Abstract
The detailed nature of N-3 binding at the multi-copper active site in native laccase is investigated through a combination of low-temperature magnetic circular dichroism (LTMCD) and absorption spectroscopies. This combination of techniques allows charge-transfer spectral features associated with N-3 binding to the paramagnetic type 2 Cu(II) to be differentiated from those associated with binding to the antiferromagnetically coupled, and therefore diamagnetic, binuclear type 3 Cu(II) site. Earlier absorption titration studies have indicated that N-3 binds with two different binding constants, yielding a high-affinity and a low-affinity form. The studies presented here are interpreted as strong evidence that low-affinity N-3 bridges the paramagnetic type 2 and diamagnetic type 3 binuclear Cu(II) sites in fully oxidized laccase. This assignment is further supported by features in the MCD spectrum whose intensity correlates with an EPR signal associated with uncoupled type 3 Cu(II) sites. In these sites, N-3 has displaced the endogenous bridge, thereby rendering the site paramagnetic and detectable by both LTMCD and EPR spectroscopy. High-affinity N-3 is found to bind to the paramagnetic type 2 Cu(II) site in a limited fraction of the protein molecules that contains reduced type 3 sites. Finally, the possible role of this trinuclear (type 2-type 3) Cu(II) active site in enabling the irreversible reduction of dioxygen to water is considered.
Full text
PDF

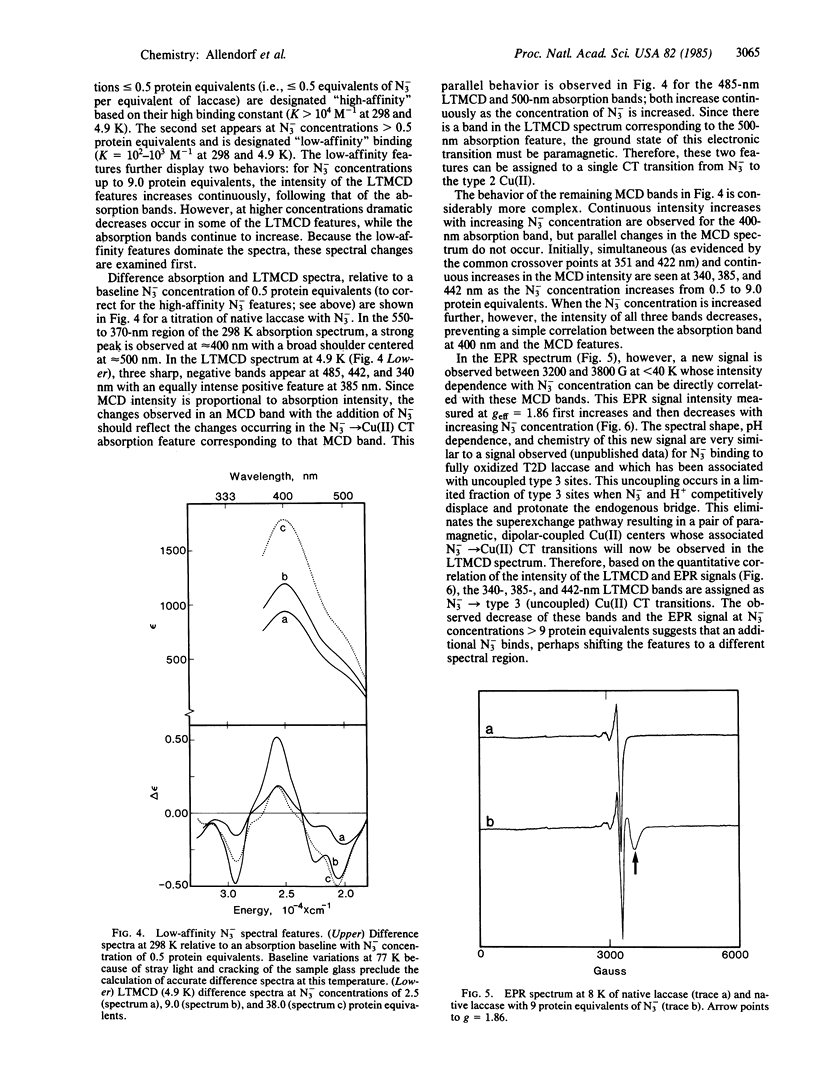
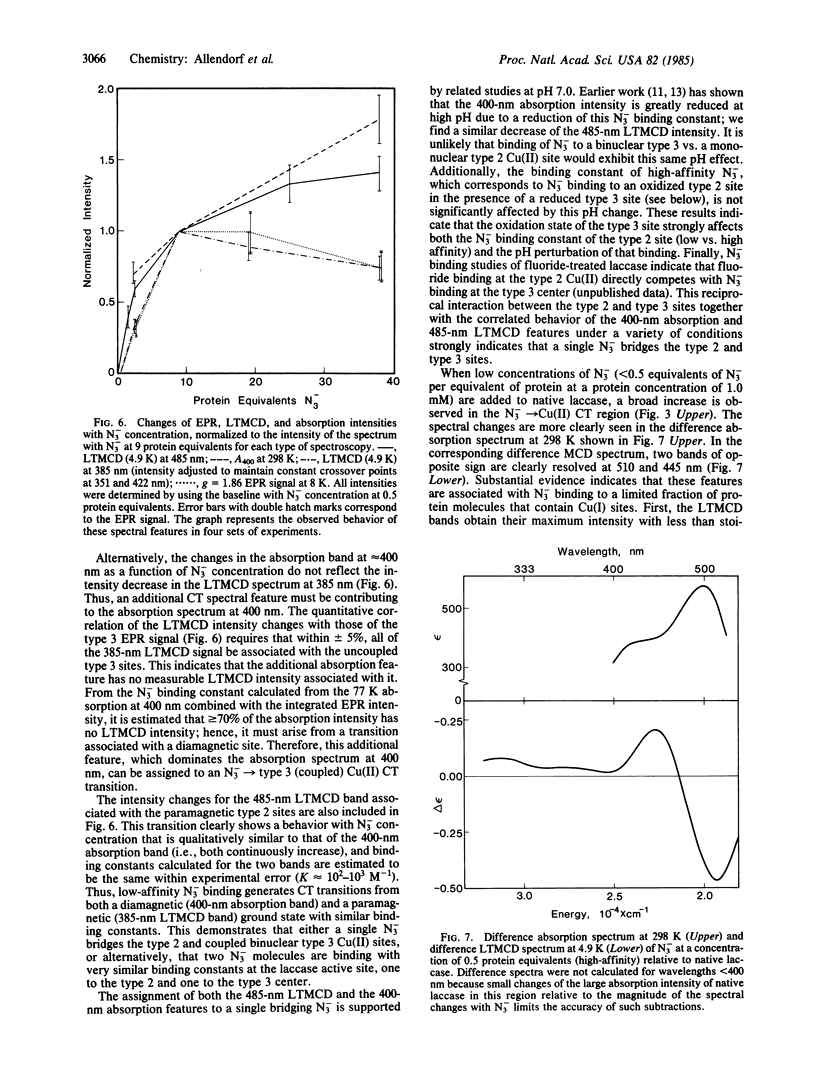

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasa R., Brändén R., Deinum J., Malmström B. G., Reinhammar B., Vänngård T. A paramagnetic intermediate in the reduction of oxygen by reduced laccase. FEBS Lett. 1976 Jan 15;61(2):115–119. doi: 10.1016/0014-5793(76)81016-2. [DOI] [PubMed] [Google Scholar]
- Andréasson L. E., Brändén R., Reinhammar B. Kinetic studies of Rhus vernicifera laccase. Evidence for multi-electron transfer and an oxygen intermediate in the reoxidation reaction. Biochim Biophys Acta. 1976 Jul 8;438(2):370–379. doi: 10.1016/0005-2744(76)90254-0. [DOI] [PubMed] [Google Scholar]
- Andréasson L. E., Reinhammar B. The mechanism of electron transfer in laccase-catalysed reactions. Biochim Biophys Acta. 1979 May 10;568(1):145–156. doi: 10.1016/0005-2744(79)90282-1. [DOI] [PubMed] [Google Scholar]
- Byers W., Curzon G., Garbett K., Speyer B. E., Young S. N., Williams R. J. Anion-binding and the state of copper in caeruloplasmin. Biochim Biophys Acta. 1973 May 17;310(1):38–50. doi: 10.1016/0005-2795(73)90006-8. [DOI] [PubMed] [Google Scholar]
- Dooley D. M., Scott R. A., Ellinghaus J., Solomon E. I., Gray H. B. Magnetic susceptibility studies of laccase and oxyhemocyanin. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3019–3022. doi: 10.1073/pnas.75.7.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickman N. C., Himmelwright R. S., Solomon E. I. Geometric and electronic structure of oxyhemocyanin: spectral and chemical correlations to met apo, half met, met, and dimer active sites. Proc Natl Acad Sci U S A. 1979 May;76(5):2094–2098. doi: 10.1073/pnas.76.5.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farver O., Frank P., Pecht I. Peroxide binding to the type 3 site in Rhus vernicifera laccase depleted of type 2 copper. Biochem Biophys Res Commun. 1982 Sep 16;108(1):273–278. doi: 10.1016/0006-291x(82)91862-9. [DOI] [PubMed] [Google Scholar]
- Farver O., Goldberg M., Pecht I. A circular dichroism study of the reactions of Rhus laccase with dioxygen. Eur J Biochem. 1980 Feb;104(1):71–77. doi: 10.1111/j.1432-1033.1980.tb04401.x. [DOI] [PubMed] [Google Scholar]
- Graziani M. T., Morpurgo L., Rotilio G., Mondovì B. Selective removal of type 2 copper from Rhus vernicifera laccase. FEBS Lett. 1976 Nov;70(1):87–90. doi: 10.1016/0014-5793(76)80732-6. [DOI] [PubMed] [Google Scholar]
- Hahn J. E., Co M. S., Spira D. J., Hodgson K. O., Solomon E. I. Quantitative Cu(I) determination using X-ray absorption edge spectroscopy: oxidation of the reduced binuclear copper site in type 2 depleted Rhus laccase. Biochem Biophys Res Commun. 1983 Apr 29;112(2):737–745. doi: 10.1016/0006-291x(83)91524-3. [DOI] [PubMed] [Google Scholar]
- Malkin R., Malmström B. G. The state and function of copper in biological systems. Adv Enzymol Relat Areas Mol Biol. 1970;33:177–244. doi: 10.1002/9780470122785.ch4. [DOI] [PubMed] [Google Scholar]
- Morpurgo L., Desideri A., Rotilio G. Heterogeneity of the Type 3 copper in Japanese-lacquer-tree (Rhus vernicifera) laccase. Biochem J. 1982 Dec 1;207(3):625–627. doi: 10.1042/bj2070625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner-Hahn J. E., Hedman B., Hodgson K. O., Spira D. J., Solomon E. I. On the spectral features associated with peroxide reactivity of the coupled binuclear copper active site in type 2 depleted and native Rhus laccase. Biochem Biophys Res Commun. 1984 Mar 15;119(2):567–574. doi: 10.1016/s0006-291x(84)80286-7. [DOI] [PubMed] [Google Scholar]
- Reinhammar B. R. Oxidation-reduction potentials of the electron acceptors in laccases and stellacyanin. Biochim Biophys Acta. 1972 Aug 17;275(2):245–259. doi: 10.1016/0005-2728(72)90045-x. [DOI] [PubMed] [Google Scholar]
- Reinhammar B., Oda Y. Spectroscopic and catalytic properties of Rhus vernicifera laccase depleted in type 2 copper. J Inorg Biochem. 1979 Oct;11(2):115–127. doi: 10.1016/s0162-0134(00)80177-4. [DOI] [PubMed] [Google Scholar]
- Reinhammar B. Purification and properties of laccase and stellacyanin from Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):35–47. doi: 10.1016/0005-2728(70)90059-9. [DOI] [PubMed] [Google Scholar]
- Solomon E. I., Dooley D. M., Wang R. H., Gray H. B., Credonio M., Mogno F., Romani G. L. Letter: Susceptibility studies of laccase and oxyhemocyanin using an ultrasensitive magnetometer. Antiferromagnetic behavior of the type 3 copper in Rhus laccase. J Am Chem Soc. 1976 Feb 18;98(4):1029–1031. doi: 10.1021/ja00420a035. [DOI] [PubMed] [Google Scholar]
- Spira D. J., Solomon E. I. Nitrite reactivity of the binuclear copper site in T2D Rhus laccase: preparation of half met-NO2- T2D laccase and its correlation to half met-NO2- hemocyanin and tyrosinase. Biochem Biophys Res Commun. 1983 Apr 29;112(2):729–736. doi: 10.1016/0006-291x(83)91523-1. [DOI] [PubMed] [Google Scholar]
- Spira D. J., Winkler M. E., Solomon E. I. Preparation and characterization of a stable half met derivative of type 2 depleted Rhus laccase: exogenous ligand binding to the type 3 site. Biochem Biophys Res Commun. 1982 Jul 30;107(2):721–726. doi: 10.1016/0006-291x(82)91550-9. [DOI] [PubMed] [Google Scholar]
- Winkler M. E., Spira D. J., LuBein C. D., Thamann T. J., Solomon E. I. Anion binding to oxidized type 2 depleted and native laccase: a spectroscopically effective model for exogenous ligand binding to the type 3--type 2 active site. Biochem Biophys Res Commun. 1982 Jul 30;107(2):727–734. doi: 10.1016/0006-291x(82)91551-0. [DOI] [PubMed] [Google Scholar]


