Abstract
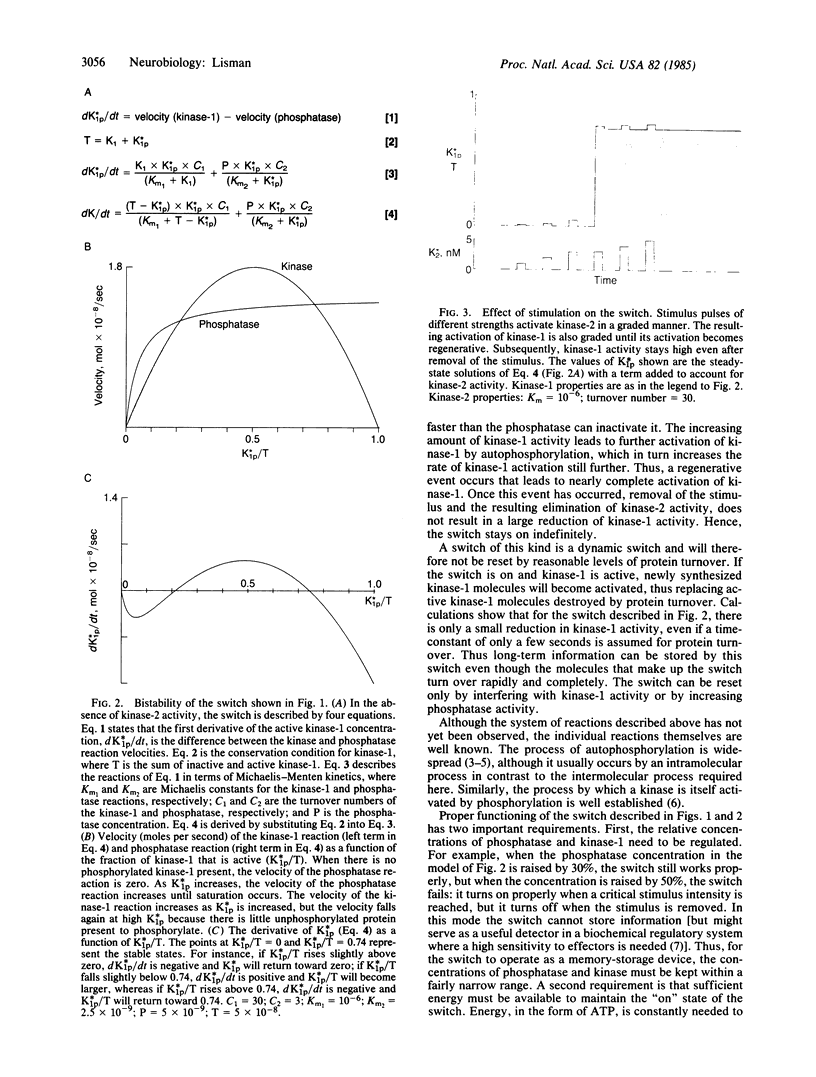
A mechanism is proposed for a molecular switch that can store information indefinitely, despite the complete turnover of the molecules that make up the switch. The design of the switch is based on known types of biochemical reactions. Central to the mechanism is a kinase that is activated by phosphorylation and capable of intermolecular autophosphorylation. It is shown that such a kinase and an associated phosphatase form a bistable chemical switch that can be turned on by an external stimulus and that is not reset by protein turnover.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crick F. Memory and molecular turnover. Nature. 1984 Nov 8;312(5990):101–101. doi: 10.1038/312101a0. [DOI] [PubMed] [Google Scholar]
- DeLange R. J., Kemp R. G., Riley W. D., Cooper R. A., Krebs E. G. Activation of skeletal muscle phosphorylase kinase by adenosine triphosphate and adenosine 3',5'-monophosphate. J Biol Chem. 1968 May 10;243(9):2200–2208. [PubMed] [Google Scholar]
- El-Maghrabi M. R., Claus T. H., Pilkis J., Fox E., Pilkis S. J. Regulation of rat liver fructose 2,6-bisphosphatase. J Biol Chem. 1982 Jul 10;257(13):7603–7607. [PubMed] [Google Scholar]
- Erlichman J., Rangel-Aldao R., Rosen O. M. Reversible autophosphorylation of type II cAMP-dependent protein kinase: distinction between intramolecular and intermolecular reactions. Methods Enzymol. 1983;99:176–186. doi: 10.1016/0076-6879(83)99051-1. [DOI] [PubMed] [Google Scholar]
- Foster J. L., Guttmann J., Rosen O. M. Autophosphorylation of cGMP-dependent protein kinase. J Biol Chem. 1981 May 25;256(10):5029–5036. [PubMed] [Google Scholar]
- Grab D. J., Carlin R. K., Siekevitz P. Function of a calmodulin in postsynaptic densities. II. Presence of a calmodulin-activatable protein kinase activity. J Cell Biol. 1981 Jun;89(3):440–448. doi: 10.1083/jcb.89.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. The epidermal growth factor receptor gene and its product. Nature. 1984 Oct 4;311(5985):414–416. doi: 10.1038/311414a0. [DOI] [PubMed] [Google Scholar]
- KREBS E. G., LOVE D. S., BRATVOLD G. E., TRAYSER K. A., MEYER W. L., FISCHER E. H. PURIFICATION AND PROPERTIES OF RABBIT SKELETAL MUSCLE PHOSPHORYLASE B KINASE. Biochemistry. 1964 Aug;3:1022–1033. doi: 10.1021/bi00896a003. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., McGuinness T. L., Greengard P. Evidence that the major postsynaptic density protein is a component of a Ca2+/calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1984 Feb;81(3):945–949. doi: 10.1073/pnas.81.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B., Bennett M. K., Erondu N. E. Biochemical and immunochemical evidence that the "major postsynaptic density protein" is a subunit of a calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7357–7361. doi: 10.1073/pnas.80.23.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Goldbeter A., Stock J. B. Amplification and adaptation in regulatory and sensory systems. Science. 1982 Jul 16;217(4556):220–225. doi: 10.1126/science.7089556. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Koshland D. E., Jr A protein with kinase and phosphatase activities involved in regulation of tricarboxylic acid cycle. Nature. 1982 Dec 2;300(5891):458–460. doi: 10.1038/300458a0. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- MONOD J., JACOB F. Teleonomic mechanisms in cellular metabolism, growth, and differentiation. Cold Spring Harb Symp Quant Biol. 1961;26:389–401. doi: 10.1101/sqb.1961.026.01.048. [DOI] [PubMed] [Google Scholar]
- Nestler E. J., Greengard P. Protein phosphorylation in the brain. Nature. 1983 Oct 13;305(5935):583–588. doi: 10.1038/305583a0. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles C. D. The molecular biology of platelet-derived growth factor. Cell. 1983 Jul;33(3):653–655. doi: 10.1016/0092-8674(83)90008-9. [DOI] [PubMed] [Google Scholar]




