Abstract
Aims
Recent metabolomics and animal model studies show trimethylamine-N-oxide (TMAO), an intestinal microbiota-dependent metabolite formed from dietary trimethylamine-containing nutrients such as phosphatidylcholine (PC), choline, and carnitine, is linked to coronary artery disease pathogenesis. Our aim was to examine the prognostic value of systemic choline and betaine levels in stable cardiac patients.
Methods and results
We examined the relationship between fasting plasma choline and betaine levels and risk of major adverse cardiac events (MACE = death, myocardial infraction, stroke) in relation to TMAO over 3 years of follow-up in 3903 sequential stable subjects undergoing elective diagnostic coronary angiography. In our study cohort, median (IQR) TMAO, choline, and betaine levels were 3.7 (2.4–6.2)μM, 9.8 (7.9–12.2)μM, and 41.1 (32.5–52.1)μM, respectively. Modest but statistically significant correlations were noted between TMAO and choline (r = 0.33, P < 0.001) and less between TMAO and betaine (r = 0.09, P < 0.001). Higher plasma choline and betaine levels were associated with a 1.9-fold and 1.4-fold increased risk of MACE, respectively (Quartiles 4 vs. 1; P < 0.01, each). Following adjustments for traditional cardiovascular risk factors and high-sensitivity C-reactive protein, elevated choline [1.34 (1.03–1.74), P < 0.05], and betaine levels [1.33 (1.03–1.73), P < 0.05] each predicted increased MACE risk. Neither choline nor betaine predicted MACE risk when TMAO was added to the adjustment model, and choline and betaine predicted future risk for MACE only when TMAO was elevated.
Conclusion
Elevated plasma levels of choline and betaine are each associated with incident MACE risk independent of traditional risk factors. However, high choline and betaine levels are only associated with higher risk of future MACE with concomitant increase in TMAO.
Keywords: Gut microbiota, Cardiovascular disease, Nutrition, Choline, Myocardial infarction
Introduction
Choline is a semi-essential nutrient for humans, meaning that while it can be endogenously synthesized, additional dietary intake of choline is also required or else a deficiency state will eventually occur. A common nutrient in many animal and some plant products, choline is found in dietary sources such as egg yolk, meats, liver, fish, dairy products, nuts, and soybean.1–3 Choline is a component of phosphatidylcholine (PC, also known as lecithin), the major phospholipid in cell membranes. It also is a precursor of the neurotransmitter acetylcholine, and following oxidation to betaine, choline functions as a methyl group donor in pathways that produce S-adenosylmethionine.4 As a methyl donor, choline and betaine can influence DNA and histone methylation.5 Choline deficiency has been linked to neurological impairment,6 and targeting enzymes related to choline metabolism has been postulated to provide promising therapeutic opportunities for tumour growth arrest.7 The connection between dietary choline and betaine and heart disease in subjects is unclear. While high intake of dietary choline and betaine was reportedly associated with diminished inflammation in one study,8 epidemiological studies have reported a limited association between high choline and betaine intake and cardiovascular morbidity that was attenuated following adjustments for other cardiovascular risk markers.9,10
There is a growing appreciation that intestinal microbiota can participate in metabolic phenotypes such as obesity and insulin resistance.11–13 Recently, through a series of animal and human studies, our group has identified a direct mechanistic link between intestinal microbiota-dependent metabolism of trimethylamine (TMA)-containing dietary nutrients such as choline, PC, and carnitine and production of trimethylamine-N-oxide (TMAO), a metabolite shown to both alter cholesterol and sterol metabolism in multiple compartments and to directly enhanced atherosclerosis in animal models.14–16 Gut microbiota have been shown to play an obligate role in TMAO formation from ingested choline, PC, and carnitine in both animal models and humans.14,16 Moreover, elevated levels of TMAO have been shown to predict increased prevalent cardiovascular disease (CVD) risk, and increased incident risk of major adverse cardiovascular events (MACE = myocardial infarction, stroke, and death) in large-scale human clinical studies.14,16,17 Betaine, an oxidation product of choline, is generally regarded as safe for dietary ingestion. Surprisingly, although a TMA-containing species, whether orally ingested betaine produces TMAO or not is unknown. Moreover, while elevated whole-blood choline levels have been reported to predict increased cardiovascular risk,18–21 the relationship between TMAO and the clinical prognostic value of choline or betaine is unknown. Herein, we show that oral ingestion of d9-betaine results in generation of circulating levels of d9-TMAO in a gut microbiota-dependent fashion. We further examine the relationship between circulating levels of choline and betaine and development of cardiovascular morbidity and mortality, and the impact of TMAO on this relationship.
Methods
Study population
We prospectively recruited sequential stable subjects (n = 3903) undergoing elective, non-urgent coronary angiography at the Cleveland Clinic without any evidence of acute coronary syndrome (cardiac troponin I ≤0.03 ng/mL). All subjects were followed over a period of 3 years from time of enrollment including adjudication of MACE(which includes the future development of all-cause mortality, non-fatal myocardial infarction, or non-fatal stroke). Fasting blood glucose, high-sensitivity (hs) C-reactive protein, and lipid profiles were measured on the Abbott Architect ci8200 platform (Abbott Diagnostics, Abbott Park, IL, USA).
Mass spectrometry analyses of trimethylamine-N-oxide, choline, and betaine
Plasma was immediately prepared from venous blood drawn into ethylenediaminetetraacetic acid tubes and then stored at −80°C until analysis. Trimethylamine-N-oxide, choline, and betaine levels in plasma were determined using stable isotope dilution high-performance liquid chromatography with on line electrospray ionization tandem mass spectrometry (LC/MS/MS) on either an AB SCIEX 5000 or AB SCIEX 5500 triple quadrupole mass spectrometer using d9-(trimethyl)-labelled internal standards as described previously.14
Metabolic challenges in mice
Whole blood (50 μL) was collected via the saphenous vein from C57BL/6J mice prior to gavage for the measurement of baseline analyte levels. Mice were administered an oral dose of stable isotope-labelled betaine or choline. The gavage consisted of 150 μL of either 150 mM betaine-N,N,N-trimethyl-d9 (d9-betaine, CDN Isotopes) or 150 mM choline-N,N,N-trimethyl-d9 (d9-choline, Cambridge Isotope Lab) followed by post-gavage blood collection at the indicated times. Where indicated, gut microbiota were suppressed in mice as previously described14 by placement of a cocktail of antibiotics in drinking water for 3 weeks prior to the d9-betaine challenge. Ethylenediaminetetraacetic acid plasma was generated following centrifugation at 1000 g for 20 min at 4°C, and d9-labelled parent compounds and metabolites were measured by LC/MS/MS using choline-1,1,2,2-d4 (d4-choline, Cambridge Isotope Lab) as an internal standard. The chromatographic gradient employed was as previously reported14 and specific parent to daughter transitions in positive ion multiple reaction monitoring mode were 85→66 for d9-TMAO, 113→69 for d9-choline, 127→68 for d9-betaine, and 108→60 for d4-choline. In additional studies, the role of gut flora in TMAO formation from dietary betaine was determined in C57BL/6J mice by performance of oral (gavage) d9-betaine challenge. Mice were housed in conventional cages in the absence or presence of a cocktail of broad spectrum antibiotics added to the drinking water previously shown to suppress gut flora.14
Statistical analysis
Student's t-test or Wilcoxon-rank sum test for continuous variables and χ2 test for categorical variables were used to examine the difference between groups. Kaplan–Meier analysis with Cox proportional hazards regression was used for time-to-event analysis to determine hazard ratio (HR) and 95% confidence intervals (95% CI) for MACE. Stratification between median levels of choline, betaine, and TMAO levels was performed. Adjustments were made for individual traditional risk factors including age, sex, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, smoking, diabetes mellitus, and hs C-reactive protein to predict incident 3-year MACE risks. All analyses performed used R 2.8.0 (Vienna, Austria). P < 0.05 was considered statistically significant.
Results
Baseline characteristics and relation of plasma choline and betaine levels and cardiovascular risks
Table 1 shows the baseline characteristics of the study population. The median (inter-quartile ranges) for choline, betaine, and TMAO plasma levels was 9.8 (7.9–12.2)μM, 41.1 (32.5–52.1)μM, and 3.7 (2.4–6.2)μM, respectively. Modest but statistically significant correlations were noted between TMAO and choline (r = 0.33, P < 0.001) and between TMAO and betaine (r = 0.09, P < 0.001).
Table 1.
Baseline characteristics
| Characteristic | Value |
|---|---|
| Age (years) | 63 ± 11 |
| Male sex (%) | 64 |
| History of cardiovascular disease (%) | 65 |
| History of coronary artery disease (%) | 61 |
| History of myocardial infarction (%) | 41 |
| Hypertension (%) | 72 |
| Diabetes mellitus (%) | 31 |
| Cigarette smoking (%) | 65 |
| LDL cholesterol (mg/dL) | 96 (78–116) |
| HDL cholesterol (mg/dL) | 34 (28–41) |
| Triglycerides (mg/dL) | 118 (85–169) |
| High-sensitivity C-reactive protein (mg/L) | 2.4 (1.1–5.9) |
| Betaine (μM) | 9.8 (7.9–12.2) |
| Choline (μM) | 41.1 (32.5–52.1) |
| TMAO (μM) | 3.7 (2.4–6.2) |
Values expressed in means ± standard deviation or median (inter-quartile range).
As illustrated in the Kaplan–Meier analyses shown in Figures 1 and 2, a graded increase in risk for MACE was observed with increasing plasma levels of either choline or betaine. Higher plasma choline or betaine levels were each associated with increased risk for MACE (1.9-fold and 1.4-fold, respectively; Quartile 4 vs. 1, P < 0.01, each; Table 2). This is lower than the 2.5-fold increased risk for MACE observed with increasing TMAO levels observed in the cohort (Quartile 4 vs. 1, P < 0.01), similar to previous reports.17 Following adjustments for traditional risk factors and hs C-reactive protein, elevated choline [hazard ratio, 1.34 (95% CI, 1.03–1.74, P < 0.05)], and betaine levels [hazard ratio, 1.33 (95% CI: 1.03–1.73, P < 0.05)] each predicted increased MACE risk (Table 2).
Figure 1.
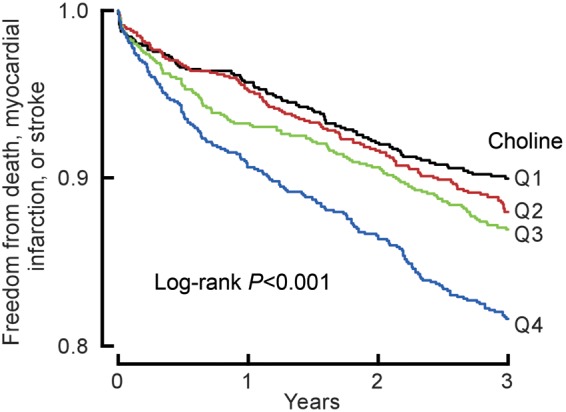
Kaplan–Meier estimates of freedom from major adverse cardiac events (death, myocardial infarction, or stroke) and plasma levels of choline. Data are shown for 3903 participants in the clinical-outcome study according to quartiles of plasma levels of choline. The P-value is for all comparisons.
Figure 2.
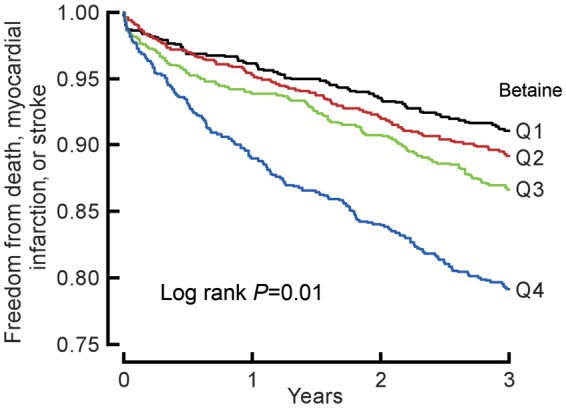
Kaplan–Meier estimates of freedom from major adverse cardiac events (death, myocardial infarction, or stroke) and plasma levels of betaine. Data are shown for 3903 participants in the clinical-outcome study according to quartiles of plasma levels of betaine. The P-value is for all comparisons.
Table 2.
Risk of a major adverse cardiac event at 3 years according to quartiles of choline and betaine level
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|---|---|---|---|---|
| Choline level | ||||
| Range (μM) | <7.89 | 7.89–9.79 | 9.79–12.23 | ≥12.23 |
| Event rate | 90/963 = 9.4 | 112/982 = 11.4 | 121/978 = 12.4 | 172/980 = 17.6 |
| Unadjusted HR | 1 | 1.20 (0.91–1.58) | 1.32 (1.01–1.74)* | 1.92 (1.49–2.48)** |
| Adjusted HR | 1 | 1.06 (0.80–1.40) | 1.09 (0.83–1.44) | 1.34 (1.03–1.74)* |
| Betaine level | ||||
| Range (μM) | <32.46 | 32.46–41.1 | 41.1–52.13 | ≥52.13 |
| Event rate | 111/974 = 11.4 | 120/977 = 12.3 | 111/976 = 11.4 | 153/976 = 15.7 |
| Unadjusted HR | 1 | 1.09 (0.84–1.41) | 0.99 (0.76–1.29) | 1.41 (1.11–1.8)** |
| Adjusted HR | 1 | 1.09 (0.83–1.41) | 1.01 (0.77–1.33) | 1.33 (1.03–1.73)* |
Adjusted for traditional risk factors include age, sex, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, smoking, diabetes mellitus, log (hs C-reactive protein).
*P < 0.05; **P < 0.01.
Interaction between choline, betaine, and trimethylamine-N-oxide in predicting future major adverse cardiac event risk
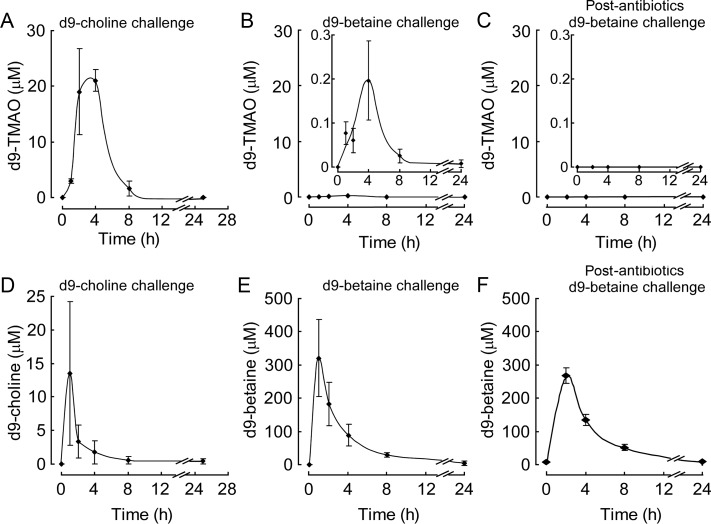
Choline, betaine, and TMAO were previously shown to be metabolites of dietary PC and are each independently associated with the prevalence of CVD.14 In addition, dietary choline serves as a substrate to ultimately produce TMAO.14 To directly test whether dietary betaine can serve as a substrate for generation of TMAO similar to choline, we performed oral (gavage) challenges in mice with synthetic isotope-labelled choline or betaine. First, C57BL/6J mice were fed isotope-labelled choline (d9-trimethyl-choline) via gavage. After an initial lag period, a rapid increase in plasma levels of the d9-isotopologue of TMAO was observed that peaks at ∼4 h following ingestion, and then disappears rapidly within the subsequent 4 h period. In comparison, plasma d9-choline levels peaked earlier (within 1 h) following ingestion (Figure 3A and D). Although there are reports that some bacterial species can cleave betaine to produce TMA,22 to date, there have been no demonstrations that TMAO can be produced from oral ingestion of betaine in animals. We next examined whether oral betaine ingestion can produce TMAO. C57BL/6J mice were similarly challenged with isotope-labelled betaine (d9-trimethyl-betaine) via gavage, and plasma levels of analytes monitored before vs. following ingestion. Similar to the time course observed with d9-choline ingestion, time dependent increases in the plasma levels of the anticipated d9-isotopologue of TMAO were observed following oral ingestion of d9-betaine, with peak level observed within 4 h. Further, following oral ingestion, d9-betaine levels increased rapidly (peak level within 1 h), and then disappeared gradually (Figure 3E). These results directly demonstrate for the first time that oral betaine can serve as a precursor for TMAO formation. Of note, however, compared with comparable oral challenge of d9-choline,14 betaine was a far poorer precursor for TMAO, as it produced 100-fold less d9-TMAO (Figure 3A and B). To assess the requirement of gut flora in dietary betaine-dependent formation of TMAO, parallel studies were performed in mice following suppression of gut flora with an oral cocktail of poorly absorbed antibiotics (3 weeks). Notably, no detectable d9-TMAO was observed in plasma following oral d9-betaine challenge (Figure 3C), consistent with gut flora involvement in oral betaine metabolism into TMAO.
Figure 3.
Deuterium-labelled trimethylamine-N-oxide production from orally ingested deuterium-labelled choline or betaine in mice and an obligatory role for gut flora in generation of plasma trimethylamine-N-oxide. (A) d9-choline or (B) d9-betaine was administered by gastric gavage in C57BL/6J mice, and plasma levels of d9-trimethylamine-N-oxide were monitored at the indicated times as described under ‘Methods’ section. (D) Plasma concentrations of d9-choline or (E) d9-betaine were determined by LC/MS/MS following oral ingestion of d9-choline or d9-betaine, respectively, as described under ‘Methods’ section. (C and F) d9-trimethylamine-N-oxide production and plasma d9-betaine concentration after oral d9-betaine administration in mice following suppression of gut flora with antibiotics (3 weeks). Data are presented as means ± standard error from two (A and D) and three (B and C, E and F) female mice.
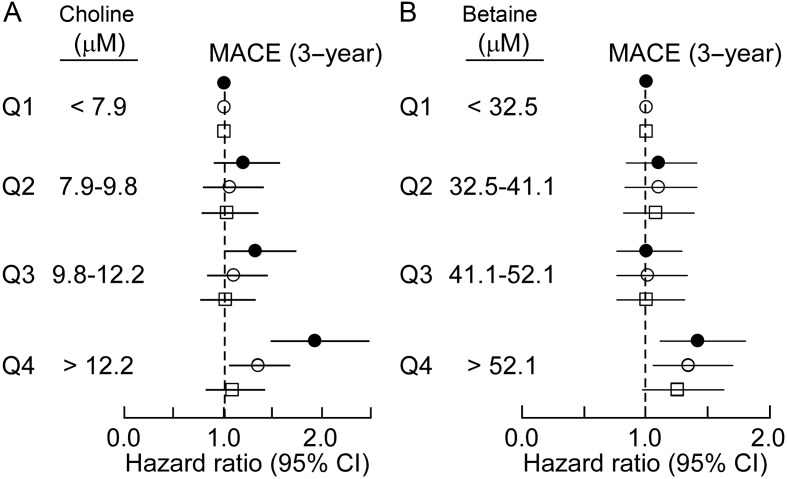
To further investigated the interaction between plasma levels of either choline or betaine and TMAO vs. incident risk for MACE, we constructed a model that included plasma levels of all three PC metabolites within the cohort of sequential subjects presenting for diagnostic cardiac evaluation (n = 3903; Table 1). Remarkably, only TMAO predicted MACE risk when all three metabolites were included in the model [HR: 1.73 (1.3–2.31), P < 0.01]. Further, while choline and betaine each predicted future risk for MACE within 3 years following adjustments for traditional cardiovascular risk factors, addition of TMAO to the model completely attenuated the association for choline and significantly attenuate the association of betaine for MACE risk (Figure 4A and B). Interestingly, if we separated plasma levels of choline, betaine, and TMAO into two ranges, below median value as low and equal to or above median value as high, cox regression analysis adjusted for multiple traditional risk factors reveals that choline and betaine predict increased MACE risk, but only among those with concomitant elevated TMAO levels and not in those with low TMAO levels (Figures 5 and 6).
Figure 4.
Relationship between plasma choline and betaine concentration and risk for major adverse cardiac events (A). Forest plot of the hazard ratio of major adverse cardiac events (3 year) and quartiles of choline levels both unadjusted (closed circles) and after adjusting for traditional cardiovascular risk factors and high-sensitivity (hs) C-reactive protein (open circles), or traditional cardiovascular risk factors plus hs C-reactive protein and trimethylamine-N-oxide (open squares). (B). Forest plot of the hazard ratio of major adverse cardiac events and quartiles of betaine unadjusted (closed circles) and after adjusting for traditional cardiovascular risk factors and hs C-reactive protein (open circles), or traditional cardiovascular risk factors plus hs C-reactive protein and trimethylamine-N-oxide (open squares). Bars represent 95% confidence intervals.
Figure 5.
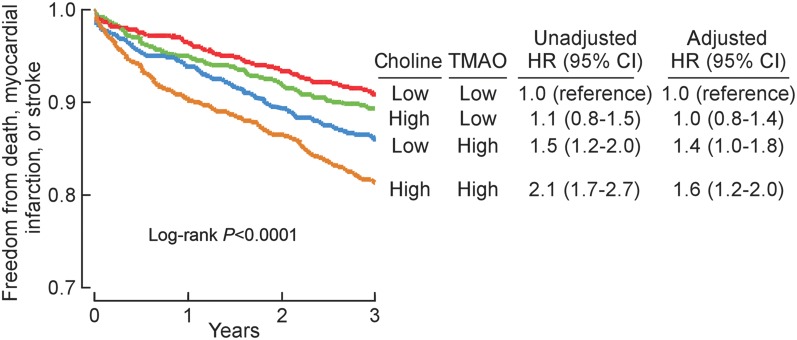
Relationship between plasma choline concentration and risk for major adverse cardiac events in the context of plasma trimethylamine-N-oxide levels. Kaplan–Meier plot and hazard ratio with 95% confidence intervals for unadjusted model, or following adjustments for traditional risk factors and hs C-reactive protein as in Figure 4A. Median plasma concentration of choline (9.8 μM) and trimethylamine-N-oxide (3.7 μM) within the cohorts were used to stratify subjects as ‘high’ (≥median) or ‘low’ (<median) values.
Figure 6.
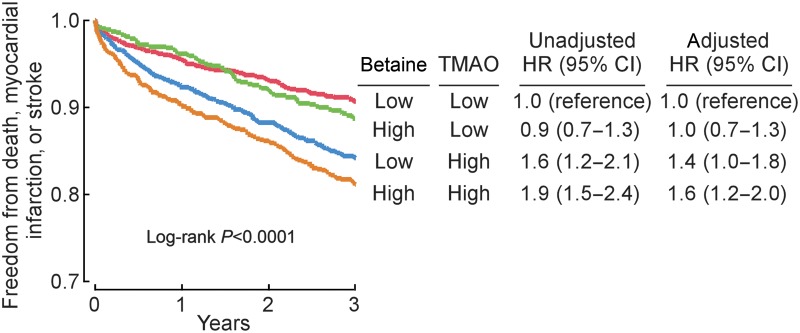
Relationship between plasma betaine concentration and risk for major adverse cardiac events in the context of plasma trimethylamine-N-oxide levels. Kaplan–Meier plot and hazard ratio with 95% confidence intervals for unadjusted model, or following adjustments for traditional risk factors and C-reactive protein as in Figure 4B. Median plasma concentration of betaine (41.1 μM) and trimethylamine-N-oxide (3.7 μM) within the cohorts were used to stratify subjects as ‘high’ (≥median) or ‘low’ (<median) values.
Discussion
Choline, betaine, and TMAO are all metabolites formed following ingestion of dietary PC.14,17 Moreover, systemic levels of choline, betaine, and TMAO each are independently associated with the prevalence of CVD.14 However, recent animal model studies demonstrate that the mechanism through which dietary PC or choline enhances atherosclerosis requires the presence of intact intestinal microbiota and formation of TMAO.14 In the present animal study, we demonstrated for the first time the production of TMAO from dietary betaine administration, albeit at a significantly less efficient rate (∼100-fold) than that from dietary choline. Importantly, we show that the TMAO formed from oral betaine has an obligatory requirement for intact gut flora, similar to previous observations with dietary choline. Prior mechanistic studies in mice reveal that TMAO itself inhibits reverse cholesterol transport, and also alters cholesterol and sterol metabolism in multiple different compartments, including suppression of bile acid pool size and altered composition.16 Given (i) the direct pro-atherogenic effects observed with direct dietary administration of TMAO;14–16 (ii) the precursor → product metabolite relationships between both PC and choline and betaine, as well as either choline14 or betaine (proved for first time in this study) and TMAO formation; and (iii) the observation in animal models that the pro-atherogenic effects of dietary choline is only observed with intact gut microbiota where TMAO could be formed, we sought in the present clinical studies to test several hypotheses. First, we tested the hypothesis that circulating choline and betaine levels were associated with an incident risk for MACE. Second, we sought to test whether any associations between either choline or betaine and CVD risks would be attenuated by inclusion of TMAO into the models since it is TMAO that appears to mechanistically serve as the culprit in enhancing atherosclerosis. Thus, an important finding of this study is that elevated fasting plasma choline and betaine levels each individually were associated with greater risk for developing future MACE among stable patients undergoing evaluation with elective coronary angiography. More importantly, however, is that the observed heightened cardiovascular risks in those with elevated choline and/or betaine levels were observed only among those with concomitantly elevated fasting plasma TMAO levels. The results of these human clinical observation studies are thus consistent with the previously recognized mechanistic role of TMAO in accelerated atherosclerosis, and imply that increased production of the intestinal microbiota-dependent product of PC metabolism, TMAO, rather than the presence of or exposure to its substrate (i.e. choline and/or betaine), is likely the major driver for the development of future cardiovascular events in humans.
It is important to emphasize that one cannot (and should not) simply eliminate dietary choline as a means of trying to lower CVD risks since dietary choline deficiency, while rare, promotes a disease state.2 At the same time, studies looking at acute increase in dietary intake (meal) of choline-rich foods show that they do not always lead to substantial increases in urinary TMA or TMAO in healthy subjects,23 perhaps consistent with a wide variability in intestinal microbial communities between individuals, as well as the substantial whole body pool size of TMAO relative to the amount formed following a single meal. Following ingestion of foods that lead to a detectable rise in urinary TMA/TMAO excretion, the kidneys are highly efficient in eliminating TMAO in order to maintain a steady state of circulating choline levels. Furthermore, enterohepatic circulation of bile, which is exceedingly high in total choline content, likely contributes both to the maintenance of intestinal microbial colonies by providing a steady source of substrates for their metabolism,24 and blunts the impact of acute changes in dietary choline on plasma TMAO levels. It is interesting to note that several prior studies have suggested circulating total choline levels may serve as a marker for acute coronary syndrome risk.18–21 The mechanisms for this association, however, have been speculative including suggestion that activation of inflammatory responses and the clotting cascade during acute coronary events may lead to release of choline from membrane phospholipids.19
As we continue to explore the intricate relationship between various PC metabolites and atherogenesis, the role of intestinal microbiota has emerged as a key regulator.14,16,17 Recent animal studies have explored the contributions of distinct flavin monooxygenases, a family of hepatic enzymes, in converting TMA to TMAO.15 We have also established the obligatory role of intestinal microbiota in the production of TMAO from dietary choline and other TMA nutrients in humans,16,17 and the association between elevated fasting levels of TMAO and higher incident risks of long-term MACEs.17 Interestingly, the administration of antibiotics in humans in our prior studies had limited effects on circulating choline and betaine levels, despite abolition of TMAO levels, upon ingestion of stable-isotope-labelled d9-PC.17 Hence, the presence of elevated plasma levels of choline and betaine, which individually are associated with poorer prognosis, only confer this added risk when associated with concomitant TMAO elevation. In other words, subjects in whom one observes the absence of high TMAO levels despite high choline and betaine levels (i.e. low TMAO producers) are significantly less likely to have an adverse cardiovascular event than those with intestinal microbiota that readily produces TMA and TMAO (i.e. high TMAO producers).
Conclusion
Elevated plasma levels of choline and betaine are each associated with poor prognosis even after adjusting for traditional cardiovascular risk factors. However, high choline and betaine levels are associated with higher risk of future MACE only with concomitant increased level of TMAO.
Funding
This work was supported by grants P20HL113452, R01HL103866 from the National Heart, Lung, and Blood Institute of the National Institutes of Health and the Office of Dietary Supplements. Clinical samples used in this study were from GeneBank, a study supported by National Institutes of Health grant numbers P01HL076491, P01HL098055, UL1TR 000439, and R01HL103931. Z.W. was partially supported by American Heart Association grant 12SDG12050473. S.L.H is also partially supported by the Leonard Krieger endowment and a grant from the Foundation LeDucq. Mass spectrometry instrumentation used was housed within the Cleveland Clinic Mass Spectrometry Facility with partial support through a Center of Innovation by AB SCIEX.
Conflict of interest: Z.W. and S.L.H. are named as co-inventors on pending patents held by the Cleveland Clinic relating to cardiovascular diagnostics. Z.W. reports that he has the right to receive royalty payment for inventions or discoveries related to cardiovascular diagnostics from Liposciences, Inc. W.H.W.T. has no relationships to disclose. S.L.H. reports having been paid as a consultant for the following companies: Cleveland Heart Lab, Esperion, Lilly, Liposcience Inc., Merck and Co., Inc., Pfizer Inc. and Proctor and Gamble. S.L.H. reports receiving research funds from Abbott, Cleveland Heart Lab, Liposcience Inc., Pfizer Inc., Proctor and Gamble and Takeda. Dr. S.L.H. reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from the companies shown below: Cleveland Heart Lab., Esperion, Frantz Biomarkers, LLC, Liposcience Inc.
References
- 1.Pinotti L, Rebucci R, Fusi E, Rossi L, Baldi A. Milk choline, alpha-tocopherol and neutrophil chemotaxis in the periparturient dairy cow. Vet Res Commun. 2003;27(Suppl. 1):265–268. doi: 10.1023/b:verc.0000014156.88095.1b. [DOI] [PubMed] [Google Scholar]
- 2.Zeisel SH, da Costa KA. Choline: an essential nutrient for public health. Nutr Rev. 2009;67:615–623. doi: 10.1111/j.1753-4887.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu DM, Wahlqvist ML, Chang HY, Yeh NH, Lee MS. Choline and betaine food sources and intakes in Taiwanese. Asia Pac J Clin Nutr. 2012;21:547–557. [PubMed] [Google Scholar]
- 4.Li Z, Vance DE. Phosphatidylcholine and choline homeostasis. J Lipid Res. 2008;49:1187–1194. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Zeisel SH. Dietary choline deficiency causes DNA strand breaks and alters epigenetic marks on DNA and histones. Mutat Res. 2012;733:34–38. doi: 10.1016/j.mrfmmm.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paoletti L, Elena C, Domizi P, Banchio C. Role of phosphatidylcholine during neuronal differentiation. IUBMB Life. 2011;63:714–720. doi: 10.1002/iub.521. [DOI] [PubMed] [Google Scholar]
- 7.Glunde K, Jacobs MA, Bhujwalla ZM. Choline metabolism in cancer: implications for diagnosis and therapy. Expert Rev Mol Diagn. 2006;6:821–829. doi: 10.1586/14737159.6.6.821. [DOI] [PubMed] [Google Scholar]
- 8.Detopoulou P, Panagiotakos DB, Antonopoulou S, Pitsavos C, Stefanadis C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: the ATTICA study. Am J Clin Nutr. 2008;87:424–430. doi: 10.1093/ajcn/87.2.424. [DOI] [PubMed] [Google Scholar]
- 9.Bidulescu A, Chambless LE, Siega-Riz AM, Zeisel SH, Heiss G. Repeatability and measurement error in the assessment of choline and betaine dietary intake: the Atherosclerosis Risk in Communities (ARIC) study. Nutr J. 2009;8:14. doi: 10.1186/1475-2891-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bidulescu A, Chambless LE, Siega-Riz AM, Zeisel SH, Heiss G. Usual choline and betaine dietary intake and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. BMC Cardiovasc Disord. 2007;7:20. doi: 10.1186/1471-2261-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Wang B, Zhang M, Rantalainen M, Wang S, Zhou H, Zhang Y, Shen J, Pang X, Wei H, Chen Y, Lu H, Zuo J, Su M, Qiu Y, Jia W, Xiao C, Smith LM, Yang S, Holmes E, Tang H, Zhao G, Nicholson JK, Li L, Zhao L. Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci USA. 2008;105:2117–2122. doi: 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, Fearnside J, Tatoud R, Blanc V, Lindon JC, Mitchell SC, Holmes E, McCarthy MI, Scott J, Gauguier D, Nicholson JK. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci USA. 2006;103:12511–6. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett BJ, Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, Didonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Body R, Griffith CA, Keevil B, McDowell G, Carley S, Ferguson J, Mackway-Jones K. Choline for diagnosis and prognostication of acute coronary syndromes in the Emergency Department. Clin Chim Acta. 2009;404:89–94. doi: 10.1016/j.cca.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 19.Danne O, Lueders C, Storm C, Frei U, Mockel M. Whole blood choline and plasma choline in acute coronary syndromes: prognostic and pathophysiological implications. Clin Chim Acta. 2007;383:103–109. doi: 10.1016/j.cca.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Danne O, Mockel M, Lueders C, Mugge C, Zschunke GA, Lufft H, Muller C, Frei U. Prognostic implications of elevated whole blood choline levels in acute coronary syndromes. Am J Cardiol. 2003;91:1060–1067. doi: 10.1016/s0002-9149(03)00149-8. [DOI] [PubMed] [Google Scholar]
- 21.LeLeiko RM, Vaccari CS, Sola S, Merchant N, Nagamia SH, Thoenes M, Khan BV. Usefulness of elevations in serum choline and free F2-isoprostane to predict 30-day cardiovascular outcomes in patients with acute coronary syndrome. Am J Cardiol. 2009;104:638–643. doi: 10.1016/j.amjcard.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 22.Moller B, Hippe H, Gottschalk G. Degradation of various amine compounds by mesophilic clostridia. Arch Microbiol. 1986;145:85–90. doi: 10.1007/BF00413032. [DOI] [PubMed] [Google Scholar]
- 23.Zhang AQ, Mitchell SC, Smith RL. Dietary precursors of trimethylamine in man: a pilot study. Food Chem Toxicol. 1999;37:515–520. doi: 10.1016/s0278-6915(99)00028-9. [DOI] [PubMed] [Google Scholar]
- 24.Robinson BS, Snoswell AM, Setchell BP. The enterohepatic recycling of bile choline in sheep. Comp Biochem Physiol A Comp Physiol. 1987;88:283–289. doi: 10.1016/0300-9629(87)90485-3. [DOI] [PubMed] [Google Scholar]