). Consequently, high ED diets may lead to “passive over-consumption” and increases in body weight. The WHO suggests that increased consumption of energy-dense diets contributes to the obesity epidemic (7). Likewise, the 2005 USDA Dietary Guidelines for Americans and the CDC recommend consuming low-ED diets as an important part of a weight management strategy (8, 9).
Despite these recommendations, surprisingly little is known about the long-term relationship between ED diets and subsequent weight gain in free-living populations (10). Whereas some cross-sectional studies have found a positive association between ED and body mass index (BMI) or body weight (11–15), others have not (16–18). In addition, despite a positive short-term relationship between ED and energy intake, it is unclear whether compensation (i.e. reducing subsequent energy intake) occurs over the long term. So far, only two prospective studies examined the relationship between ED and weight change and the results are inconsistent (19, 20). Therefore, the objective of this study was to assess the long-term relationship between changes of dietary ED and age-related weight gain in a large prospective cohort of young and middle-aged women.
METHODS
Study population
The Nurses' Health Study II is a prospective cohort study of 116,671 female US nurses aged 24 to 44 years at study initiation in 1989. This cohort was followed using biennial mailed questionnaires with a follow-up rate exceeding 90% for every 2-year period. Participants completed self-administered food frequency questionnaires (FFQ) in 1991, 1995, and 1999. For the present analysis, women were excluded from the baseline population if they did not complete dietary questionnaires in 1991, if more than nine food items were left blank, if they reported unreasonable caloric intakes (<500 or >3500 kcal/d), if they had a history of diabetes or cardiovascular disease or reported a diagnosis of cancer (except non-melanoma skin cancer) before 1999, if they had no data on physical activity assessed in 1991 or 1997, or if they were pregnant at the time of the 1991, 1995, or 1999 questionnaire administration. Those who did not provide information on weight at any time period were also excluded. These exclusions left a total of 50,026 women for the analyses. The Harvard School of Public Health and Brigham and Women's Hospital Human Subjects Committees approved the study protocol.
Dietary assessment
In 1991, the mailed questionnaire included a 133-item semiquantitative FFQ to obtain dietary information. Women were asked how often they had consumed a commonly used unit or portion size of each food on average over the previous year. The participants chose from 9 possible responses ranging from never to 6 or more times per day. Similar FFQs were used to collect dietary information in 1995 and 1999. Dietary ED was calculated by dividing each subject's daily intake (kcal) by the reported weight (g) of all foods consumed based on the serving size and daily frequency of consumption. Caloric and non-caloric beverages were excluded from the calculation (21) based on the consideration that energy intake from beverages is regulated differently from energy intake from foods (22). In addition, this method of calculating dietary ED has been demonstrated to provide the best correlations to measures of obesity in previous cross-sectional studies (12, 21). However, we included the caloric beverages in a secondary analysis. We calculated the energy intake by multiplying the frequency of consumption by the calorie content of each food item and then adding the contribution from all food items. To assess the weight of all food consumed, we multiplied the frequency of consumption by the specified portion size of each food item (in grams) and then summed all values. The food-composition database used to calculate the caloric values is based primarily on US Department of Agriculture data (23) and was supplemented with the manufacturers' data. The validity and reliability of FFQs similar to those used in the Nurses' Health Study II have been described elsewhere (24).
Assessment of non-dietary exposures
Through biennial questionnaires, we collected information on age, weight, smoking status, oral contraceptive use, hormone replacement therapy, and pregnancies. The validity of self-reported weight was evaluated previously in the original Nurses' Health Study (25). The correlation coefficient between self-reported weight measurements and the average of 2 technician measurements was 0.97.
Physical activity was assessed with the 1991 and 1997 questionnaires and was computed as metabolic equivalent (MET) index per week using the time spent engaging in various forms of exercise, and multiplying by the MET score specific for each activity. The MET-hours for all activities were combined to obtain a value of total weekly MET-hours, which adequately correlated with energy expenditure measured in diaries or by recalls (0.62 and 0.79, respectively) (26).
Statistical analysis
We calculated Pearson correlation coefficients between dietary ED and consumption of each food item. We presented the top 15 positive and negative correlations. We used multiple approaches to assess the relationship between dietary ED and body weight or BMI. In all analyses, we modeled the main exposure variable in quintiles (by including four dichotomous indicator variables in the model) to avoid a linearity assumption and to reduce the effect of outliers. First, we calculated the least-squares means for changes in body weight from 1991 to 1995, 1995 to 1999, and 1991 to 1999 across quintiles of changes in dietary ED for each period. We adjusted for age, alcohol intake, physical activity, smoking, and other lifestyle and dietary confounders at baseline for each time period. We also adjusted for changes in these covariates and changes in soft drink consumption, which were associated with weight gain in this cohort (27) and other cohorts (28). Tests of linear trend across quintiles of dietary ED were performed by assigning the median values for each quintile and treating them as continuous variables.
We also classified participants according to categories of changes in ED across three time periods (1991–1995, 1995–1999, 1991–1999): consistent in the lowest quintile, consistent in the highest quintile, moving from the lowest to the highest quintiles, moving from the highest to the lowest quintiles and others, and we estimated the mean weight change for each of these groups.
To take into account the effects of overall dietary patterns, we conducted additional analyses to adjust for changes in prudent and Western dietary pattern based on the results from principal component analysis of the 39 predefined food groups using the PROC FACTOR procedure in SAS (29, 30).
To differentiate the effects of overall dietary ED and specific ED values of individual food items, we ranked the foods from the lowest to the highest ED values, and then represented them graphically in the x-axis. For each food item, we fitted a linear regression model with weight change as the outcome and tertiles of change in the consumption of each food as the exposure, also adjusting for relevant confounders. We represented graphically in the y-axis the regression coefficients (β) and their 95% CI of the weight gain for the highest tertile of change in each food item consumption during follow-up (1991–1999) relative to the first tertile of change.
All p values presented are two-sided, and p values < 0.05 were considered statistically significant.
RESULTS
The mean 8-y (1991–1999) weight gain was 5.44 kg (SD: 7.37) among 50,026 women (mean age: 36.5; SD: 4.6). The mean baseline ED was 1.15 kcal/g (SD: 0.39). During 8 years of follow-up, the average change in dietary ED was +0.47 kcal/g (SD: 0.44). Participants with higher dietary ED consumed more calories and lower total weight of food than participants with lower ED (Table 1). Saturated fat, trans fat, and the glycemic index were positively correlated with dietary ED (r: +0.16; +0.15; +0.16, respectively), whereas vegetable protein intake, vegetables, and fruit consumption were inversely associated with ED (r: −0.30; −0.27, −0.17, respectively). Total fat was minimally correlated with dietary ED (r=0.08). A higher dietary ED was associated with higher consumption of soda and red meat and lower consumption of fruit, vegetables, legumes, whole grains, and white meat (poultry) (Table 2).
Table 1.
Characteristics at baseline (1991) and changes during follow-up (1991–1999) according to quintiles (Q) of dietary energy density (kcal/g) (1991) in 50,026 women.
| Quintiles of baseline energy density (1991) | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |
| N | 10,005 | 10,005 | 10,006 | 10,005 | 10,005 |
| Energy density (only food) (Kcal/g), mean (SD) | 0.7 (0.2) | 0.9 (0.1) | 1.1 (0.1) | 1.3 (0.1) | 1.7 (0.3) |
| Change in energy density (only food) (Kcal/g), mean (SD) | +0.9 (0.3) | +0.6 (0.3) | +0.5 (0.3) | +0.2 (0.3) | 0.0 (0.4) |
| Energy density (food and caloric beverages) (Kcal/g), mean (SD) | 0.8 (0.1) | 1.0 (0.1) | 1.1 (0.1) | 1.2 (0.1) | 1.4 (0.3) |
| Change in energy density (food and caloric beverages) (kcal/g), mean (SD) | +0.4 (0.3) | +0.2 (0.3) | +0.1 (0.3) | 0.0 (0.3) | −0.3 (0.4) |
| Total energy intake (Kcal/day), mean (SD) | 1,497 (447) | 1,762 (488) | 1,839 (511) | 1,877 (521) | 1,884 (541) |
| Change total energy intake (kcal/day), mean (SD) | +164 (494) | +59 (511) | +30 (512) | −13 (519) | −42 (533) |
| Beverages energy intake (Kcal/day), mean (SD) | 560 (254) | 487 (230) | 423 (218) | 366 (205) | 287 (193) |
| Changes in beverages energy intake (Kcal/day), mean (SD) | −344 (271) | −232 (262) | −149 (252) | −72 (249) | +47 (263) |
| Amount of food (g), mean (SD) | 1,421 (567) | 1,363 (471) | 1,259 (400) | 1,142 (343) | 944 (301) |
| Change in amount of food (g), mean (SD) | −448 (563) | −338 (458) | −238 (396) | −166 (348) | −59 (304) |
| Amount of food and caloric beverages (g), mean (SD) | 1,925 (664) | 1,835 (595) | 1,706 (540) | 1,568 (503) | 1,332 (484) |
| Change in amount of food and caloric beverages (g), mean (SD) | −508 (697) | −278 (633) | −107 (596) | +825 (768) | +277 (615) |
| Age (years), mean (SD) | 36.7 (4.6) | 36.7 (4.6) | 36.5 (4.5) | 36.5 (4.5) | 36.0 (4.7) |
| Weight (kg), mean (SD) | 68.0 (14.9) | 67.1 (14.7) | 65.8 (14.4) | 65.2 (14.1) | 64.5 (14.6) |
| BMI (kg/m2), mean (SD) | 25.0 (5.2) | 24.7 (5.2) | 24.2 (5.0) | 24.0 (4.9) | 23.7 (5.1) |
| Physical activity (METs-h/wk), mean (SD) | 25.0 (30.1) | 22.4 (27.9) | 20.4 (25.4) | 18.3 (24.3) | 15.8 (21.9) |
| Currently smoking (%) | 13.6 | 10.7 | 10.1 | 9.5 | 11.9 |
| Currently using oral contraceptives (%) | 2.4 | 2.5 | 2.5 | 2.3 | 1.9 |
| Currently receiving hormone replacement (%) | 11.2 | 10.4 | 10.5 | 10.8 | 11.0 |
| Dietary intake, mean (SD) | |||||
| Alcohol (g/day) | 4.1 (7.4) | 3.6 (6.38) | 3.3 (5.7) | 3.0 (5.6) | 2.3 (4.8) |
| Carbohydrates (energy percentage) | 4.9.1 (8.3) | 49.9 (7.5) | 49.9 (7.0) | 49.8 (6.8) | 50.0 (7.2) |
| Protein (energy percentage) | 20.1 (3.9) | 19.6 (3.4) | 19.3 (3.2) | 19.1 (3.1) | 18.5 (3.3) |
| Total fat (energy percentage) | 31.0 (6.2) | 31.1(5.7) | 31.5 (5.3) | 31.9 (5.1) | 32.4 (5.3) |
| Saturated fat (energy percentage) | 10.8 (2.6) | 10.9 (2.4) | 11.1 (2.2) | 11.4 (2.2) | 11.9 (2.3) |
| Monounsaturated fat (energy percentage) | 11.6 (2.7) | 11.8 (2.5) | 11.9 (2.3) | 12.1 (2.2) | 12.4 (2.3) |
| Polyunsaturated fat (energy percentage) | 5.8 (1.5) | 5.8 (1.4) | 5.7 (1.3) | 5.6 (1.30) | 5.4 (1.3) |
| Polyunsaturated/Saturated ratio | 0.6 (0.2) | 0.6 (0.2) | 0.5 (0.1) | 0.5 (0.1) | 0.5 (0.1) |
| Trans fat (energy percentage) | 1.5 (0.6) | 1.6 (0.6) | 1.6 (0.6) | 1.7 (0.6) | 1.8 (0.6) |
| Total fiber (g/day) | 17.4 (7.9) | 19.3 (7.8) | 19.2 (7.5) | 18.4 (6.8) | 16.0 (6.2) |
| Dietary consumption, mean (SD) | |||||
| Water as a drink (g/day) | 145.5 (251.4) | 165.4 (264.0) | 174.9 (269.4) | 175.1 (268.7) | 179.9 (286.0) |
| Fruits (g/day) | 181.4 (127.44) | 199.3 (131.5) | 192.7 (119.1) | 176.0 (107.6) | 130.3 (87.6) |
| Vegetables (g/day) | 186.4 (130.8) | 182.1 (106.1) | 167.0 (70.2) | 147.6 (74.1) | 111.4 (59.8) |
| Legumes (g/day) | 45.0 (39.8) | 46.1 (34.0) | 43.8 (31.5) | 40.5 (28.0) | 31.6 (23.8) |
| Sweets (g/day) | 38.0 (33.0) | 49.7 (37.4) | 55.7 (41.0) | 59.2 (42.2) | 60.8 (45.7) |
| Low-fat dairy products (g/day) | 42.6 (67.0) | 49.7 (75.6) | 51.6 (78.9) | 52.9 (84.8) | 53.7 (95.0) |
| Whole-fat dairy products (g/day) | 115.9 (229.7) | 89.5 (154.7) | 81.0 (124.4) | 74.8 (108.4) | 61.6 (85.7) |
| White meat (g/day) | 61.4 (46.1) | 65.4 (45.1) | 64.6 (43.7) | 62.3 (41.5) | 54.0 (38.1) |
| Red meat (g/day) | 52.4 (36.4) | 61.4 (38.6) | 64.8 (39.1) | 65.9 (39.1) | 66.1 (40.9) |
| Processed meat (g/day) | 58.0 (38.6) | 68.4 (40.9) | 72.7 (41.7) | 74.3 (41.8) | 75.3 (43.8) |
| Fish (g/day) | 45.3 (37.6) | 51.9 (39.7) | 52.4 (41.0) | 51.8 (37.8) | 45.5 (39.3) |
| Whole grains (g/day) | 176.7 (218.2) | 191.5 (210.2) | 170.0 (172.6) | 141.1 (137.7) | 107.7 (91.7) |
| Refined grains (g/day) | 83.3 (50.3) | 98.2 (54.0) | 101.7 (53.8) | 101.5 (53.2) | 95.3 (50.7) |
| Low-calorie carbonated soda (g/day) | 184.7 (342.3) | 195.2 (307.7) | 206.1 (306.4) | 222.2 (312.1) | 314.5 (397.5) |
| High-calorie carbonated soda (g/day) | 67.0 (168.5) | 94.3 (195.6) | 110.3 (203.7) | 128.3 (215.0) | 158.9 (250.9) |
| Nuts (g/day) | 1.4 (4.8) | 1.6 (4.7) | 1.6 (4.6) | 1.6 (5.1) | 1.4 (4.6) |
Table 2.
Top 15 positive and top 15 negative Pearson correlation coefficients between food items and dietary energy density among 50,026 women.
| POSITIVE | NEGATIVE | |||
|---|---|---|---|---|
| ORDER | FOOD ITEMS | PEARSON COEFFICIENT CORRELATION | FOOD ITEMS | PEARSON COEFFICIENT CORRELATION |
| 1 | Chocolate bars | +0.19 | Kale, mustard greens, or chard greens | −0.22 |
| 2 | Crackers | +0.18 | Iceberg or head lettuce | −0.20 |
| 3 | Processed meats, e.g. sausage, salami, bologna, etc. | +0.17 | Peaches, apricots, or plums | −0.19 |
| 4 | Pancake or waffles | +0.16 | Brussels sprouts | −0.18 |
| 5 | Beef, pork, or lamb as a sandwich or mixed dish, e.g. stew, casserole, lasagne, etc | +0.15 | Romaine or leaf lettuce | −0.17 |
| 6 | Brownies | +0.14 | Chowder or cream soup | −0.17 |
| 7 | Pork as a main dish, e.g. ham or chops | +0.14 | Celery | −0.16 |
| 8 | Hamburger | +0.14 | Green peppers | −0.16 |
| 9 | Cookies, ready made | +0.13 | Other cooked breakfast cereal | −0.15 |
| 10 | Sweet roll, coffee cake, or other pastry, home baked | +0.13 | Avocado | −0.15 |
| 11 | Doughnuts | +0.12 | String beans | −0.15 |
| 12 | Cookies, home baked | +0.12 | Dark orange (winter) squash | −0.14 |
| 13 | Sherbet, ice milk, or frozen yogurt | +0.11 | Cabbage or coleslaw | −0.14 |
| 14 | Cake, ready made | +0.10 | Spinach, cooked | −0.13 |
| 15 | Pie, homemade | +0.09 | Bananas | −0.11 |
For both 4-year periods, participants with the greatest increase in dietary ED (quintile 5) experienced a statistically significant greater weight gain as compared with those with the greatest decrease in ED (quintile 1) (Table 3). A similar association was observed for the 8-year period. Adjusting for dietary pattern scores did not appreciably alter the results.
Table 3.
Mean weight change (kg) according to quintiles (Q) of change in dietary energy density (Kcal/g) among 50,026 women.
| Quintiles of change in dietary energy density |
||||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | p for trend1 | |
|
| ||||||
| N | 10,005 | 10,005 | 10,006 | 10,005 | 10,005 | |
| Period 1991 – 1995 | ||||||
| Median change in energy density (Kcal/g) | −0.48 | −0.19 | −0.02 | +0.16 | +0.44 | |
| Crude weight change [kg, mean (SE)] | 2.58 (0.06) | 2.97 (0.06) | 3.30 (0.06) | 3.39 (0.06) | 4.17 (0.06) | <0.001 |
| Multivariate 12-adjusted weight change [kg, mean (SE)] | 2.57 (0.06) | 2.98 (0.06) | 3.31 (0.06) | 3.41 (0.06) | 4.15 (0.06) | <0.001 |
| Multivariate 23-adjusted weight change [kg, mean (SE)] | 2.69 (0.06) | 3.04 (0.06) | 3.33 (0.06) | 3.35 (0.06) | 4.00 (0.06) | <0.001 |
| Period 1995 – 1999 | ||||||
| Median change in energy density (Kcal/g) | 0.00 | +0.29 | +0.48 | +0.67 | +0.97 | |
| Crude weight change [kg, mean (SE)] | 1.59 (0.06) | 1.81 (0.06) | 1.96 (0.06) | 2.41 (0.06) | 3.00 (0.06) | <0.001 |
| Multivariate 12-adjusted weight change [kg, mean (SE)] | 1.54 (0.06) | 1.82 (0.06) | 1.97 (0.06) | 2.44 (0.06) | 3.00 (0.06) | <0.001 |
| Multivariate 23-adjusted weight change [kg, mean (SE)] | 1.63 (0.06) | 1.86 (0.06) | 1.98 (0.06) | 2.39 (0.06) | 2.90 (0.06) | <0.001 |
| Period 1991 – 1999 | ||||||
| Median change in energy density (Kcal/g) | −0.05 | +0.25 | +0.46 | +0.67 | +0.99 | |
| Crude weight change [kg, mean (SE)] | 4.35 (0.07) | 4.83 (0.07) | 5.28 (0.07) | 5.95 (0.07) | 6.78 (0.07) | <0.001 |
| Multivariate 12-adjusted weight change [kg, mean (SE)] | 4.40 (0.07) | 4.90 (0.07) | 5.31 (0.07) | 5.92 (0.07) | 6.65 (0.07) | <0.001 |
| Multivariate 23-adjusted weight change [kg, mean (SE)] | 4.57 (0.07) | 5.02 (0.07) | 5.35 (0.07) | 5.81 (0.07) | 6.42 (0.07) | <0.001 |
p value from the models in which the change in energy density was modelled as a continuous variable with the use of the median value of each quintile.
Multivariate model adjusted for age (continuous), baseline alcohol intake (0, 0.1 to 4.9, 5.0 to 9.9, 10+ g/day), physical activity (quintiles metabolic equivalent score), smoking (never, past, current, missing), postmenopausal hormone use (no, current or past, missing), oral contraceptive use (no, current, missing), cereal fiber intake (quintiles), trans fat intake (quintiles), and baseline BMI (continuous).
Multivariate model adjusted for multivariate model 1 + changes in confounders between time periods (except BMI) and change in intake of sugar-sweetened soft drinks.
When we classified participants according to categories of ED change, women who moved from the highest to the lowest quintiles of ED experienced the least weight gain, whereas women who moved from the lowest to the highest quintiles of ED had the greatest weight gain (Table 4). Weight gain was not lower for women who maintained a low-energy density as compared with women who maintained a high ED during follow-up.
Table 4.
Mean weight change (kg) according to categories of change in dietary energy density (Kcal/g) among 50,026 women.
| Categories of change in dietary energy density |
|||||
|---|---|---|---|---|---|
| Consistent in the Lowest Quintiles | Consistent in the Highest Quintiles | Moved from the Lowest to the Highest Quintiles | Moved from the Highest to the Lowest Quintiles | Other | |
| Period 1991 – 1995 | |||||
| No. participants | 12,144 | 12,084 | 4,186 | 4,071 | 17,541 |
| Crude weight change [kg, mean (SE)] | 3.57 (0.05) | 3.07 (0.05) | 4.29 (0.09) | 2.26 (0.09) | 3.23 (0.05) |
| Multivariate 11-adjusted weight change [kg, mean (SE)] | 3.49 (0.05) | 3.13 (0.05) | 4.26 (0.09) | 2.24 (0.09) | 3.25 (0.05) |
| Multivariate 22-adjusted weight change [kg, mean (SE)] | 3.63 (0.06) | 2.99 (0.05) | 4.08 (0.09) | 2.42 (0.09) | 3.24 (0.04) |
|
| |||||
| Period 1995 – 1999 | |||||
| No. participants | 10,670 | 10,689 | 5,649 | 5,196 | 17,822 |
| Crude weight change [kg, mean (SE)] | 2.00 (0.06) | 2.15 (0.06) | 3.05 (0.08) | 1.41 (0.08) | 2.18 (0.04) |
| Multivariate 11-adjusted weight change [kg, mean (SE)] | 2.04 (0.06) | 2.09 (0.06) | 3.05 (0.08) | 1.40 (0.08) | 2.20 (0.04) |
| Multivariate 22-adjusted weight change [kg, mean (SE)] | 2.33 (0.06) | 1.80 (0.06) | 2.90 (0.08) | 1.62 (0.08) | 2.18 (0.04) |
|
| |||||
| Period 1991 – 1999 | |||||
| No. participants | 9,861 | 9,951 | 6,346 | 5,914 | 17,954 |
| Crude weight change [kg, mean (SE)] | 5.34 (0.08) | 5.33 (0.08) | 6.94 (0.09) | 4.20 (0.09) | 5.43 (0.05) |
| Multivariate 11-adjusted weight change [kg, mean (SE)] | 5.30 (0.08) | 5.37 (0.08) | 6.78 (0.09) | 4.30 (0.09) | 5.45 (0.05) |
| Multivariate 22-adjusted weight change [kg, mean (SE)] | 5.81 (0.08) | 4.90 (0.08) | 6.41 (0.09) | 4.67 (0.09) | 5.44 (0.05) |
Multivariate model adjusted for age (continuous), baseline alcohol intake (0, 0.1 to 4.9, 5.0 to 9.9, 10+ g/day), physical activity (quintiles metabolic equivalent score), smoking (never, past, current, missing), postmenopausal hormone use (no, current or past, missing), oral contraceptive use (no, current, missing), cereal fiber intake (quintiles), trans fat intake (quintiles), and baseline BMI (continuous).
Multivariate model adjusted for multivariate model 1 + changes in confounders between time periods (except BMI) and change in intake of sugar-sweetened soft drinks.
The amount of weight change over time varied considerably according to the ED of individual foods and beverages (Figure 1). The top food/beverage items that were associated with the most weight gain include soda beverages, white bread, brownies, caffeine-free soda, French fried potatoes, fruit punches, chowder, crackers, candy bars, doughnuts, and bacon. Some foods with relatively high ED values such as oil and vinegar salad dressing, olive oil salad dressing, and nuts were not associated with greater weight gain or were associated with slightly reduced weight.
Figure 1.
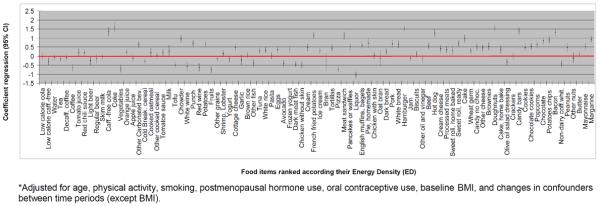
Adjusted* estimates (regression coefficients (β) and 95% CIs) for 8-year weight change (kg) for the highest tertile of change in consumption of each food item versus the lowest tertile. Food items are ranked according to their energy density from the lowest (left) to the highest (right) energy density.
DISCUSSION
In this large longitudinal study, an increase in total dietary ED was associated with a significantly greater weight gain during 8 years of follow-up in healthy middle-aged women. However, the magnitude of weight change varied considerably according to ED values of individual foods and beverages.
Several cross-sectional studies have evaluated the association between dietary ED and body weight. In the Multiethnic Cohort, Howarth and colleagues (11) calculated ED from responses to a FFQ in 191,023 participants. After adjusting for potential confounders, a higher ED was associated with a higher current BMI in each ethnic-gender group. Using national data of US adults older than 20 years from the 1999–2002 National Health and Nutrition Examination Survey (n=9,688), dietary ED was independently associated with higher BMI in women and trended toward a significant association in men (12). Ledikwe and colleagues (13) conducted a cross-sectional survey of adults (n=7,356) from the 1994–1996 Continuing Survey of Food Intakes by Individuals. The authors found that the ED for obese subjects tended to be slightly higher than that for normal-weight people. A similar association was found among participants from the NHANES III (14) and in a free-living sample of Chinese adults (15). However, three cross-sectional studies reported no substantial association between dietary ED and weight, percentage of body fat, or BMI (16–18). These null findings were based on studies with relatively small sample sizes. In addition, cross-sectional studies may have been affected by reverse causation bias: individuals who increased their weight may have reduced their dietary ED in the belief that it will help them to reduce energy intakes and lead to weight loss.
Limited prospective data are available on the relationship between dietary ED and subsequent weight gain. Iqbal et al. (19) examined this association among Danish participants of the MONICA study (n=862 men and 900 women). In the overall cohort, ED at baseline was not substantially associated with 5-year weight gain. In women, ED was positively associated with 5-year weight gain among the obese and inversely associated with weight gain in normal-weight women, whereas no significant interaction with baseline weight was observed among men. However, the researchers measured dietary intake at baseline only and assumed this to be constant over time. Our results showed that women who increased their dietary ED most had the greatest weight gain, whereas those who decreased their ED had lower weight gain. Interestingly, women who maintained a high ED experienced lower weight gain compared to women who maintained a low ED. Participants with constantly high ED might compensate better the energy intake from dietary ED. Another possibility is that the trajectory of weight gain for those participants has already reached a steady state after long-term consumption of a high ED diet.
In the post-hoc analyses of the PREMIER trial, Ledikwe and colleagues (20) found that reductions in dietary ED were associated with greater weight loss in overweight and obese participants with prehypertension or hypertension (n=658) during 6 months of follow-up. The reductions in ED were primarily achieved by the implementation of the DASH diet with higher amount of fruits and vegetables. In a one-year randomized trial, Ello-Martin and colleagues (31) found that reductions in dietary ED by reducing fat intake and increasing intakes of water-rich foods, particularly fruits and vegetables led to greater weight reduction in obese women. However, interpretation of this study was complicated by lack of a control group with high ED and a substantial increase in physical activity levels in both intervention groups.
A major consideration in evaluating ED and obesity in epidemiologic studies is whether or not to include beverages in the calculation of dietary ED. We conducted secondary analyses by including caloric beverages in the definition of ED and found somewhat weaker associations. Because of the large amount of water in beverages, they have very low ED even when the amount of sugar and calories is high (e.g., sugar-sweetened beverages). Thus, the meaning of the total dietary ED based on both solid foods and beverages is complicated and should therefore be interpreted cautiously.
Translating the results from short-term studies on the relationship between ED and body weight into dietary practice is not easy because of the large variability of ED values of individual foods and beverages that may comprise a high- or a low-ED diet. Freely selected high-ED diets may differ considerably in foods and nutrient composition between populations. Energy-dense diets are often high in refined grains, added sugars, and added fats (12). In fact, foods that presented the highest positive correlations with dietary ED were in general confectionery products that are low in fiber and rich in saturated and trans fats such as cookies, crackers, pancakes, waffles, and processed meats. Therefore, they have been associated with increased energy intake and poor diet quality (32, 33). However, diets with moderately high-fat content are not necessarily energy-dense. For example, the traditional Mediterranean diet is largely based on energy-diluted foods such as vegetables, fruits, and legumes. Indeed, the consumption of olive oil-based salad dressings in our cohort was negatively associated with dietary ED (r: −0.10). An increase in consumption of nuts (that are energy-dense) was not associated with higher weight gain either. These findings are consistent with results from a Mediterranean cohort where higher consumption of olive oil was not associated with weigh gain (34), while higher consumption of nuts was actually associated with less weight gain in the same cohort (35). Moreover, many processed foods high in fat and sugar are energy-dense, but at the same time palatable, convenient, and inexpensive. Thus, ecological analyses have suggested a close relationship between high-ED diets and low-energy cost (dollars per mega joule), which may contribute to higher obesity rates among socioeconomically disadvantaged populations (36).
The results of this study suggest that dietary ED reflects a poor diet quality characterized by higher intakes of saturated and trans fats and a higher glycemic load. The positive association between changes in dietary ED and weight gain observed in this study thus appears to represent the effects of an overall dietary pattern rather than physiological effects of ED per se. When each food and beverage was evaluated individually, the amount of weight changes over time varied considerably according to the ED of individual foods and beverages. Changes in the consumption of some foods/beverages with low ED values such as soda, fruit punches, and potatoes were associated with greater weight gain, whereas changes in consumption of some foods with relatively higher ED values such as olive oil and nuts were not associated with weight gain. Therefore, it would be misleading to select foods solely based on ED values of individual foods and beverages. Also, because total dietary ED was not associated with total fat, reduction in total fat intake does not necessarily lead to reduction in total ED. Based on our data, the best way to reduce total dietary ED is to reduce consumption of foods with a high amount of saturated fats and refined carbohydrates and to increase the consumption of fruits and vegetables. Furthermore, reducing consumption of soft drinks, which are low in ED, has been associated with lower weight gain in both children and adults (37).
In conclusion, an increase in dietary ED during 8 years was independently associated with a significantly higher weight gain in healthy middle-aged women. These results suggest that reducing overall dietary ED by decreasing consumption of foods with a high amount of saturated fats and refined carbohydrates and increasing fruits and vegetables may be beneficial for weight maintenance.
ACKNOWLEGMENTS
This study was funded by NIH Grants CA50385 and P30 DK46200. M.B-R. was also supported by a grant from the Spanish Ministry of Education. M.B-R acknowledges the financial support received by Fundacion Caja Madrid, and Amigos de la Universidad de Navarra. F.B.H. is the recipient of an American Heart Association–established Investigator Award. The funding organizations had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; and in the preparation, review, or approval of the manuscript.
REFERENCES
- 1).World Health Organization Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech. Rep. Ser. 2000;894:i–253. [PubMed] [Google Scholar]
- 2).Hossain P, Kawar B, El Nahas M. Obesity and Diabetes in the developing world-a growing challenge. N Engl J Med. 2007;356:213–5. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 3).World Health Organization . Fact sheet n° 311. 2006. Obesity and overweight. [Google Scholar]
- 4).Haslam D, Sattar N, Lean M. Obesity-time to wake up. BMJ. 2006;333:640–2. doi: 10.1136/bmj.333.7569.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Ello-Martin JA, Ledikwe JH, Rolls BJ. The influence of food portion size and energy density on energy intake: implications for weight management. Am J Clin Nutr. 2005;82:236S–241S. doi: 10.1093/ajcn/82.1.236S. [DOI] [PubMed] [Google Scholar]
- 6).Bell EA, Castellanos VH, Pelkman CL, Thorwart ML, Rolls BJ. Energy density of foods affects energy intake in normal-weight women. Am J Clin Nutr. 1998;67:412–20. doi: 10.1093/ajcn/67.3.412. [DOI] [PubMed] [Google Scholar]
- 7).World Health Organization/Food and Agriculture Organization Diet, nutrition and the prevention of chronic diseases: a report of the WHO Study Group on Diet, Nutrition and Prevention of Noncommunicable Diseases. Nutr Rev. 2002;49:291–301. doi: 10.1111/j.1753-4887.1991.tb07370.x. [DOI] [PubMed] [Google Scholar]
- 8).US Department of Health and Human Services and US Department of Agriculture . Dietary Guidelines for Americans, 2005. 6th ed US Government Printing Office; Washington, DC: 2005. [Google Scholar]
- 9).National Center for Chronic Disease Prevention and Health Promotion [Accessed March 1, 2007];Research to Practice Series, No. 1: Can eating fruits and vegetables help people to manage their weight? at: http://www.cdc.gov/nccdphp/dnpa/nutrition/pdf/rtp_practitioner_10_07.pdf.
- 10).Drewnowski A, Almiron-Roig E, Marmonier C, Lluch A. Dietary energy density and body weight: is there a relationship? Nutr Rev. 2004;62:403–13. doi: 10.1111/j.1753-4887.2004.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 11).Howarth NC, Murphy SP, Wilkens LR, Hankin JH, Kolonel LN. Dietary energy density is associated with overweight status among 5 ethnic groups in the Multiethnic Cohort Study. J Nutr. 2006;136:2243–8. doi: 10.1093/jn/136.8.2243. [DOI] [PubMed] [Google Scholar]
- 12).Mendoza JA, Drewnowski A, Christakis DA. Dietary energy density is associated with obesity and the metabolic syndrome in US adults. Diabetes Care. 2007;30:974–9. doi: 10.2337/dc06-2188. [DOI] [PubMed] [Google Scholar]
- 13).Ledikwe JH, Blanck HM, Kahn LK, Serdula MK, Seymour JD, Tohill BC, Rolls BJ. Dietary energy density is associated with energy intake and weight status in US adults. Am J Clin Nutr. 2006;83:1362–8. doi: 10.1093/ajcn/83.6.1362. [DOI] [PubMed] [Google Scholar]
- 14).Kant AK, Graubard BI. Energy density of diets reported by American adults: association with food group intake, nutrient intake and body weight. Int J Obes. 2005;29:950–6. doi: 10.1038/sj.ijo.0802980. [DOI] [PubMed] [Google Scholar]
- 15).Stookey JD. Energy density, energy intake and weight status in a large free-living sample of Chinese adults: exploring the underlying roles of fat, protein, carbohydrate, fiber and water intakes. Eur J Clin Nutr. 2001;55:349–59. doi: 10.1038/sj.ejcn.1601163. [DOI] [PubMed] [Google Scholar]
- 16).Cuco G, Arija V, Marti-Henneberg, Fernandez-Ballart J. Food and nutritional profile of high energy density consumers in an adult Mediterranean population. Eur J Clin Nutr. 2001;55:192–9. doi: 10.1038/sj.ejcn.1601144. [DOI] [PubMed] [Google Scholar]
- 17).De Castro JM. Dietary energy density is associated with increased intake in free-living humans. J Nutr. 2004;134:335–41. doi: 10.1093/jn/134.2.335. [DOI] [PubMed] [Google Scholar]
- 18).Yao M, McCrory MA, Tucker KL, Gao S, Fuss P, Roberts SB. Relative influence of diet and physical activity on body composition in urban Chinese adults. Am J Clin Nutr. 2003;77:1409–16. doi: 10.1093/ajcn/77.6.1409. [DOI] [PubMed] [Google Scholar]
- 19).Iqbal SI, Helge JW, Heitmann BL. Do energy density and dietary fiber influence subsequent 5-year weight changes in adult men and women? Obesity. 2006;14:106–14. doi: 10.1038/oby.2006.13. [DOI] [PubMed] [Google Scholar]
- 20).Ledikwe JH, Rolls BJ, Smiciklas-Wright H, et al. Reductions in dietary energy density are associated with weight loss in overweight and obese participants in the PREMIER trial. Am J Clin Nutr. 2007;85:1212–21. doi: 10.1093/ajcn/85.5.1212. [DOI] [PubMed] [Google Scholar]
- 21).Ledikwe JH, Blanck HM, Kahn LK, Serdula MK, Seymour JD, Tohill BC, Rolls BJ. dietary energy density determined by eight calculation methods in a nationally representative United States population. J Nutr. 2005;135:273–8. doi: 10.1093/jn/135.2.273. [DOI] [PubMed] [Google Scholar]
- 22).Mattes RD. Dietary compensation by humans for supplementar energy provided as ethanol or carbohydrate in fluids. Physiol Behav. 2006;59:179–87. doi: 10.1016/0031-9384(95)02007-1. [DOI] [PubMed] [Google Scholar]
- 23).US Department of Agriculture, Agricultural Research Service Nutrient Data Laboratory: home page. [Accessed March 1, 2007];USDA nutrient database for standard reference, release 10. 1993 at: Internet: http://www.ars.usda.gov/main/site_main.htm?modecode=12354500.
- 24).Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–6. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 25).Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–73. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 26).Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–9. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 27).Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927–34. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 28).Bes-Rastrollo M, Sanchez-Villegas A, Gomez-Gracia E, Martinez JA, Pajares RM, Martinez-Gonzalez MA. Predictors of weight gain in a Mediterranean cohort: the Seguimiento Universidad de Navarra Study. Am J Clin Nutr. 2006;83:362–70. doi: 10.1093/ajcn/83.2.362. [DOI] [PubMed] [Google Scholar]
- 29).Hatcher L. A Step-by-Step approach using the SAS System for factor analysis and structural equation modeling. SAS Institute Inc; Cary, NC: 1994. [Google Scholar]
- 30).Schulze MB, Fung TT, Manson JE, Willett WC, Hu FB. Dietary patterns and changes in body weight in women. Obesity. 2006;14:1444–53. doi: 10.1038/oby.2006.164. [DOI] [PubMed] [Google Scholar]
- 31).Ello-Martin JA, Roe LS, Ledikwe JH, Beach AM, Rolls BJ. Dietary energy density in the treatment of obesity: a year-long trial comparing 2 weight-loss diets. Am J Clin Nutr. 2007;85:1465–77. doi: 10.1093/ajcn/85.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Andrieu E, Darmon N, Drewnowski A. Low-cost diets: more energy, fewer nutrients. Eur J Clin Nutr. 2006;60:434–6. doi: 10.1038/sj.ejcn.1602331. [DOI] [PubMed] [Google Scholar]
- 33).Ledikwe JH, Blanck HM, Khan LK, Serdula MK, Seymour JD, Tohill BC, Rolls BJ. Low-energy density diets are associated with high diet quality in adults in the United States. J Am Diet Assoc. 2006:1172–80. doi: 10.1016/j.jada.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 34).Bes-Rastrollo M, Sanchez-Villegas A, de la Fuente C, de Irala J, Martinez JA, Martinez-Gonzalez MA. Olive oil consumption and weight change: the SUN prospective cohort study. Lipids. 2006;41:249–56. doi: 10.1007/s11745-006-5094-6. [DOI] [PubMed] [Google Scholar]
- 35).Bes-Rastrollo M, Sabate J, Gomez-Gracia E, Alonso A, Martinez JA, Martinez-Gonzalez MA. Nut consumption and weight gain in a Mediterranean cohort: The SUN study. Obesity. 2007;15:107–16. doi: 10.1038/oby.2007.507. [DOI] [PubMed] [Google Scholar]
- 36).Drewnowski A, Darmon N. The economics of obesity: dietary energy density and energy cost. Am J Clin Nutr. 2005;82:265S–73S. doi: 10.1093/ajcn/82.1.265S. [DOI] [PubMed] [Google Scholar]
- 37).Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–88. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]


