Abstract
Purpose
To evaluate the outcome of ductal carcinoma in situ (DCIS) patients who underwent surgery followed by radiation therapy (RT).
Materials and Methods
We retrospectively reviewed 106 DCIS patients who underwent surgery followed by postoperative RT between 1994 and 2006. Ninety-four patients underwent breast-conserving surgery, and mastectomy was performed in 12 patients due to extensive DCIS. Postoperative RT was delivered to whole breast with 50.4 Gy/28 fx. Tumor bed boost was offered to 7 patients (6.6%). Patients with hormonal receptor-positive tumors were treated with hormonal therapy.
Results
The median follow-up duration was 83.4 months (range, 33.4 to 191.5 months) and the median age was 47.8 years. Ten patients (9.4%) had resection margin <1 mm and high-grade and estrogen receptor-negative tumors were observed in 39 (36.8%) and 20 (18.9%) patients, respectively. The 7-year ipsilateral breast tumor recurrence (IBTR)-free survival rate was 95.3%. Resection margin (<1 or ≥1 mm) was the significant prognostic factor for IBTR in univariate and multivariate analyses (p < 0.001 and p = 0.016, respectively).
Conclusion
Postoperative RT for DCIS can achieve favorable treatment outcome. Resection margin was the important prognostic factor for IBTR in the DCIS patients who underwent postoperative RT.
Keywords: DCIS, Postoperative RT, IBTR
Introduction
Ductal carcinoma in situ (DCIS) of the breast refers to malignant epithelial cells confined to the myoepithelial layer of the ducts and lobules [1]. Due to the increased use of screening mammography, the diagnosis of DCIS has increased dramatically [2]. DCIS now represents approximately 20% to 30% of newly diagnosed breast carcinomas in the United States [3]. In Korea, the incidence of DCIS increased over three-fold from 1996 to 2011, that is, from 4.2% to 13.3% [4].
DCIS of the breast is a broad spectrum of disease with variable malignant potentials, and the optimal form of local therapy is being debated. Mastectomy, breast-conserving surgery (BCS), and BCS plus whole breast radiation therapy (RT) are included in recent management options. Mastectomy is a curative option with local recurrence rate of 1% to 2%, which is lower than breast conserving approach in historical series [5,6]. However, it requires immediate reconstruction surgery for better cosmetic outcome. From randomized controlled trials, whole breast RT after BCS reduced the ipsilateral breast tumor recurrence (IBTR) rate, and BCS followed by whole breast RT is now the standard treatment in most DCIS patients [7,8,9,10]. However, in recent years, considering tumor grade, lesion size, and surgical resection margin, identification of a certain subset of patients with favorable prognosis is emphasized for omission of RT after complete excision [11,12].
Although the incidence of DCIS of the breast has increased recently in Korea, the updated analyses of treatment outcomes and prognostic factors for IBTR were not enough. Kim et al. [13] reported the results of a retrospective review of DCIS patients treated with BCS plus postoperative RT from 1995 to 2001. With the median follow-up of 43 months, 5-year local relapse-free survival and overall survival rates were 91% and 100%, respectively.
The purpose of the present study is to investigate the outcome of DCIS patients who underwent surgery (mastectomy or BCS) followed by postoperative RT. We also analyzed the patterns of failure and clinical and pathological factors related to IBTR.
Materials and Methods
1. Study population
After obtaining an approval of Institutional Review Board, we retrospectively reviewed the medical records of 106 DCIS patients who underwent postoperative RT after surgery between January 1994 and December 2006. Patients with prior diagnoses of cancer of any organs, synchronous invasive breast cancer, and DCIS with microinvasion were excluded. Tumor size, surgical resection margin status, histologic grade, hormonal receptor status, and lymph node metastasis were determined from pathology reports.
2. Treatment
Surgical treatment options included both mastectomy and BCS. All of the patients underwent whole breast RT after BCS with an opposed tangential technique. In the cases of subtotal mastectomy, postoperative RT was delivered in accordance with the cases of BCS. Post-mastectomy RT was offered to one patient who had positive resection margin after total mastectomy. Whole breast RT dose was 50.4 Gy/28 fx. Tumor bed boost was given to selected patients with 9 Gy/5 fx based on physician's discretion mainly taking the close or positive resection margin into consideration. Hormonal therapy was offered to patients with hormonal receptor-positive tumors.
3. Statistical analysis
The date of surgical resection of primary tumor was used as the date of diagnosis. Overall survival (OS) was defined as the interval from the date of diagnosis to the date of death. IBTR-free survival was defined as the interval from the date of diagnosis to the date of the diagnosis of tumor recurrence in the ipsilateral breast. SPSS ver. 20.0 (SPSS Inc., Chicago, IL, USA) statistical software was used for statistical analyses. Kaplan-Meier analysis was used to estimate OS and IBTR-free survival, and the survival differences according to a variety of risk factors were evaluated by two-sided log-rank test. All of the analyses were declared statistically significant if the p-value was <0.05. In multivariate analyses, Cox proportional hazards regression was used to adjust for confounding covariates.
Results
Patient, tumor, and treatment characteristics are listed in Table 1. The median age was 47.8 years (range, 25.1 to 68.3 years), and the median tumor size was 2.5 cm (range, 0.2 to 8.5 cm). Seventy-six patients (71.7%) had resection margin of ≥10 mm, and <1 mm was observed in 10 patients (9.4%). High grade tumors were found in 39 patients (36.8%), and 67 patients (63.2%) had low or intermediate grade tumors. Estrogen receptor and progesterone receptor-positive tumors were observed in 73 (68.9%) and 70 (66.0%) patients, respectively.
Table 1.
Patient, tumor, and treatment characteristics
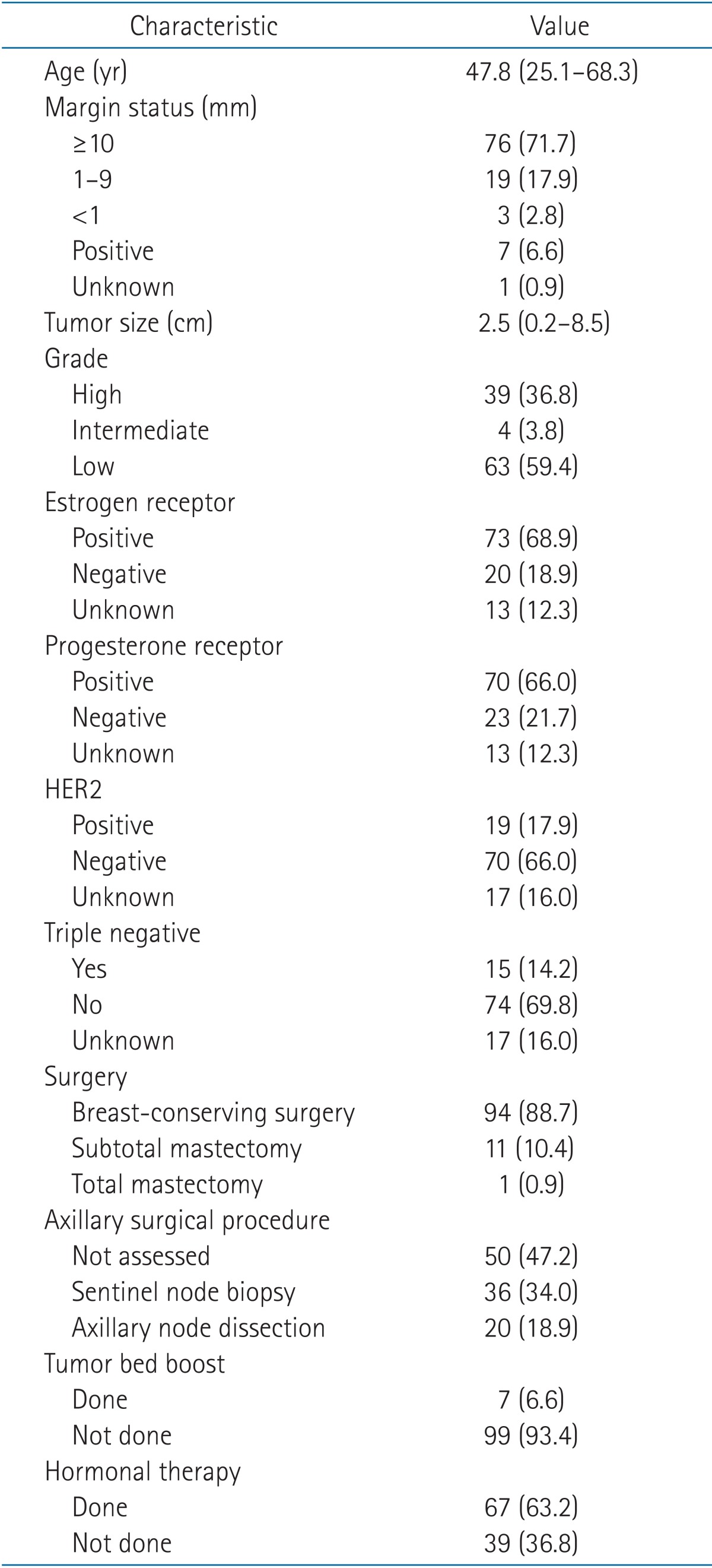
Values are presented as mean (range) or number (%).
HER2, human epidermal growth factor receptor 2.
Total or subtotal mastectomy was performed in 12 patients (11.3%) due to extensive DCIS. Fifty-six patients (52.9%) had sentinel node biopsy or axillary node dissection. The median interval between surgery and RT was 5.0 weeks (range, 2.4 to 11.3 weeks). Tumor bed boost was offered to 7 patients (6.6%), of whom 5 patients had positive resection margin and 2 patients had clear resection margin. Sixty-seven patients (63.2%) received hormonal therapy.
The median follow-up duration was 83.4 months (range, 33.4 to 191.5 months). At the time of analysis, 11 patients (10.4%) had relapse. Seven and three patients had recurred tumor in the ipsilateral and contralateral breast, respectively. Distant metastasis with peritoneal seeding was observed in one patient.
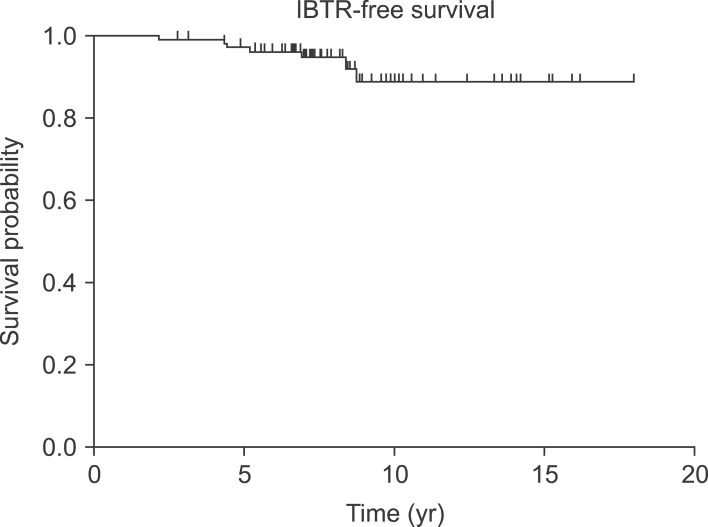
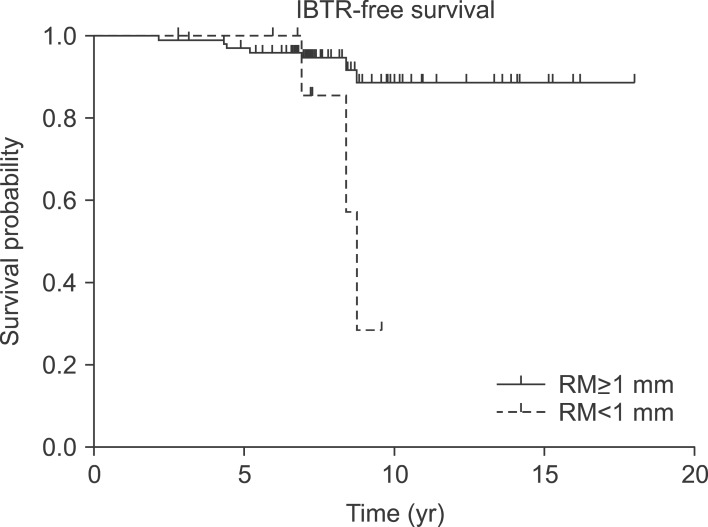
The 7-year OS was 97.2%, and the 7-year IBTR-free survival was 95.3% (Fig. 1). Resection margin (<1 or ≥1 mm) was the statistically significant factor in univariate (p < 0.001) and multivariate (p = 0.016) analyses for IBTR-free survival (Table 2). The 7-year IBTR-free survival for patients with resection margin <1 and ≥1 mm were 90.0% and 96.8%, respectively (Fig. 2).
Fig. 1.
Ipsilateral breast tumor recurrence (IBTR)-free survival.
Table 2.
Univariate and multivariate analyses for IBTR
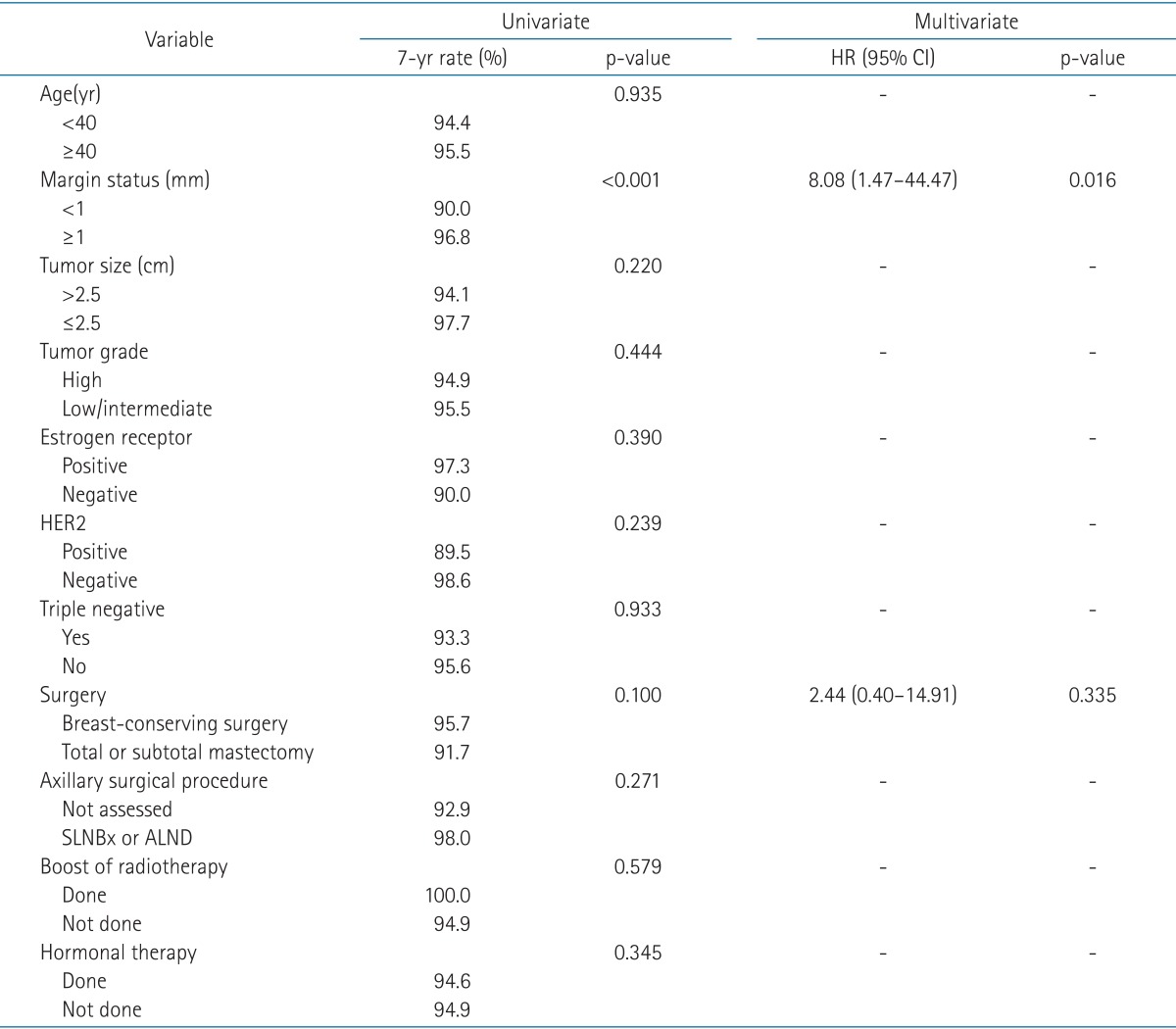
IBTR, ipsilateral breast tumor recurrence; HR, hazard ratio; CI, confidence interval; HER2, human epidermal growth factor receptor 2; SLNBx, sentinel lymph node biopsy; ALND, axillary lymph node dissection.
Fig. 2.
Ipsilateral breast tumor recurrence (IBTR)-free survival according to margin status (<1 or ≥1 mm).
Characteristics of the 7 patients with IBTR are listed in Table 3. All of the patients had no evidence of disease (NED) status at the time of analysis. Six patients initially underwent BCS, and one patient had total mastectomy. Among these patients, close (<1 mm) or positive resection margins were observed in 3 patients. The histological diagnosis of the recurrence was DCIS in 3 cases and infiltrating duct carcinoma in 4 cases. Four patients had recurred tumors in the same quadrant compared to each original tumor site. The median time to IBTR was 5.19 years (range, 2.14 to 8.75 years). Two patients had the second salvage surgery due to repeated events of IBTR.
Table 3.
Clinicopathologic characteristics and outcomes of patients with IBTR (n = 7)
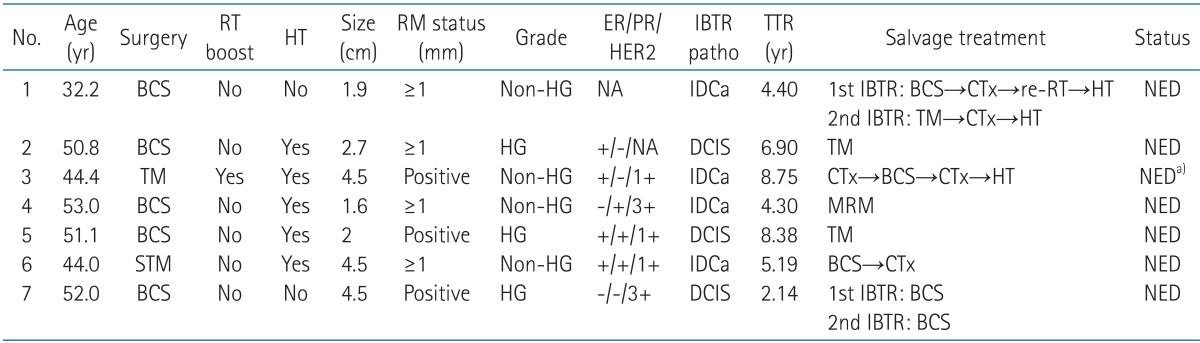
IBTR, ipsilateral breast tumor recurrence; RT, radiation therapy; HT, hormonal therapy; RM, resection margin; TTR, time to recurrence; BCS, breast-conserving surgery; HG, high grade; NA, not available; IDCa, infiltrating duct carcinoma; CTx, chemotherapy; NED, no evidence of disease; TM, total mastectomy; MRM, modified radical mastectomy; STM, subtotal mastectomy.
a)The patient had NED status for the breast cancer, but dedifferentiated liposarcoma in abdominopelvic cavity was diagnosed during the salvage treatment.
Discussion and Conclusion
We analyzed the outcomes and patterns of failure of DCIS patients who underwent surgery followed by RT with or without hormonal therapy in a single institution. The 7-year IBTR-free survival of the present study was 95.3%, which is comparable to the historical randomized controlled trials. In addition, all of the patients with IBTR had NED status with salvage treatment at the time of analysis.
Among the clincopathological risk factors, margin status (<1 or ≥1 mm) was a significant risk factor for IBTR in univariate and multivariate analyses. Saverio et al. [14] retrospectively analyzed 259 patients. Postoperative RT was delivered to 73 patients (28.2%), and the mean follow-up was 130 months. In the multivariate analysis for local recurrence, margin width (p = 0.002), pathologic classification (p = 0.024), and hormonal therapy (p = 0.009) were statistically significant. Dunne et al. [15] performed a meta-analysis of trials examining DCIS patients treated with BCS plus RT. The authors emphasized the importance of negative surgical margin, and a threshold of 2 mm was considered to be appropriate for the patients treated with postoperative RT. Also, in a recent meta-analysis of 21 studies (n = 7,564), negative margin was significantly associated with lower risk of IBTR compared to positive margin both in the group of postoperative RT (odds ratio [OR], 0.46; 95% confidence interval [CI], 0.35 to 0.59) and without RT (OR, 0.34; 95% CI, 0.24 to 0.47) [16].
In the present study, tumor bed boost was not significantly associated with IBTR. However, the number of patients (n = 7) who underwent tumor bed boost was not enough to evaluate the significance. In our data, we could not draw a conclusion on the impact of tumor bed boost.
Although the benefit of tumor bed boost in invasive carcinoma has been established from randomized controlled trials, the use of boost in DCIS is more controversial. Wong et al. [17] reported the improved local control in DCIS patients who underwent tumor bed boost following whole breast irradiation. The authors reviewed 220 cases of DCIS, and tumor bed boost was delivered to 36% of patients. Although positive and <0.1 cm margins were more frequently included in the boost group, the rate of local recurrence was lower than no boost group with the median follow-up of 46 months (p = 0.03). However, in a recent population-based analysis, Rakovitch et al. [18] could not find a lower risk of local recurrences associated with tumor bed boost. In 1,895 cases including 561 patients with boost, the 10-year rate of local recurrence was 13% in the boost group compared to 12% in the patients without boost (p = 0.3). Also in multivariate analysis, tumor bed boost was not a significant risk factor for local control (hazard ratio [HR], 0.82; 95% CI, 0.59 to 1.15).
In recent years, the possibility of an overestimation of the benefit of postoperative RT has been suggested. The ECOG 5194 is a prospective single-arm study of DCIS patients treated with local excision alone [12]. The study population was categorized into two groups: low- or intermediate-grade (LIG) group (tumor size >0.3 cm but <2.5 cm and surgical margins ≥3 mm) and high-grade (HG) group (tumor size from 0.3 to 1.0 cm with surgical margins ≥3 mm). The 7-year IBTR rates were 10.5% and 18% in LIG and HG group, respectively. The authors suggested that omission of RT can be considered in LIG group.
Motwani et al. [19] performed a retrospective analysis to validate the message of the ECOG 5194. With the same patient eligibility criteria as the ECOG 5194, all of the study population (n = 263) underwent postoperative RT after BCS. The 7-year IBTR rates were 4.4% and 2% in LIG and HG group, respectively. The authors demonstrated that longer follow-up is necessary to consider the omission of RT based on the patient criteria of ECOG 5194. More recently, results of RTOG 9804 trial were presented [20]. The good-risk DCIS group was defined as no initial symptoms, not high-grade tumors, tumor size <2.5 cm, and margin ≥3 mm. The 7-year rates of local recurrence were 0.9% and 6.4% for the RT and observation arms, respectively (p = 0.0005). This study concluded that the local failure rate was significantly decreased with postoperative RT in the good-risk subset of patients.
Our study included 22 patients (LIG group, 19 patients; HG group, 3 patients) who met the ECOG 5194 eligibility criteria, and the remaining patients had larger tumor sizes or closer surgical resection margins (data not shown). At the time of analysis, only one patient experienced IBTR in the LIG group, and there was no IBTR event in the HG group. Although the data were not adequate to evaluate the feasibility of omission of RT, routine omission of postoperative RT for a low-risk group of DCIS may not be justified based on the patient eligibility of the ECOG 5194. Further prospective studies are needed to obtain the lower risk group with tolerable IBTR rate who may not require postoperative RT.
Our analysis is a single institution retrospective study with median follow-up of 6.95 years. Longer follow-up is needed to estimate the long-term outcome. Also, selection bias is expected due to the heterogeneities of the study population in the clinical and pathological characteristics and treatment method.
In conclusion, we could confirm the favorable treatment outcome of postoperative RT for DCIS, which is comparable to previous studies. Resection margin was the important prognostic factor related to IBTR. Further studies are needed to determine which group of DCIS patients may not require postoperative RT.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Bane A. Ductal carcinoma in situ: what the pathologist needs to know and why. Int J Breast Cancer. 2013;2013:914053. doi: 10.1155/2013/914053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masson S, Bahl A. The management of ductal carcinoma in situ: current controversies and future directions. Clin Oncol (R Coll Radiol) 2013;25:275–282. doi: 10.1016/j.clon.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Siziopikou KP. Ductal carcinoma in situ of the breast: current concepts and future directions. Arch Pathol Lab Med. 2013;137:462–466. doi: 10.5858/arpa.2012-0078-RA. [DOI] [PubMed] [Google Scholar]
- 4.Korean Breast Cancer Society. Breast Cancer Facts & Figures. Seoul: Korean Breast Cancer Society; 2013. [Google Scholar]
- 5.Owen D, Tyldesley S, Alexander C, et al. Outcomes in patients treated with mastectomy for ductal carcinoma in situ. Int J Radiat Oncol Biol Phys. 2013;85:e129–e134. doi: 10.1016/j.ijrobp.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Hwang ES. The impact of surgery on ductal carcinoma in situ outcomes: the use of mastectomy. J Natl Cancer Inst Monogr. 2010;2010:197–199. doi: 10.1093/jncimonographs/lgq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103:478–488. doi: 10.1093/jnci/djr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuzick J, Sestak I, Pinder SE, et al. Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol. 2011;12:21–29. doi: 10.1016/S1470-2045(10)70266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmberg L, Garmo H, Granstrand B, et al. Absolute risk reductions for local recurrence after postoperative radiotherapy after sector resection for ductal carcinoma in situ of the breast. J Clin Oncol. 2008;26:1247–1252. doi: 10.1200/JCO.2007.12.7969. [DOI] [PubMed] [Google Scholar]
- 10.Donker M, Litiere S, Werutsky G, et al. Breast-conserving treatment with or without radiotherapy in ductal carcinoma in situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol. 2013;31:4054–4059. doi: 10.1200/JCO.2013.49.5077. [DOI] [PubMed] [Google Scholar]
- 11.Wong JS, Kaelin CM, Troyan SL, et al. Prospective study of wide excision alone for ductal carcinoma in situ of the breast. J Clin Oncol. 2006;24:1031–1036. doi: 10.1200/JCO.2005.02.9975. [DOI] [PubMed] [Google Scholar]
- 12.Hughes LL, Wang M, Page DL, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:5319–5324. doi: 10.1200/JCO.2009.21.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KJ, Huh SJ, Park W, et al. Treatment outcome and analysis of the prognostic factors of ductal carcinoma in situ treated with breast conserving surgery and radiotherapy. J Korean Soc Ther Radiol Oncol. 2004;22:11–16. [Google Scholar]
- 14.Di Saverio S, Catena F, Santini D, et al. 259 Patients with DCIS of the breast applying USC/Van Nuys prognostic index: a retrospective review with long term follow up. Breast Cancer Res Treat. 2008;109:405–416. doi: 10.1007/s10549-007-9668-7. [DOI] [PubMed] [Google Scholar]
- 15.Dunne C, Burke JP, Morrow M, Kell MR. Effect of margin status on local recurrence after breast conservation and radiation therapy for ductal carcinoma in situ. J Clin Oncol. 2009;27:1615–1620. doi: 10.1200/JCO.2008.17.5182. [DOI] [PubMed] [Google Scholar]
- 16.Wang SY, Chu H, Shamliyan T, et al. Network meta-analysis of margin threshold for women with ductal carcinoma in situ. J Natl Cancer Inst. 2012;104:507–516. doi: 10.1093/jnci/djs142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong P, Lambert C, Agnihotram RV, David M, Duclos M, Freeman CR. Ductal carcinoma in situ: the influence of the radiotherapy boost on local control. Int J Radiat Oncol Biol Phys. 2012;82:e153–e158. doi: 10.1016/j.ijrobp.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 18.Rakovitch E, Narod SA, Nofech-Moses S, et al. Impact of boost radiation in the treatment of ductal carcinoma in situ: a population-based analysis. Int J Radiat Oncol Biol Phys. 2013;86:491–497. doi: 10.1016/j.ijrobp.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 19.Motwani SB, Goyal S, Moran MS, Chhabra A, Haffty BG. Ductal carcinoma in situ treated with breast-conserving surgery and radiotherapy: a comparison with ECOG study 5194. Cancer. 2011;117:1156–1162. doi: 10.1002/cncr.25623. [DOI] [PubMed] [Google Scholar]
- 20.McCormick B, Winter K, Hudis C. RTOG 9804: a prospective randomized trial for "good risk" ductal carcinoma in situ (DCIS), comparing radiation (RT) to observation (OBS); 2012 American Society of Clinical Oncology Annual Meeting; 2012 Jun 1-5; Chicago, IL, USA. Alexandria, VA: ASCO; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]