Abstract
Recent studies reveal a potential contribution of intestinal microbes in the expression of certain human cardio-metabolic diseases. The mechanisms through which intestinal microbiota and/or their metabolic products alter systemic homoeostasis and cardio-metabolic disease risks are just beginning to be dissected. Intervention studies in humans aiming to either selectively alter the composition of the intestinal microbiota or to pharmacologically manipulate the microbiota to influence production of their metabolites are crucial next steps. The intestinal microbiome represents a new potential therapeutic target for the treatment of cardio-metabolic diseases.
Keywords: Gutmicrobiota, Vascular inflammation, Atherothrombosis, Insulin resistance, Obesity, TMAO, Therapy
Introduction
The human gut microbiome is a complex ecosystem, which harbours a staggering number of microbes—∼100 trillion—representing an estimated 5000 species.1 The collective genome of gut microbiota, termed the metagenome, contains close to 5 million genes. The recent development of high-throughput sequencing makes it feasible to examine the metagenome derived from stool samples, allowing for comprehensive analyses of intestinal microbiota composition. When metagenomic analysis is combined with clinical phenotypic data, it is known as a metagenome-wide association study.2 Despite the diversity between individuals, studies with serial stool collections show that the unique core gut microbiota composition of an individual remains remarkably stable over time,3,4 pointing towards intestinal bacteria as a potential risk factor for human cardio-metabolic disease. Indeed, in recent years the gut microbiome has increasingly been acknowledged as a novel contributor affecting host metabolism. Mounting evidence in mice and humans is accumulating showing that gut microbiota are linked with both cardiovascular health and the onset and development of metabolic disorders, such as type 2 diabetes mellitus (T2DM) and obesity.5 In this review, we will describe how specific changes in intestinal microbiota can affect host metabolism, and how these findings may give rise to novel therapeutic targets for obesity, diabetes, and cardiovascular disease (CVD).
Gut microbiota in obesity and type 2 diabetes mellitus
The prevalence of obesity and T2DM is increasing at an alarming pace worldwide, making obesity a primary public health concern. The pathophysiology of obesity, insulin resistance (IR), and T2DM are complex, and involve a combination of both genetic and environmental factors. Ingestion of food is a major environmental exposure, and it is processed through the filter of the intestinal microbial community. It is thus not surprising that there is growing appreciation that intestinal microbiota contribute to how a given individual experiences this environmental exposure, and accordingly, cardio-metabolic phenotypes.6 The human gut microbiota benefits the host in numerous ways, playing a pivotal role in both innate immune and metabolic functions. Gut microbiota can also provide functions not intrinsically available to humans, such as the synthesis of vitamin K, or biotransformation of other nutrients. Intestinal microbiota enables their host to extract calories from otherwise indigestible components of our diets, such as plant polysaccharides. It is suggested that gut microbiota also plays a role in harvesting energy from food and controlling energy homoeostasis, implicating gut microbiota as a potential contributor to the development of obesity and T2DM.7
Recent studies in both mice and humans show that an obese phenotype is associated with changes in the intestinal microbial composition compared with lean counterparts. Turnbaugh and colleagues8,9 first demonstrated that the obesity phenotype was a transmissible trait by showing caecal microbiome transplantation into germ-free mice (who have sterile intestines) resulted in efficient transmission of the obesity phenotype into recipients. The mechanism(s) involved appear to involve more efficient energy harvest from food with the obesity-related caecal microbiome. Bäckhed et al.10 have extended these observations and reported that germ-free mice are relatively protected from obesity following the institution of a Western high-fat sugar-rich diet. Conversely, colonization of germ-free mice with caecum-derived microbiota from normal mice (called ‘conventional’ because they are housed in conventional cages) resulted in a significant increase in the total body fat content without any change in dietary caloric intake.8 A potential mechanism for the more efficient energy harvest from food is suggested to occur via intestinal production of short-chain fatty acids (SCFA). Intestinal microbes ferment non-digestible carbohydrates in order to yield energy, leading to the production of SCFA in the form of acetate (60%), propionate (25%), and butyrate (15%)9; however, the concentration of faecal SCFA is highly dependable on the amount of daily dietary fibre intake.5 Short-chain fatty acids are readily absorbed in the plasma of the host via the intestinal epithelium, and thus can serve as an energy source, predominantly via metabolism in the liver.9,10 The role for intestinal bacteria in the production of SCFA is clearly demonstrated by the observation that germ-free rats and mice are characterized by reduced levels of intestinal SCFAs with a concomitant increase in faecal excretion of non-digestible carbohydrates.6 Moreover, both mouse models (ob/ob) and human disease states (e.g. metabolic syndrome) characterized by IR also tend to have decreased intestinal SCFA levels with a concomitant reduced excreted energy content in their faeces.9,11,12 The complexity of the relationship between gut microbiota composition and human metabolism is attested to by the observation that not only composition, but also relative proportions, as well as functional capacity (e.g. SCFA production) of bacteria, may determine the propensity towards obesity and IR.
Research into the role of gut microbiota in obesity and IR in humans has focused on the relative proportions of specific bacterial taxa, predominantly Bacteroidetes and Firmicutes.11–16 In this respect, two recent landmark papers elegantly demonstrated the diagnostic and clinical value of faecal microbiota composition in the development of obesity-associated IR and T2DM.17,18 Both studies also showed that T2DM subjects were characterized by a reduction in the number of Clostridiales bacteria, which produce the SCFA butyrate (e.g. Roseburia species and Faecalibacterium prausnitzii). Hence, these studies lend support to a role of butyrate-producing bacteria as regulators of human glucose and lipid metabolism, possibly via alterations in intestinal permeability driving chronic inflammation.5 Whereas Qin et al.17 reported an enrichment of Proteobacteria predicting T2DM; Karlsson et al.18 identified an enrichment of Lactobacillus gasseri and Streptococcus mutans (abundant in the mouth and upper intestinal tract) as a predictor of T2DM. Potential causality of these bacterial strains and T2DM is supported by the compositional changes of faecal microbiota, particularly of the SCFA butyrate-producing strains, following both faecal transplantation of lean donors as well as a gastric bypass procedure (Roux-en-Y gastric bypass),12,19,20 with both of these interventions being associated with a marked improvement of IR.
Gut microbiota, inflammation, and lipid metabolism
The majority of obese subjects will eventually develop chronic (visceral) adipose tissue inflammation leading to production of pro-inflammatory cytokines21 and subsequent IR.22,23 Recent data have shown that variations in intestinal microbiota were associated with pro-inflammatory changes in adipose gene expression.24 In fact, several other lines of evidence point towards a direct relation between the intestine and visceral adipose tissue inflammation, as the macrophage infiltration (crown-like cells) in adipose tissue was directly correlated with a pro-inflammatory gene expression profile.25,26 As macrophages are part of our innate immune system, these findings imply that translocation of intestinal bacteria could play a role in the development of chronic inflammatory state. The innate immune system is capable of sensing various types of bacterial components via pattern recognition receptors, such as toll-like receptors (TLRs). It is known that alterations in the gut microbiota composition drive activation of TLRs by bacterial endotoxins [e.g. lipopolysaccharide (LPS)] and subsequent obesity.27 Thus, chronic low-dose LPS exposure in mice resulted in hepatic IR, hepatic steatosis, adipose tissue macrophages infiltration, dyslipidaemia, fasting hyperglycaemia, hyperinsulinaemia, and eventually obesity. Similar changes were seen in mice fed a high-fat diet,28,29 whereas mice deficient in TLR4 (the receptor that is directly involved in LPS binding) were, however, completely resistant to the development of these high-fat-diet-induced changes.27 It should be noted, however, that besides LPS, the intestinal microbiota is a source of many other pro-inflammatory bacterial, capsule-derived compounds (e.g. peptidoglycans, lipoproteins, and flagellins) that are able to activate innate inflammatory pathways.30,31 Recent murine data have shown that SCFAs are indeed involved in intestinal cell homoeostasis thus preventing increased permeability.32,33 Combined with the afore-mentioned bacteria-derived SCFAs affecting both intestinal permeability as well glucose and lipid metabolism,5 these observations provide the basis for a bi-directional cross-talk between the intestinal microbiota SCFA metabolism and bacterial translocation in the development of chronic adipose tissue inflammation and IR.
Gut microbiota and atherothrombosis
Cardiovascular disease represents the leading cause of mortality and morbidity in Western societies. Moreover, obesity, non-alcoholic fatty liver disease, and metabolic dyslipidaemia are associated with a further increase in CVD, independent from classic risk factors.34 Chronic low-grade inflammation, induced by intestinal microbiota-derived endotoxaemia, has been suggested as a potential contributing factor for both obesity and atherosclerosis.35 Obesity and chronic inflammation are associated with hypercoagulability, presumably as a result of both increased production of pro-coagulant vitamin K-dependent clotting factors (II, VII, IX, and X), as well as adipose tissue inflammation-associated reduction in fibrinolytic capacity.36 Considerable evidence shows that endotoxaemia, which is associated with enhanced intestinal bacterial translocation, elicits activation of the coagulation and inflammatory cascades.37 Solid evidence for the effect of the intestinal microbiota in hypercoagulability is however limited. A study in germ-free mice showed that gut microbiota can regulate tissue factor level expression in the intestinal microvasculature,38 but data are lacking in humans. Subsequent papers suggested that intestinal translocation can be facilitated via co-transport on dietary fat-derived chylomicrons promoting an inflammatory response,39,40 whereas parenteral infusion of lipids did not convey this effect.37 Increased levels of plasma endotoxin have also been linked to the development of CVD,41 and repetitive endotoxin injections in both mice and rabbits are reported to accelerate cholesterol-induced atherosclerosis.35,42,43 More specific, translocation of intact bacteria and subsequent captivation by vascular macrophages was also suggested in humans reporting bacterial DNA from the Porphyromonas gingivalis in 79% of the analysed carotid atherosclerotic plaques.44,45 Currently, only one prospective randomized controlled trial with antibiotic treatment in humans was executed and failed to reduce cardiovascular event rates.46 However, whether or not the chronic antibiotics exposure resulted in expansion of alternative resistant bacterial strains is unknown and moreover bacterial resistance to specific antibiotics precludes potential use in the clinic of long-term oral antibiotic treatment.
Recent studies identify a new pathway through which gut microbiota participate in CVD pathogenesis. In the setting of specific dietary nutrients characterized by a trimethylamine group [e.g. choline, phosphatidylcholine (PC), and carnitine], gut microbiota are shown to participate in the formation of a pro-atherogenic compound called trimethylamine-N-oxide (TMAO).47,48 Identified initially though metabolomics studies comparing analytes in plasma from subjects who experience a heart attack, stroke, or death in the 3 years following blood collection vs. age- and gender-matched subjects who do not experience a cardiovascular event, subsequent animal model and human clinical studies have shown that TMAO is directly related to atherosclerosis. Trimethylamine-N-oxide was shown to directly influence the propensity of macrophages to accumulate cholesterol and form foam cells in atherosclerotic lesions, as well as to alter cholesterol and sterol metabolism within multiple compartments including the liver and intestines49 Further, systemic levels of TMAO in several clinical cohorts have now been shown to independently associate with incident risks of heart attack, stroke, and death in both primary and secondary prevention subjects.47,50–51 Trimethylamine-N-oxide is produced in a two-step process, starting with degradation of dietary trimethylamines like free choline, PC, or carnitine by specific intestinal bacterial strains into the precursor trimethylamine (TMA). Trimethylamine is then efficiently absorbed into the circulation and converted by one or more members of the hepatic flavin monooxygenase (FMO) family, in particular FMO3, into TMAO.50,51 Flavin monooxygenase 3 is a known enzymatic source for TMAO in humans, based on the recent recognition of the aetiology of an uncommon genetic disorder called trimethylaminuria (also known as fish malodour syndrome). Patients with this metabolic condition are characterized by a genetically impaired capacity to convert TMA into TMAO. Studies using germ-free mice or mice treated with broad-spectrum antibiotics, suppressing the intestinal flora, demonstrate an essential role for gut microbiota in TMA and TMAO formation.47,50 Moreover, conventionalization of germ-free mice results in an increase and recovery of the plasma levels of TMAO, indicating towards an obligate role for intestinal microbiota in the generation of TMA from dietary choline including eggs, milk, liver, and red meat.49 As noted, another source of TMAO is dietary l-carnitine (a TMA structure similar to choline). Carnitine is an abundant nutrient in red meat. Recent studies show that gut microbiota also play a role in TMAO production from dietary l-carnitine in both mice and humans. Moreover, a strong correlation is observed between increased plasma concentrations of l-carnitine and TMAO levels in relation to increased risk of incident major adverse cardiac events in subjects, even following multivariate analyses.49,50 Of note, a recent meta-analysis suggested that the use of l-carnitine in patients immediately following an acute myocardial infarction was associated with a significant reduction in lethal ventricular arrhythmias and development of angina.52 However, review of the meta analyses demonstrates concerns about its validity, since 11 of the 13 studies included in the meta-analyses failed to show clinical benefit with short-term carnitine supplementation, including the two largest trials, one of which had over 1000 subjects. Moreover, the 2 of 13 studies that did show a benefit were each small (<100 subjects), making the positive results hypothesized in the meta-analyses difficult to reconcile with negative placebo-controlled trial results of the major studies included in the analyses. Regardless, it should be noted that the relationship proposed between dietary carnitine and atherosclerotic CVD pathogenesis refers to a process that takes decades to develop (atherosclerosis), and which appears at least in part to exert its pro-atherogenic effect via alterations in cholesterol and sterol metabolism. In contrast, the carnitine supplementation studies examined thus far have only focused on short-term effects in the post-myocardial infarction setting, where alternative phenotypes such as arrhythmias have been the major ones examined.
Carnitine is almost exclusively found in red meat. Therefore, examination of epidemiology studies examining meat ingestion and cardiovascular risks is of interest. Numerous large-scale studies have been performed. Perhaps one of the largest and most carefully performed is the results recently reported from composite of two prospective studies, from the Health Professionals Follow-up Study (n = 37 698; followed 1986–2008) and women from the Nurses’ Health Study (n = 83 644; followed 1980–2008) who were free of CVD and cancer at baseline, and for whom dietary intake was assessed by validated food frequency questionnaires every 4 years53 For each 1-serving-per-day (3 oz.) of meat over the duration of follow-up, which varied between 20 and 28 years on average for the two studies, a 13% increase in all-cause mortality for unprocessed red meat, and 20% increase in mortality for processed red meat was observed. In contrast, however, in a very recent similar pooled analyses of eight Asian prospective cohort studies, higher red meat consumption was reported to be inversely associated with cardiovascular mortality.54 It also should be noted that another source of TMAO is seafood, including some but not all fish.55 Multiple epidemiological studies have shown beneficial effects of regular seafood consumption on the incidence of CVD,56 making recommendations about intake of specific dietary nutrients or meats/proteins and cardiovascular risks premature at present. Interestingly, in animal model studies, dietary TMAO directly augmented atherosclerosis, as did dietary choline or carnitine in the presence of intact gut microbes (and hence formation of TMAO). Moreover, both TMAO and choline or carnitine with intact gut microbiota and TMAO formation significantly inhibited the reverse cholesterol transport pathway, suggesting a plausible mechanistic explanation for the tight associations noted between TMAO, choline, and carnitine and incident cardiovascular risks.51 Also, (small) intestinal transit time of choline and carnitine comprising food particles might be impaired in obese subjects57 thus resulting in different TMA levels in the colon. In conjunction with the effect (suppression) on bile acid synthesis and composition by the TMAO pathway, this demonstrates the multiple distinct mechanistic links between TMAO producing diet—microbe pathways and atherothrombotic risk. Although a causal role of l-carnitine in CVD disease in humans is provocative, and requires further examination, discovery of this new pathway suggests that targeting the gut microbiota, either through efforts to change their composition, or through targeted small molecule or molecular approaches, may serve as a novel therapeutic for the prevention or treatment of CVD.
Intestinal microbiota as future diagnostic and therapeutic targets
The faecal intestinal microbiota is increasingly regarded an important participant in the development of obesity and its comorbidities. As currently available evidence is mainly based on in vitro and relatively small animal and human cohort studies, further work is required in order to dissect how altered intestinal microbiota can be used in a diagnostic or therapeutic setting. For example, long-term, prospective, and multi-ethnic cohort studies are needed to determine whether there are in fact substantial differences in predictors of faecal sample-derived microbiota between ethnic groups taking diet and gender into account, as both factors can influence the composition of the gut microbiota.6 Moreover, as intestinal uptake of dietary lipids and carbohydrates driving human obesity are mainly taking place in the small intestine5 rather than the distal colon, it is important to define the correlation between small intestinal microbiota composition in relation to that of the faecal sample. Moreover, environmental factors (e.g. composition of diet) introduce profound changes in intestinal microbiota composition,58 and the logistics of faecal sample (non-frozen) transport, handling and DNA isolation also influence results.59 A role for quantifying proportions of bacterial strains in faeces for use as a diagnostic tool and in cardiovascular risk prediction is thus neither recommended nor likely to provide useful clinical information in the near term future. In line, using faecal transplantation studies as a working tool might enable us to identify causally involved intestinal bacterial strains in human cardio-metabolism which may give rise to the development of novel, microorganism-based intervention strategies. Although promising, the links of specific gut microbial taxa with CVD phenotypes are associative, which makes it too early to induce real changes in clinical practice of cardiovascular risk assessment.18
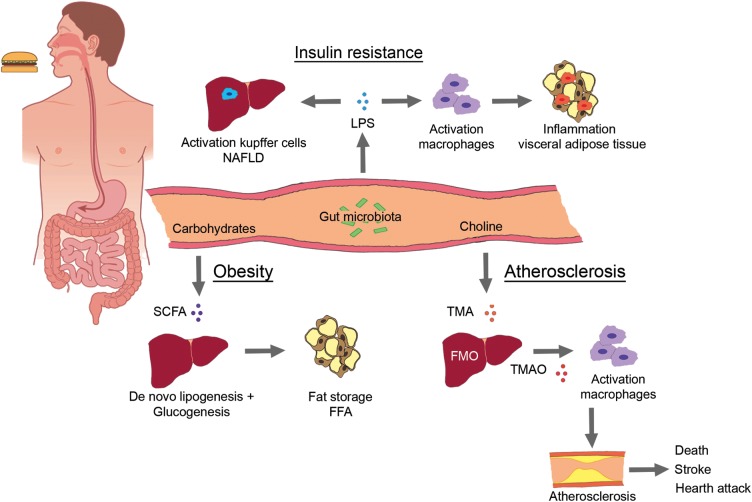
In contrast to quantifying bacterial species within faeces, perhaps a more promising and clinically viable diagnostic is the quantification of blood levels of a microbial-dependent product that is biologically active and at least in animal models promotes atherosclerosis (i.e. TMAO). Indeed, while results are relatively new, those reported suggest great promise for TMAO as a strong cardiovascular diagnostic or prognostic marker. In multiple large clinical studies, plasma levels of TMAO were observed to predict prospective CVD risks, including incident heart attack, stroke and death over the ensuing 3-year follow-up period. The prognostic value of TMAO remained robust following multivariable adjustments for traditional risk factors and other cardiovascular diagnostic markers.47,49,50 Indeed, in one study (n = 4007 subjects), addition of TMAO to traditional risk factors was shown to substantially reclassify incident risks for major adverse cardiovascular events, and significantly augment prognostic value.49 Clinical studies thus far with TMAO have all been in relatively higher risk populations, and not within community-based cohorts with longer-term follow-up. Further studies are needed to determine the generalizability of the results with TMAO as a prognostic marker in alternative clinical cohorts, as well as whether plasma TMAO levels can either serve as a means of monitoring dietary efforts, or are modifiable with alternative interventions (Figure 1).
Figure 1.
Three major pathways via which intestinal microbiota can alter human cardio-metabolism. (i) Chronic bacterial translocation (due to increased intestinal permeability) can drive systemic inflammation leading to macrophage influx into (visceral) adipose tissue, activation of hepatic Kupffer cells resulting in non-alcoholic fatty liver disease and insulin resistance. (ii) Short-chain fatty acids normalize intestinal permeability and alter de novo lipogenesis and gluconeogenesis via reduction of free fatty acids production by visceral adipose tissue. (iii) trimethylamine-N-oxide can accelerate atherosclerosis and vascular inflammation via influx of macrophages and cholesterol accumulation via both up-regulation of macrophage scavenger receptors and reduction in reverse cholesterol transport.
Conclusion
Recent studies implicate involvement of gut microbiota in development of complex metabolic phenotypes including obesity, IR, and CVD. In this active research area of cardio-metabolism, there are numerous research programmes evaluating the mechanisms by which intestinal microbiota alter human glucose, lipid metabolism, and alternative CVD relevant phenotypes. In animal model studies, transmissibility of traits and causality of gut microbiota as a contributor has been established in some studies. In human studies thus far, however, published studies are associative. There is a need to apply Koch's postulates (originally used for infectious disease) to differentiate association from causality in cardio-metabolic disease states. Further research is needed in order to allow us to use the gut microbiota composition and modulation as novel diagnostic or therapeutic strategies. Most importantly, experimental findings on correlations between microbial communities and specific cardio-metabolic phenotypes should be corroborated by more detailed mechanistic investigations, and ideally, therapeutic intervention studies in humans. We have yet to see that ‘the time has come’ for gut microbiota compositional manipulation or targeting by drugs for therapeutic gain in cardio-metabolic disease. However, the highly promising studies that have arisen over the past few years argue that not too long into the future, we may yet ‘drug the microbiome’ as a target for preventing or treating human diseases and promoting cardiovascular health.2
Funding
S.V. is supported by the 2012 EU RESOLVE consortium (grant number FP7-EU 305707); E.S.G.S.: grant from CVON (GENIUS) (number CVON2011-19). S.L.H. is supported by National Institutes of Health (NIH) and Office of Dietary Supplement grants R01 HL103866, and NIH grant P20 HL113452. He is also partially supported by a gift from the Leonard Krieger Fund.
Conflict of interest: S.L.H. reports being named as co-inventor on pending patents held by the Cleveland Clinic relating to cardiovascular diagnostics. S.L.H. also reports he has the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics from Abbott Laboratories, Cleveland Heart Lab, Inc., Frantz Biomarkers, Liposcience, Inc., and Siemens. S.L.H. reports he has been paid as a consultant by the following companies: Cleveland Heart Lab, Inc., Esperion, Liposciences, Inc., Merck & Co., Inc., Pfizer, Inc., and Proctor & Gamble. S.L.H. reports he has received research funds from Abbott, Cleveland Heart Lab, Esperion and Liposciences, Inc., and Proctor & Gamble.
References
- 1.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Vos WM, Nieuwdorp M. Genomics: a gut prediction. Nature. 2013;498:48–49. doi: 10.1038/nature12251. [DOI] [PubMed] [Google Scholar]
- 3.Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, Cresci A, Silvi S, Orpianesi C, Verdenelli MC, Clavel T, Koebnick C, Zunft HJ, Doré J, Blaut M. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006;72:1027–1033. doi: 10.1128/AEM.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai F, Coyle WJ. The microbiome and obesity: is obesity linked to our gut flora? Curr Gastroenterol Rep. 2009;11:307–313. doi: 10.1007/s11894-009-0045-z. [DOI] [PubMed] [Google Scholar]
- 5.Kootte RS, Vrieze A, Holleman F, Dallinga-Thie GM, Zoetendal EG, De Vos WM, Groen AK, Hoekstra JB, Stroes ES, Nieuwdorp M. The therapeutic potential of manipulating gut microbiota in obesity and type 2 diabetes mellitus. Diab Obes Metab. 2012;14:112–120. doi: 10.1111/j.1463-1326.2011.01483.x. [DOI] [PubMed] [Google Scholar]
- 6.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 7.Tilg H. Obesity, metabolic syndrome, and microbiota: multiple interactions. J Clin Gastroenterol. 2010;44(Suppl. 1):S16–S18. doi: 10.1097/MCG.0b013e3181dd8b64. [DOI] [PubMed] [Google Scholar]
- 8.Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 10.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916. doi: 10.1053/j.gastro.2012.06.031. e7. [DOI] [PubMed] [Google Scholar]
- 13.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 14.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, Flint HJ. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes. 2008;32:1720–1724. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- 16.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring, Md.) 2010;18:190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 17.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 18.Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE, Krajmalnik-Brown R. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Doré J, Henegar C, Rizkalla S, Clément K. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gesta S, Blüher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci USA. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith JD, Borel AL, Nazare JA, Haffner SM, Balkau B, Ross R, Massien C, Alméras N, Després JP. Visceral adipose tissue indicates the severity of cardiometabolic risk in patients with and without type 2 diabetes: results from the INSPIRE ME IAA study. J Clin Endocrinol Metab. 2012;97:1517–1525. doi: 10.1210/jc.2011-2550. [DOI] [PubMed] [Google Scholar]
- 23.McArdle MA, Finucane OM, Connaughton RM, McMorrow AM, Roche HM. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endocrinol. 2013;10:52. doi: 10.3389/fendo.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong LC, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot JL, Zucker JD, Doré J, Clément K. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98:16–24. doi: 10.3945/ajcn.113.058743. [DOI] [PubMed] [Google Scholar]
- 25.Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O'Brien PE, Harrison LC. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanz Y, Santacruz A, Gauffin P. Gut microbiota in obesity and metabolic disorders. Proc Nutr Soc. 2010;69:434–441. doi: 10.1017/S0029665110001813. [DOI] [PubMed] [Google Scholar]
- 28.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 29.Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86:1286–1292. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- 30.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vijay-Kumar M, Gewirtz AT. Role of flagellin in Crohn's disease: emblematic of the progress and enigmas in understanding inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:789–795. doi: 10.1002/ibd.20734. [DOI] [PubMed] [Google Scholar]
- 32.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396–406. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 33.Lewis K, Lutgendorff F, Phan V, Söderholm JD, Sherman PM, McKay DM. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm Bowel Dis. 2010;16:1138–1148. doi: 10.1002/ibd.21177. [DOI] [PubMed] [Google Scholar]
- 34.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 35.Westerterp M, Berbée JF, Pires NM, van Mierlo GJ, Kleemann R, Romijn JA, Havekes LM, Rensen PC. Apolipoprotein C-I is crucially involved in lipopolysaccharide-induced atherosclerosis development in apolipoprotein E-knockout mice. Circulation. 2007;116:2173–2181. doi: 10.1161/CIRCULATIONAHA.107.693382. [DOI] [PubMed] [Google Scholar]
- 36.Nieuwdorp M, Stroes ESG, Meijers JCM, Büller H. Hypercoagulability in the metabolic syndrome. Curr Opin Pharmacol. 2005;5:155–159. doi: 10.1016/j.coph.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Van der Poll T, Levi M, Braxton CC, Coyle SM, Roth M, Ten Cate JW, Lowry SF. Parenteral nutrition facilitates activation of coagulation but not of fibrinolysis during human endotoxemia. J Infect Dis. 1998;177:793–795. doi: 10.1086/517811. [DOI] [PubMed] [Google Scholar]
- 38.Reinhardt C, Bergentall M, Greiner TU, Schaffner F, Ostergren-Lundén G, Petersen LC, Ruf W, Bäckhed F. Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature. 2012;483:627–631. doi: 10.1038/nature10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010;31:817–844. doi: 10.1210/er.2009-0030. [DOI] [PubMed] [Google Scholar]
- 40.Ghoshal S, Witta J, Zhong J, De Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50:90–97. doi: 10.1194/jlr.M800156-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, Willeit J. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the bruneck study. J Am Coll Cardiol. 1999;34:1975–1981. doi: 10.1016/s0735-1097(99)00448-9. [DOI] [PubMed] [Google Scholar]
- 42.Caesar R, Fåk F, Bäckhed F. Effects of gut microbiota on obesity and atherosclerosis via modulation of inflammation and lipid metabolism. J Intern Med. 2010;268:320–328. doi: 10.1111/j.1365-2796.2010.02270.x. [DOI] [PubMed] [Google Scholar]
- 43.Lehr HA, Sagban TA, Ihling C, Zahringer U, Hungerer KD, Blumrich M, Reifenberg K, Bhakdi S. Immunopathogenesis of atherosclerosis: endotoxin accelerates atherosclerosis in rabbits on hypercholesterolemic diet. Circulation. 2001;104:914–920. doi: 10.1161/hc3401.093153. [DOI] [PubMed] [Google Scholar]
- 44.Koren O, Spor A, Felin J, Fåk F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, Bäckhed F. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA. 2011;108(Suppl):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Figuero E, Sánchez-Beltrán M, Cuesta-Frechoso S, Tejerina JM, Del Castro JA, Gutiérrez JM, Herrera D, Sanz M. Detection of periodontal bacteria in atheromatous plaque by nested polymerase chain reaction. J Periodontol. 2011;82:1469–1477. doi: 10.1902/jop.2011.100719. [DOI] [PubMed] [Google Scholar]
- 46.Grayston JT, Kronmal RA, Jackson LA, Parisi AF, Muhlestein JB, Cohen JD, Rogers WJ, Crouse JR, Borrowdale SL, Schron E, Knirsch C ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637–1645. doi: 10.1056/NEJMoa043526. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bennett BJ, De Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metabolism. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiac risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang D, Xia M, Yan X, Li D, Wang L, Xu Y, Jin T, Ling W. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ Res. 2012;111:967–981. doi: 10.1161/CIRCRESAHA.112.266502. [DOI] [PubMed] [Google Scholar]
- 52.DiNicolantonio JJ, Lavie CJ, Fares H, Menezes AR, O'Keefe JH. L-carnitine in the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Mayo Clinic Proc. 2013;88:544–551. doi: 10.1016/j.mayocp.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012;172:555–563. doi: 10.1001/archinternmed.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee JE, McLerran DF, Rolland B, Chen Y, Grant EJ, Vedanthan R, Inoue M, Tsugane S, Gao YT, Tsuji I, Kakizaki M, Ahsan H, Ahn YO, Pan WH, Ozasa K, Yoo KY, Sasazuki S, Yang G, Watanabe T, Sugawara Y, Parvez F, Kim DH, Chuang SY, Ohishi W, Park SK, Feng Z, Thornquist M, Boffetta P, Zheng W, Kang D, Potter J, Sinha R. Meat intake and cause-specific mortality: a pooled analysis of Asian prospective cohort studies. Am J Clin Nutr. 2013;98:1032–1041. doi: 10.3945/ajcn.113.062638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin R, Herbard C. Chemistry and Biochemistry of Marine Food Products. Westport: A V I Publishing Company; pp. 149–175. [Google Scholar]
- 56.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish Oil, omega-3 fatty acids, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23:20–30. doi: 10.1161/01.atv.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 57.Kiely JM, Noh JH, Graewin SJ, Pitt HA, Swartz-Basile DA. Altered intestinal motility in leptin-deficient obese mice. J Surg Res. 2005;124:98–103. doi: 10.1016/j.jss.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 58.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]