Abstract
Synthetic glucocorticoids (GC) form a crucial first-line treatment for childhood acute lymphoblastic leukemia (ALL). However prolonged GC therapy frequently leads to GC-resistance with an unclear molecular mechanism. 11β-hydroxysteroid dehydrogenase (11β-HSD) 2 inactivates GCs within cells. Here, we show the association between GC sensitivity and 11β-HSD2 expression in human T-cell leukemic cell lines. 11β-HSD2 mRNA and protein levels were considerably higher in GC-resistant MOLT4F cells than in GC-sensitive CCRF-CEM cells. The 11β-HSD inhibitor, carbenoxolone pre-treatment resulted in greater cell death with prednisolone assesed by methyl-thiazol-tetrazolium assay and Caspase-3/7 assay, suggesting that 11β-HSD2 is a cause of GC-resistance in ALL.
Keywords: Glucocorticoid resistance, 11β-hydroxysteroid dehydrogenase 2, acute lymphoblastic leukemia
Introduction
Glucocorticoids (GCs) have potent pro-apoptotic effects on lymphoid cells at certain stages of differentiation that are widely exploited in the treatment of malignant lymphoproliferative disorders. The synthetic GCs dexamethasone and prednisolone form a crucial first-line treatment for both B- and T-cell subtypes of childhood acute lymphoblastic leukemia [1,2]. However, ALL cells show different degrees of GC sensitivity/resistance at diagnosis and resistance to GCs can arise during therapy [3,4]. In childhood-ALL, in vitro GC sensitivity at diagnosis correlated with the in vivo response to 7 days prednisolone pretreatment, with a poor response associated with an unfavourable prognosis [5,6,7]. Functional glucocorticoid receptor (GR) levels generally correlate with response to GC treatment. Low GR levels may be a common, though hard to detect, mechanism of GC resistance [4,8,9,10,11]. However, this does not account for all GC resistance in ALL.
11β-HSD interconverts endogenous active GC (cortisol) and intrinsically inert cortisone (which does not bind to GR) [12]. Two isozymes exist. 11β-HSD2 (encoded by HSD11B2) inactivates GCs, whereas 11β-HSD1 (encoded by HSD11B1) regenerates active GCs from inert 11keto forms [12,13]. Importantly, prednisone (inert) and prednisolone (active) are also interconverted by these enzymes [14]. In contrast, dexamethasone is poorly inactivated [14]. Previous work has shown that 11β-HSD2 is expressed in some tumours and malignant cells, although it is not expressed in the normal adult tissue from which it is derived [15,16,17]. We have therefore postulated that 11β-HSD2 is expressed in glucocorticoid-resistant leukemia cells where its activity contributes to glucocorticoid resistance. The aim of this study was to evaluate how 11β-HSD2 affects GC sensitivity in lymphoblastic leukemia.
Materials and methods
Cell culture
Human T-lymphoblastic leukemia cells, CCRF-CEM (JCRB9023) and MOLT4F (JCRB0021) were obtained from the Health Science Research Resources Bank (Osaka, Japan). Cells were maintained in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 100 IU/mL penicillin, 100 μg/mL streptomycin at 37°C, 5% CO2. Carbenoxolone (CBX, 10 μM) was added 1h before adding glucocorticoids.
Cytotoxicity assay
Cytotoxicity was assessed using the methyl-thiazol-tetrazolium (MTT) assay and Caspase-3/7 assay. Cells (1×105 cells/well) were cultured in 96-well plates. Glucocorticoid sensitivity/resistance was determined by addition of prednisolone (PRED) or dexamethasone (DEX) (9 nM–280 μM) for 96 h and cell viability measured by MTT assay, as previously described [18,19]. The 50% lethal dose (LD50) was calculated from the dose–response curve. For caspase assay, cells were treated with prednisolone (1 μM) or dexamethasone (1 μM) for 48h. 100 μl of caspase-Glo 3/7 (Promega, Madison, WI, USA) reagent was then added to each sample, cells were incubated for 1 h and caspase activity measured according to the manufacturer’s instructions.
RNA extraction and reverse transcription-PCR analysis
Cells (1×106 cells/well) were cultured for 24 h in the presence or absence of 1 μM dexamethasone in 12-well plates. RNA was extracted following homogenisation in Trizol (Invitrogen) and resuspended in RNase-free water. Reverse transcription of RNA (1 μg) used a reverse transcriptase system (Takara) according to the manufacturer’s protocol. Specific mRNA levels were quantified by real-time PCR on a LightCycler (Roche, Mannheim, Germany), according to the manufacturer’s instructions. Primers used were: 11β-HSD2, 5′-ATCCGTGCTTGGGGGCCTATGGAAC-3′, 5′-GACCCACGTTTCTCACTGACTCTGTC-3′ and GRα, 5′-ACTTACACCTGGATGACCAAAT-3′, 5′-TTCAATACTCATGGTCTTATCC-3′. 18S served as internal control.
Western blotting
Cells were washed twice in cold PBS and lysed with RIPA buffer containing protease inhibitor cocktail. Electrophoresis was carried out on 12% NuPAGE Bis-Tris gels (Invitrogen) with 20 μg protein loaded. After transfer, blots were probed with antibodies to 11β-HSD2 (a gift of Dr. Roger W. Brown [20]) or tubulin at 1:10000 dilution overnight at 4°C. After washing, membranes were incubated with goat anti-rabbit IRDye-800CW (for 11β-HSD2) or goat anti-mouse IRDye-800CW (for tubulin) secondary antibodies (both from LI-COR) for 1 h at room temperature. Blots were visualized on an Odyssey scanner (LI-COR).
Analysis of 11β-HSD2 activity
Cells were cultured in 12-well plates in the presence or absence of 10 μM CBX for 1 h. Cortisol (0.1 μM) was added and medium was collected after 24 h. Cells were removed from the medium by centrifugation (10,000 × g, 20 min, 4°C) and cell numbers per well were counted. The collected medium was loaded onto activated Sep-Pak C18 (Waters Corp., MA, USA) cartridges. The column was then washed with 5% methanol and steroids eluted with 100% methanol. The eluate was dried in a vacuum centrifuge and steroids were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) as previously described [21]. Enzyme activity is expressed as pmol cortisone produced/ h/ 106 cells.
Statistics
Data were analyzed using ANOVA. Significance was set at p < 0.05. Values are mean±SEM.
Results
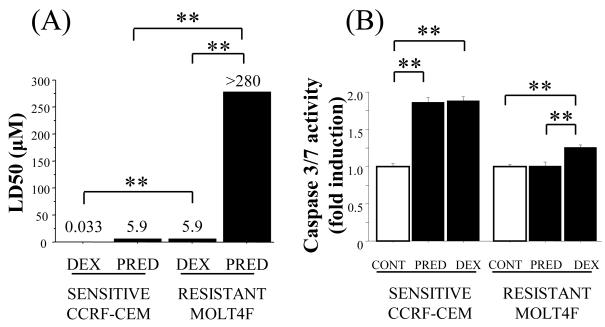
Differential sensitivity to dexamethasone and prednisolone in glucocorticoid-resistant MOLT4F cells
Dexamethasone is poorly metabolised by 11β-HSD enzymes, whereas prednisone/prednisolone are readily metabolized [14]. To determine whether glucocorticoid-sensitive CCRF-CEM [22] and glucocorticoid-resistant MOLT4F cells [23] show differential sensitivity to dexamethasone and prednisolone, MTT and caspase 3/7 assays were carried out. Both MOLT4F and CCRF-CEM cells showed measureable sensitivity to dexamethasone, although MOLT4F cells were considerably more resistant than CCRF-CEM cells (Fig. 1A). However, in contrast to CCRF-CEM cells, MOLT4F cells were completely resistant to prednisolone (LD50 >280 μM). Consistent with these data, prednisolone and dexamethasone (at 1 μM) were equally effective in the induction of caspasae 3/7 activity in CCRF-CEM cells, yet only dexamethasone induced caspase 3/7 activity in MOLT4F cells (Fig. 1B).
Figure 1.
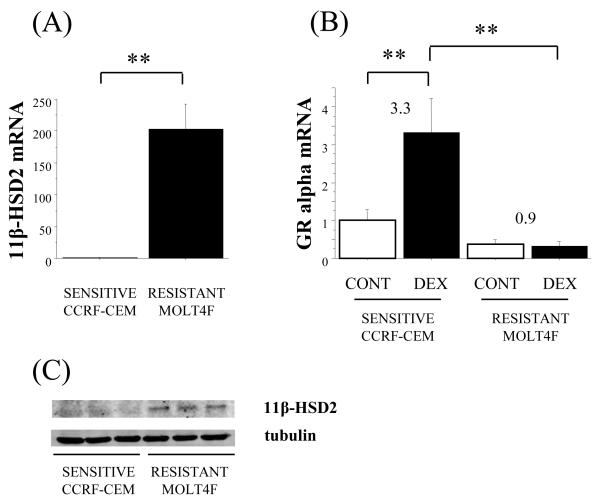
High expression of 11β-HSD2 and low expression of GR alpha in glucocorticoid-resistant MOLT4F cells
The relative resistance to prednisolone suggested the presence of 11β-HSD2 in MOLT4F cells. Quantitative (q)PCR showed that levels of 11β-HSD2 mRNA were considerably higher in MOLT4F cells than in CCRF-CEM cells (Fig. 2A). 11β-HSD1 mRNA was not detectable in MOLT4F cells (data not shown). GR alpha mRNA was also expressed in both CCRF-CEM cells and MOLT4F cells (Fig. 2B). The basal level of GR alpha mRNA was higher in CCRFCEM cells than MOLT4F cells, although this did not achieve significance. In CCRF-CEM cells, GR alpha mRNA was dramatically increased by dexamethasone treatment, whereas no induction of GR alpha occurred in MOLT4F cells (Fig. 2B) to give a 10.5 fold difference in GR alpha mRNA levels between glucocorticoid-sensitive CCRF-CEM and glucocorticoid-resistant MOLT4F cells following dexamethasone treatment (Fig. 2B). Consistent with the levels of 11β-HSD2 mRNA, western blotting showed higher levels of 11β-HSD2-immunoreative protein in MOLT4F cells than in CCRF-CEM cells (Fig. 2C), indicating that 11β-HSD2 could be inactivating prednisolone to prednisone in MOLT4F cells.
Figure 2.
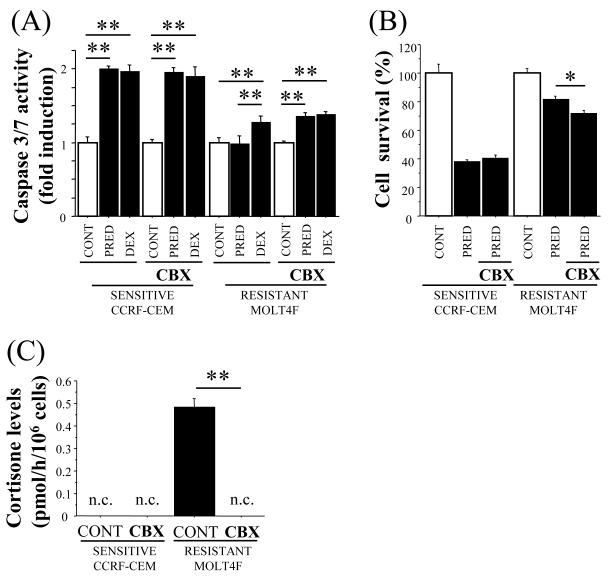
Inhibition of 11β-HSD2 increases sensitivity to prednisolone in MOLT4F cells
To determine whether 11β-HSD2 could contribute to prednisolone resistance in MOLT4F cells, cells were pre-treated with the 11β-HSD inhibitor carbenoxolone (CBX) prior to cytotoxicity assay. CBX pre-treatment significantly increased caspase 3/7 activity following prednisolone treatment in MOLT4F cells (Fig. 3A) and significantly decreased cell survival following prednisolone treatment, assayed by MTT (Fig. 3B), whereas CBX had no effect in CCRF-CEM cells (Fig. 3A and 3B). Cortisol was converted to cortisone by MOLT4F cells, but not by CCRF-CEM cells (Fig. 3C), confirming the presence of functional 11β-HSD2 in MOLT4F cells with negligible levels in CCRF-CEM cells. Moreover, conversion of cortisol (active natural glucocorticoid) to cortisone (inert natural glucocorticoid) was completely abolished by CBX pre-treatment (Fig. 3C), demonstrating inhibition of 11β-HSD2 by CBX in MOLT4F cells.
Figure 3.
Discussion
Here we have shown that high levels of 11β-HSD2 are associated with resistance to the synthetic glucocorticoid, prednisolone, in MOLT4F cells, whereas the non-metabolisable glucocorticoid, dexamethasone, could induce measurable apoptosis in these cells. Dexamethasone reportedly has 6 times the glucocorticoid potency of prednisolone [24]. However even a 40-fold higher dose of prednisolone could not induce apoptosis to the levels achieved with dexamethasone, suggesting that the prednisolone resistance in these cells is not simply due to differences in potency of the two synthetic glucocorticoids. The presence of 11β-HSD2 in MOLT4F cells could plausibly contribute to prednisolone resistance. Indeed, the conversion of active cortisol into inactive cortisone by MOLT4F cells and its inhibition by CBX demonstrates the presence of oxidative 11β-HSD activity in these cells. This was confirmed by demonstration of 11β-HSD2 mRNA and protein, but not 11β-HSD1 mRNA in these cells.
MOLT4F cells showed greater resistance to dexamethasone-induced cell death than did CCRF-CEM cells. This is unlikely to be due to 11β-HSD2, as dexamethasone is poorly inactivated by this isozyme [14]. However, dexamethasone resistance in MOLT4F cells could, at least in part, be due to the low levels of GR in these cells and failure of GR auto-induction. This is consistent with previous reports of low GR levels and/or a failure of auto-induction as a common though hard to detect cause of GC resistance in lymphoblastic leukemia [4,8,9,10,11,25], exquisitely sensitive to glucocorticoid dose [9]. Our data therefore point to at least two mechanisms of GC resistance in MOLT4F cells: a pre-receptor mechanism due to 11β-HSD2 expression (dependent on the type of glucocorticoid administered) and a receptor-mediated mechanism; low GR levels and failure of GR auto-induction.
We have previously shown differential GC regulation of 11β-HSD1 in glucocorticoid-sensitive and glucocorticoid-resistant lymphoblastic leukemia [19]. Levels of 11β-HSD1, which amplifies glucocorticoid action within cells by converting inert GC to active forms, were increased by GC treatment of glucocorticoid-sensitive acute lymphoblastic leukemia cells, whereas they were decreased or unchanged in resistant cells [19]. This may be yet another mechanism that could contribute to differential glucocorticoid sensitivity of leukemic cells. In contrast to 11β-HSD1, 11β-HSD2 is widely expressed during development and is re-expressed in tumours and transformed cell lines [17,26,27]. Indeed, many tissues switch from 11β-HSD2 to 11β-HSD1 expression as they differentiate and mature [27,28]. In transfected cells, 11β-HSD1 expression reduced proliferation, whereas transfection with 11β-HSD2 increased proliferation rate [26]. Therefore it is possible that the expression of 11β-HSD1 and 2 produce opposing patterns of cell proliferation. Alternatively, expression of these enzymes together with GR auto-induction may contribute to the differential GC-sensitivity of early lymphocyte precursors [29,30].
CCRF-CEM and MOLT4F cells may be extreme examples of GC sensitivity/resistance. Nevertheless, they add further support to the hypothesis that threshold levels of GR are required for glucocorticoid sensitivity of lymphoblastic leukemic cells. Moreover, whilst 11β-HSD2 is unlikely to be a contributory factor in dexamethasone resistance, it may contribute to prednisolone resistance. It is now critical to establish whether 11β-HSD2 can contribute to, or is a marker of, glucocorticoid resistance in primary cells. If this is true for primary leukaemic cells then dexamethasone would be of greater therapeutic benefit than prednisolone in the initial treatment of lymphoblastic leukemia. Indeed, replacement of prednisolone (or prednisone) by dexamethasone in induction therapy markedly reduced risk of relapse with better clinical outcome [31,32,33]. In addition, further research with primary cells is required to investigate whether levels of GR, 11β-HSD2 and 11β-HSD1 could be useful prognostic makers in childhood acute lymphoblastic leukemia.
References
- 1.Kofler R. The molecular basis of glucocorticoid-induced apoptosis of lymphoblastic leukemia cells. Histochem Cell Biol. 2000;114:1–7. doi: 10.1007/s004180000165. [DOI] [PubMed] [Google Scholar]
- 2.Tissing WJ, Meijerink JP, den Boer ML, Pieters R. Molecular determinants of glucocorticoid sensitivity and resistance in acute lymphoblastic leukemia. Leukemia. 2003;17:17–25. doi: 10.1038/sj.leu.2402733. [DOI] [PubMed] [Google Scholar]
- 3.Kofler R, Schmidt S, Kofler A, Ausserlechner MJ. Resistance to glucocorticoid-induced apoptosis in lymphoblastic leukemia. J Endocrinol. 2003;178:19–27. doi: 10.1677/joe.0.1780019. [DOI] [PubMed] [Google Scholar]
- 4.Ploner C, Schmidt S, Presul E, et al. Glucocorticoid-induced apoptosis and glucocorticoid resistance in acute lymphoblastic leukemia. J Steroid Biochem Mol Biol. 2005;93:153–160. doi: 10.1016/j.jsbmb.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Schrappe M, Arico M, Harbott J, et al. Philadelphia chromosome-positive (Ph+) childhood acute lymphoblastic leukemia: good initial steroid response allows early prediction of a favorable treatment outcome. Blood. 1998;92:2730–2741. [PubMed] [Google Scholar]
- 6.Dördelmann M, Reiter A, Borkhardt A, et al. Prednisone response is the strongest predictor of treatment outcome in infant acute lymphoblastic leukemia. Blood. 1999;94:1209–1217. [PubMed] [Google Scholar]
- 7.Schrappe M, Reiter A, Zimmermann M, et al. Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995. Berlin-Frankfurt-Munster. Leukemia. 2000;14:2205–2222. doi: 10.1038/sj.leu.2401973. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz CL, Thompson EB, Gelber RD, et al. Improved response with higher corticosteroid dose in children with acute lymphoblastic leukemia. J Clin Oncol. 2001;19:1040–1046. doi: 10.1200/JCO.2001.19.4.1040. [DOI] [PubMed] [Google Scholar]
- 9.Gruber G, Carlet M, Türtscher E, et al. Levels of glucocorticoid receptor and its ligand determine sensitivity and kinetics of glucocorticoid-induced leukemia apoptosis. Leukemia. 2009;23:820–823. doi: 10.1038/leu.2008.360. [DOI] [PubMed] [Google Scholar]
- 10.Pui CH, Costlow ME. Sequential studies of lymphoblast glucocorticoid receptor levels at diagnosis and relapse in childhood leukemia: an update. Leuk Res. 1986;10:227–229. doi: 10.1016/0145-2126(86)90046-9. [DOI] [PubMed] [Google Scholar]
- 11.Tissing WJ, Lauten M, Meijerink JP, et al. Expression of the glucocorticoid receptor and its isoforms in relation to glucocorticoid resistance in childhood acute lymphocytic leukemia. Haematologica. 2005;90:1279–1281. [PubMed] [Google Scholar]
- 12.Seckl JR, Chapman KE. The 11ß-hydroxysteroid dehydrogenase system, a determinant of glucocorticoid and mineralocorticoid action. Eur J Biochem. 1997;249:361–364. doi: 10.1111/j.1432-1033.1997.t01-1-00361.x. [DOI] [PubMed] [Google Scholar]
- 13.Chapman KE, Coutinho AE, Gray M, Gilmour JS, Savill JS, Seckl JR. The role and regulation of 11β-hydroxysteroid dehydrogenase type 1 in the inflammatory response. Mol Cell Endocrinol. 2009;301:123–131. doi: 10.1016/j.mce.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 14.Diederich S, Eigendorff E, Burkhardt P, et al. 11β-Hydroxysteroid dehydrogenase types 1 and 2: an important pharmacokinetic determinant for the activity of synthetic mineralo and glucocorticoids. J Clin Endocrinol Metab. 2002;87:5695–5701. doi: 10.1210/jc.2002-020970. [DOI] [PubMed] [Google Scholar]
- 15.Coulter CL, Smith RE, Stowasser M, Sasano H, Krozowski ZS, Gordon RD. Expression of 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD-2) in the developing human adrenal gland and human adrenal cortical carcinoma and adenoma. Mol Cell Endocrinol. 1999;154:71–77. doi: 10.1016/s0303-7207(99)00077-5. [DOI] [PubMed] [Google Scholar]
- 16.Koyama K, Myles K, Smith R, Krozowski Z. Expression of the 11β-hydroxysteroid dehydrogenase type II enzyme in breast tumors and modulation of activity and cell growth in PMC42 cells. J Steroid Biochem Mol Biol. 2001;76:153–159. doi: 10.1016/s0960-0760(00)00157-6. [DOI] [PubMed] [Google Scholar]
- 17.Rabbitt EH, Gittoes NJ, Stewart PM, Hewison M. 11β-hydroxysteroid dehydrogenases, cell proliferation and malignancy. J Steroid Biochem Mol Biol. 2003;85:415–421. doi: 10.1016/s0960-0760(03)00224-3. [DOI] [PubMed] [Google Scholar]
- 18.Hongo T, Yajima S, Sakurai M, Horikoshi Y, Hanada R. In vitro drug sensitivity testing can predict induction failure and early relapse of childhood acute lymphoblastic leukemia. Blood. 1997;89:2959–2965. [PubMed] [Google Scholar]
- 19.Sai S, Nakagawa Y, Sakaguchi K, et al. Differential regulation of 11β-hydroxysteroid dehydrogenase-1 by dexamethasone in glucocorticoid-sensitive and -resistant childhood lymphoblastic leukemia. Leuk Res. 2009;33:1696–1698. doi: 10.1016/j.leukres.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Brown RW, Chapman KE, Kotelevtsev Y, et al. Cloning and production of antisera to human placental 11β-hydroxysteroid dehydrogenase type 2. Biochem J. 1996;313:1007–1017. doi: 10.1042/bj3131007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujisawa Y, Nakagawa Y, Li RS, Liu YJ, Ohzeki T. Diabetic pregnancy in rats leads to impaired glucose metabolism in offspring involving tissue-specific dysregulation of 11beta-hydroxysteroid dehydrogenase type 1 expression. Life Sci. 2007;81:724–731. doi: 10.1016/j.lfs.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Riml S, Schmidt S, Ausserlechner MJ, Geley S, Kofler R. Glucocorticoid receptor heterozygosity combined with lack of receptor auto-induction causes glucocorticoid resistance in Jurkat acute lymphoblastic leukemia cells. Cell Death Differ. 2004;11(Suppl 1):S65–72. doi: 10.1038/sj.cdd.4401413. [DOI] [PubMed] [Google Scholar]
- 23.Dyson JE, Quirke P, Bird CC, McLaughlin JB, Surrey CR. Relationship between cell ploidy and glucocorticoid induced death in human lymphoid cell lines. Br J Cancer. 1984;49:731–738. doi: 10.1038/bjc.1984.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito C, Evans WE, McNinch L, et al. Comparative cytotoxicity of dexamethasone and prednisolone in childhood acute lymphoblastic leukemia. J Clin Oncol. 1996;14:2370–2376. doi: 10.1200/JCO.1996.14.8.2370. [DOI] [PubMed] [Google Scholar]
- 25.Thompson EB, Smith JR, Bourgeois S, Harmon JM. Glucocorticoid receptors in human leukemias and related diseases. Klin Wochenschr. 1985;63:689–698. doi: 10.1007/BF01733111. [DOI] [PubMed] [Google Scholar]
- 26.Rabbitt EH, Lavery GG, Walker EA, Cooper MS, Stewart PM, Hewison M. Prereceptor regulation of glucocorticoid action by 11β-hydroxysteroid dehydrogenase: a novel determinant of cell proliferation. FASEB J. 2002;16:36–44. doi: 10.1096/fj.01-0582com. [DOI] [PubMed] [Google Scholar]
- 27.Brown RW, Diaz R, Robson AC, et al. The ontogeny of 11 beta-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology. 1996;137:794–797. doi: 10.1210/endo.137.2.8593833. [DOI] [PubMed] [Google Scholar]
- 28.Speirs HJ, Seckl JR, Brown RW. Ontogeny of glucocorticoid receptor and 11β- hydroxysteroid dehydrogenase type-1 gene expression identifies potential critical periods of glucocorticoid susceptibility during development. J Endocrinol. 2004;181:105–116. doi: 10.1677/joe.0.1810105. [DOI] [PubMed] [Google Scholar]
- 29.Brewer JA, Sleckman BP, Swat W, Muglia LJ. Green fluorescent protein-glucocorticoid receptor knockin mice reveal dynamic receptor modulation during thymocyte development. J Immunol. 2002;169:1309–1318. doi: 10.4049/jimmunol.169.3.1309. [DOI] [PubMed] [Google Scholar]
- 30.Purton JF, Monk JA, Liddicoat DR, et al. Expression of the glucocorticoid receptor from the 1A promoter correlates with T lymphocyte sensitivity to glucocorticoid-induced cell death. J Immunol. 2004;173:3816–3824. doi: 10.4049/jimmunol.173.6.3816. [DOI] [PubMed] [Google Scholar]
- 31.Bostrom BC, Sensel MR, Sather HN, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children’s Cancer Group. Blood. 2003;101:3809–3817. doi: 10.1182/blood-2002-08-2454. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell CD, Richards SM, Kinsey SE, et al. Benefit of dexamethasone compared with prednisolone for childhood acute lymphoblastic leukaemia: results of the UK Medical Research Council ALL97 randomized trial. Br J Haematol. 2005;129:734–745. doi: 10.1111/j.1365-2141.2005.05509.x. [DOI] [PubMed] [Google Scholar]
- 33.Schrappe M, Zimmermann M, Moricke A, et al. Dexamethasone in induction can eliminate one third of all relapses in childhood acute lymphoblastic leukemia (ALL): Results of an international randomized trial in 3655 patients (Trial AIEOP-BFM ALL 2000) [abstract] Blood. 2008;112:7. [Google Scholar]