Abstract
Prolactin (PRL) is a secretory cytokine produced by various tissues. Binding to the cognate PRL receptor (PRLR), it activates intracellular signaling via janus kinase (JAK), extracellular signal-regulated kinase (ERK) and signal transducer and activator of transcription (STAT) proteins. PRL regulates diverse activities under normal and abnormal conditions, including malignancies. Previous clinical data suggest serum PRL levels are elevated in colorectal cancer (CRC) patients. In this study, we first determined the expression of PRL and PRLR in colon cancer tissue and cell lines. Higher levels of PRLR expression were observed in the cancer cells and cell lines compared with normal colonic epithelial cells. Incubation of colon cancer cells with PRL-induced JAK2, STAT3 and ERK1/2 phosphorylation and increased expression of Jagged 1, which is a Notch-1 receptor ligand. Notch signaling regulates CRC stem cell population. We observed increased accumulation of the cleaved/active form of Notch-1 receptor (Notch intracellular domain) and increased expression of Notch responsive genes HEY1, HES1 and stem cell marker genes DCLK1, LGR5, ALDH1 and CD44. Finally, inhibiting PRL induced JAK2-STAT3 and JAK2-ERK1/2 using AG490 and PD98059, respectively, leads to complete abrogation of Notch signaling, suggesting a role for this pathway in regulating CRC stem cells. Together, our results demonstrate that cytokine signaling induced by PRL is active in colorectal cancers and may provide a novel target for therapeutic intervention.
Introduction
Colorectal cancer (CRC) remains one of the leading causes of cancer-related deaths in both economically developed and developing countries. It is the second leading cause of cancer deaths in both males and females in United States (1). The precancerous predisposition of colorectal epithelial polyps is no longer disputed. A plethora of morphologic and molecular studies have carefully analyzed the progression of a non-cancerous polyp into invasive cancerous lesions. These process have been shown to be complex and are influenced by various intrinsic and extrinsic factors, such as hormones (2).
Prolactin (PRL), a cytokine hormone, accumulates in the tissue microenvironment and elicits its action in an autocrine or paracrine manner to regulate diverse physiological activities that include reproduction, growth, development, metabolism and immunomodulation (3–6). Binding of PRL to the single-pass, transmembrane PRL receptor (PRLR) induces several intracellular signaling cascades that are mediated via Jak-STAT (7,8) and Jak-Ras-MAPK components (9).
PRL acts as a mitogen by promoting cell proliferation, inhibiting apoptosis and inducing chemoattraction in breast cancer cells (5,10,11). Blood PRL levels were found elevated in patients with hepatocellular carcinoma (12,13) and ovarian cancer (14). Cultured, immortalized ovarian epithelial cells and endometrial cells treated with exogenous PRL demonstrated increased proliferation and inhibition of chemotherapy-induced cell death (15). Autocrine PRL induces PRLR-mediated Jak2-STAT signaling in prostate cancer (16–19) and modulates the stem cell/basal cell population (17).
PRL and PRLR have been expressed along the gastrointestinal tract in fetal and neonatal stages during development (20). In adult rats, PRL induces active potassium-ion transport in distal colon and chloride-ion transport in proximal and transverse colon (21). IEC-6 colon crypt epithelial cells treated with PRL had increased expression of nutrient and mineral transport channel proteins, without inducing proliferation (22). Elevated serum levels of PRL have been identified in patients with colorectal malignancies (23–26). In addition, increase in PRL and PRLR expression was noted in CRC cell lines and tumor samples (27).
Cancer stem cells (CSCs), initially identified in hematological disorders as tumor-initiating cells when isolated and transplanted in NOD-SCID mice (28), are long-lived, self-renewing population of cells that initiate and sustain tumor growth and can be identified by unique set of marker proteins such as doublecortin like kinase 1 (DCLK1) (29–31), leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5) (32–35), CD44 (36) and CD133 (37), which also serve as protein markers for normal colon stem cells. These cells are resistant to therapeutic interventions and cause tumor relapse and metastasis (38,39). Identifying cellular factors that regulate stem cell population are critical in understanding the process of neoplastic transformation and in developing novel therapeutics to target the CSC pool. Isolated primary mouse hippocampal cells treated with exogenous PRL showed increased number of stem cells (40). Similarly, in mouse models, inducing PRL under the control of prostate-specific probasin promoter leads to expansion in the basal cell compartment (17,41), which constitutes the stem cell population of the prostate gland. Although these data suggest that PRL can affect tissue stem cell population, its effects on CSCs have not been determined.
Notch signaling pathway is active in intestinal crypts (43) and is involved in regulating stem cell hierarchy and cell fate determination (42). Constitutive Notch activation is necessary for intestinal stem cell maintenance (44) and deregulation of the pathway has been observed in colorectal and other forms of cancer (45). There are four members in the Notch receptor family: from Notch 1 to Notch 4. Binding of specific ligands such as Jagged (JAG) 1, 2, or Delta 1, 3, 4, to the Notch receptor results in a conformational change in the receptor. Subsequent activation of the γ-secretase complex, which is composed of presenilin, nicastrin, anterior pharynx defective 1 (APH 1) and presenilin enhancer 2, cleaves the Notch receptor to release the Notch intracellular domain (NICD) (46,47). The NICD then translocates into the nucleus, interacts with co-factors recombining binding protein suppressor of hairless and mastermind-like, bind to target sequences and activate the transcription of genes such as Hes1, Hey1 and stem cell responsive genes (48) such as c-Myc, all of which can be used to assess the degree of Notch signal activation. Interestingly, extracellular signal-regulated kinase (ERK) can modulate Notch signaling by regulating the expression of its ligand JAG 1 (49).
This study is aimed at determining the role of PRL signaling in colon cancer cells. We show that presence of PRL induces Jak2-ERK1/2 mediated activation of Notch signaling, leading to an increase in spheroid formation and changes in CSC population. Furthermore, PRL signaling in these cells can be suppressed with specific inhibitors of Jak2 and ERK1/2.
Materials and methods
Cells
Colon cancer cell lines HT29, HCT116, SW480, SW620, DLD1 and normal intestinal epithelial fetal human colon (FHC) cells were obtained from ATCC (Manassas, VA). The cells were well characterized and used by multiple investigators. They were cultured in the recommended media supplemented with 10% fetal bovine serum (Sigma Aldrich, MO) and 1% antibiotic–antimycotics solution (Mediatech Inc, VA) at 37°C in a humidified atmosphere of 5% CO2. The cells were cultured in serum-free media overnight prior to treatment with PRL (500ng/ml). Where indicated, cells were pretreated with 50 μM Jak2 inhibitor AG490 or 10 μM ERK1/2 inhibitor PD98059 (Selleckchem, TX).
Spheroid assay
Cells were seeded at a limiting dilution of 1500 cells/ml (total 2ml = 3000 cells/well in a 6-well dish) in DMEM supplemented with or without PRL (0–500ng/ml) and inhibitors AG490 or PD98059, in addition to epidermal growth factor (5ng/ml), fibroblast growth factor (5ng/ml), heparin (1ng/ml) and B12 supplements (0.25×) and plated on ultra-low attachment plates (BD Biosciences, NY). An important point to note is that we reduced the amount of growth factors used in the culture conditions to prevent any growth-promoting effects by these growth factors, which can complicate the analyses, and to gain a better idea of the role of PRL in promoting spheroid formation. Specifically, we used one-fourth the dose of growth factors (epidermal growth factor, fibroblast growth factor), Heparin and B12 supplements that was recommended by earlier studies (50). At these concentrations of growth factors, the dilution limit of cells to grow as spheroids and not as aggregates was determined to be 1500 cells/ml of media. Colosphere formation was assessed after 4–6 days, the number and size of colonospheres was determined using Celigo (Cyntellect Inc, San Diego, CA).
Real-time reverse-transcription PCR analysis
Colon cancer cDNA panel with matched adjacent tissue controls was obtained from Origene (Rockville, MD). Total RNA from cell lines was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) following manufacturer’s instructions. 2 μg RNA was used to synthesize complimentary DNA using Superscript II reverse transcriptase and random hexanucleotide primers (Invitrogen, CA). Individual gene expression was quantified using SYBR green reagent (Molecular Probes, OR) and specific primers with glyceraldehyde 3-phosphate dehydrogenase as internal standard.
Primers for the PCR include 5'-GCATATTGCGATCCTGGAAT-3' and 5'-CGTTTGGTTTGCTCCTCAAT-3' for PRL, 5'-GGAGCTGGC TGTGGAAGTAA-3' and 5'-CTCCCACTCAGCTGCTTTCT-3' for PRLR, 5'-GT GCGGTATATTTCCTCCAA-3' and 5`-GTTCCCGTGAAGCCTTTGT-3' for JAG1, 5'-CCTCTCTTCCCTCCGGACT-3' and 5'-GGTCAGTCACTTAATAC AGCTCTCTCT-3' for HES1, 5'-GCTGGTACCCAGTGCTTTTGAG-3' and 5'-TGCAGGATCTCGGCTTTTTCT-3' for HEY1, 5'-GGTGAACGTCAAGA CCACCT-3' and 5'-GTCCTGAAGGCACATCACCT-3' for DCLK1, 5'-AACAGTCCTGTGACTCAACTCAAG-3' and 5'-TTAGAGACATGGG ACAAATGCCAC-3' for LGR5, 5'-TGTTAGCTGATGCCGACTTG-3' and 5'-TTCTTAGCCCGCTCAACACT-3' for ALDH1, 5'-CAGCCTCAAGAT CATCAGCA-3' and 5'-GTCTTCTGGGTGGCAGTGAT-3' for GAPDH.
Western blot analysis and enzyme-linked immunosorbent assay
Protein samples were prepared in radioimmunoprecipitation assay buffer (Thermo Scientific, IL). Following quantification using BCA Kit (Thermo Scientific, IL), the lysates were subjected to polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membrane (EMD Millipore, MA). Antibodies for PRLR (ab87992), DCLK1 (ab37994) and LGR5 (ab119012) were obtained from Abcam (Cambridge, MA); JAG 1 (sc6011), Hes1 (sc25392), Hey1 (sc28746) and ACTB (sc1616) were obtained from Santa Cruz Biotech Inc (Dallas, TX); Nicastrin (A00883), presenilin1 (A00881), anterior pharynx defective 1 (A00884) and Presenilin enhancer protein (A00882) were obtained from Millipore (Billerica, MA); and Jak2 (3230S), pJak2 (3776S), signal transducer and activator of transcription 3 (STAT3) (4904S), pSTAT3 (9131S), Erk1/2 (p42/44) (9102S), pErk1/2 (p-p42/44) (9101S), cMyc (5605S), NICD (4380S), CD44 (3570S) and glyceraldehyde 3-phosphate dehydrogenase (2118S) were obtained from Cell Signaling (Boston. MA). Specific proteins were detected using chemiluminescence (GE Healthcare, NJ). To determine PRL levels, cells were cultured in serum-free media and the media was subjected to enzyme-linked immunosorbent assay according to manufacturer’s instructions (Molecular Innovations, MI). Briefly, 100 μl of the provided standard and concentrated serum-free media collected from cells was added into wells pre-coated with PRL antibody in triplicates and allowed to bind for 30 min at which point the wells were washed and treated sequentially with primary antibody and streptavidin-horseradish peroxidase-bound secondary antibody. Colorimetric quantification after treating with substrate was done at 450nm.
Luciferase assay
Cells were plated and transfected with either 4XM67 pTK-Luc (Addgene plasmid 8688 ) (51) or Hes-1A/B-Luc, a kind gift from Dr. Kimberly Foreman, Loyola University, Chicago (52), which encode firefly luciferase gene under the control of the minimal thymidine kinase promoter and four STAT3 (4X STAT3 BS) or a single Hes1 (HES1 BS) binding site using Lipofectamine 2000 (Invitrogen, NY). Cells were pre-treated with PD98059 (10 μM) and/or AG490 (50 μM) for 2h prior to treating with PRL (500ng/ml). Renilla luciferase expressing pRL-TK plasmid (Clontech, Mountain View, CA) was used as internal control. Luciferase levels in the cell lysates were determined using Dual-Luciferase Reporter Assay System (Promega Corporation, Madison, WI).
Statistical analysis
Data from at least three independent experiments were expressed as the mean ± SEM and analyzed by unpaired or paired student’s t-test using GraphPad Prism 5 (La Jolla, CA). P value ≤0.05 was considered to be statically significant.
Results
PRLR but not PRL is upregulated in colon cancer cells
To determine whether PRL signaling occurs in colorectal cancer, we first analyzed the expression of PRL and PRLR in human colon cancer tissues and cell lines. Real-time PCR quantification using CRC patient samples indicates a significant increase in PRLR but not PRL transcript levels in the cancerous tissue compared with adjacent normal tissue (Figure 1A and B). A similar increase in PRLR mRNA levels and protein was observed in CRC cells compared with normal colonic epithelial cells (FHC) (Figure 1C and D). Moreover, no difference in expression of PRL mRNA was observed between normal colonic FHC cells and CRC cell lines (Figure 1E). Quantification of concentrated culture media from the cell lines by enzyme-linked immunosorbent assay indicate that all cells secrete PRL; however, the amount varies with time and in a cell-line-specific manner, ranging from 2 to 80 pg/ml after 24h (Figure 1F).
Fig. 1.
Expression of PRL and its cognate receptor in colorectal cancers. (A) Real-time PCR to evaluate expression of PRLR in colon tumors. Increased expression of PRLR is noted in tumor samples compared with adjacent controls. (B) Real-time PCR for PRL colon tumors. Data suggest no change in the expression of PRL between tumor samples and adjacent controls. (C) Real-time PCR for PRLR in colon cancer cell lines compared with normal (FHC cells). Increased PRLR expression is observed in all CRC cell lines. (D) Western blot analysis. There were higher levels of PRLR in CRC cells compared with FHC cells. (E) Real-time PCR for PRL in normal and cancer cell lines. PRL mRNA is present in all cell lines. (F) Enzyme-linked immunosorbent assay base quantification of PRL. Media collected from the cells at 12 and 24 show that FHC cells and colon cancer cells can secrete PRL; however, the amount secreted varies from 20 to 80 pg/ml in a cell-line-specific manner.
PRL treatment induces STAT3 and ERK1/2 phosphorylation
Upregulation of PRLR, particularly in CRC, compared with normal colonic cells, suggests a role for the pathway in the pathogenesis of colorectal cancer. Binding of PRL to PRLR is known to activate JAK2-STAT and JAK2-ERK1/2 pathways (7–9). The ERK/mitogen-activated protein kinase pathway is also known to be highly active in patients with Familial Adenomatous Polyposis (53). Cells were treated with recombinant PRL and western blot analyses were performed for Jak, STAT and ERK proteins. An increase in JAK2, STAT3 and ERK1/2 phosphorylation was observed within a minute of PRL treatment (Figure 2A and B). To validate PRL-mediated STAT3 activation, we transfected HCT116 and HT29 cells with 4XM67 pTK-Luc plasmid, which encodes the firefly luciferase, under the control of a minimal promoter and four tandem STAT3 binding sites (M67 sites). This construct has been previously used to demonstrate STAT3 induced gene expression (51). PRL treatment increased luciferase activity in both cell lines in a dose- and time-dependent manner (Figure 2C). Even at 100ng/ml of PRL, there was significant increase in luciferase activity as early as 6h. However, at 1 µg/ml dose, PRL-mediated induction in luciferase activity was observed even at 1 h. Pre-treating the cells with AG490, a pharmacological inhibitor of JAK2, prior to PRL treatment leads to a decrease in STAT3 and ERK1/2 phosphorylation (Fig 2D and E), even in the presence of PRL. However, pre-treatment with PD98059 alone led to increased STAT3 activation (Figure 2D). Moreover, cells treated with the combination of JAK2 and ERK1/2 inhibitors, AG490 and PD98059, resulted in complete inhibition of JAK2, ERK1/2 and STAT3 phosphorylation (Figure 2D and E). Together, the data suggest that PRL induces JAK2, ERK1/2 and STAT3 phosphorylation in colon cancer cells. Given that STAT5 phosphorylation has shown to be significantly upregulated in breast cancer cells in response to PRL (7), we also determined the level of STAT5 phosphorylation in the colon cancer cells. However, levels of total STAT5 protein were relatively low in colon cancer cells, and no significant changes in phosphorylation of the protein were observed (data not shown), suggesting that STAT3 may be a key player in PRL/PRLR signaling in colon cancer cells.
Fig. 2.
Prolactin induces JAK2, STAT3 and ERK1/2 phosphorylation. (A) Cells were treated with 500ng/ml PRL and lysates were collected at regular intervals and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis. PRL treatment increased phosphorylation of JAK2, STAT3 and ERK1/2, starting at 1min post treatment and lasting for 30min. (B) To validate STAT3 activation, a STAT3 responsive luciferase plasmid was transfected into cells and the luciferase activity analyzed after PRL treatment. A dose and time dependent increase in luciferase activity was observed following PRL treatment compared with untreated controls (*P < 0.05). (C) JAK2 and STAT3 phosphorylation is inhibited by specific inhibitors AG490 (JAK2-specific) and PD98059 (Mitogen-activated protein kinase specific) treatment. Treatment with PRL rescues the inhibition. However, PRL cannot rescue rescues JAK2 or STAT3 phosphorylation when the combination of the two inhibitors are used. (D) Cells treated with PD98059 had decreased whereas AG490 enhanced ERK1/2 phosphorylation when treated alone. Addition of PRL did not affect ERK1/2 phosphorylation in the presence of either inhibitor.
PRL induced spheroid formation and is inhibited by JAK2 and ERK inhibitors
Previous studies have demonstrated mitogenic activity for PRL in breast cancers (5). Accordingly, we determined whether PRL affects proliferation of colon cancer cells. However, PRL did not have any effect on proliferation of various colon cancer cells (data not shown). Further confirmation of this was obtained when cells were subjected to cell cycle analyses by flow cytometry following propidium iodide staining. Again, there was no difference in cell cycle progression between PRL-treated cells and control cells (data not shown). These data, taken together, suggest that PRL does not affect cell growth.
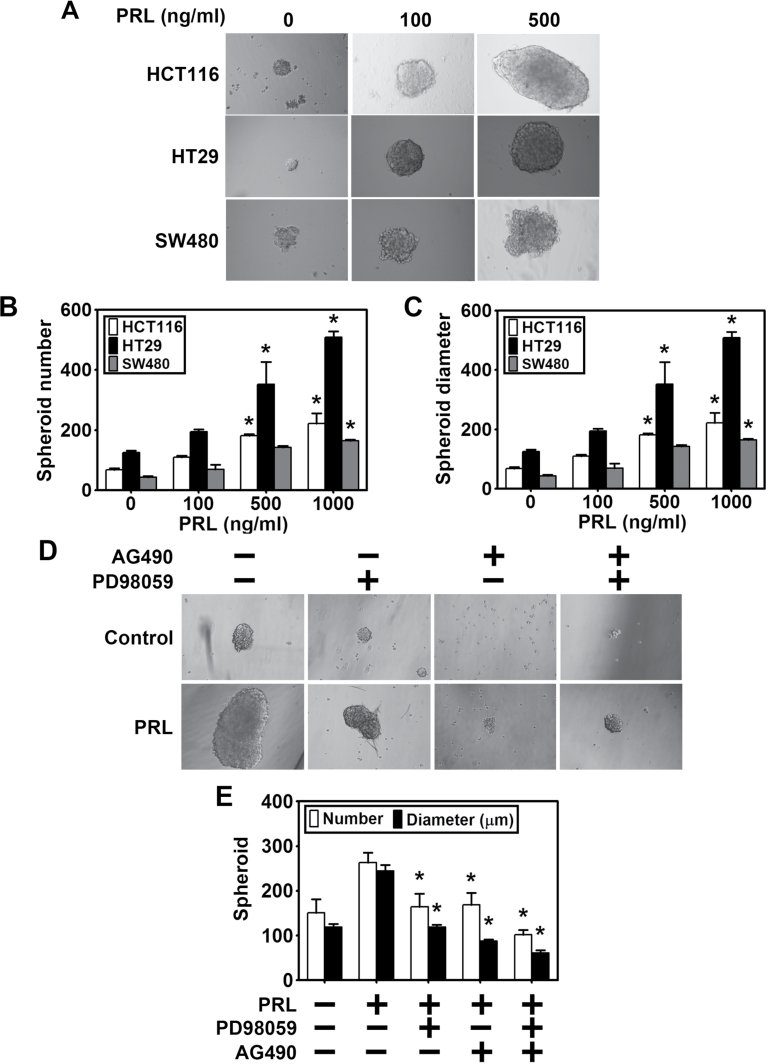
We next determined whether PRL affects spheroid formation because previous studies have demonstrated that PRL stimulates neurosphere formation when hippocampal cells are treated with PRL (40). Additionally, spheroid formation remains the best available functional assay to assess the presence of CSCs in a given cancer cell pool (54,55). We treated colon cancer cells HCT116, SW480 and HT29 with increasing concentrations of PRL (0–500ng/ml) and allowed the colospheres to form. There was a dose-dependent increase in colosphere formation (Figure 3A), with a significant increase in both number (Figure 3B) and diameter of spheroid (Figure 3C) in all the three cell lines. We also determined the effect of inhibiting JAK-STAT and ERK1/2 signaling with the two inhibitors AG490 and PD98059. Pre-incubation with the inhibitors AG490 and PD 98059, either alone or in combination, abolished colospheres formation (Figure 3D and E). Moreover, the inhibitors affected colosphere formation in the presence of PRL. There were also less number of colospheres and the size of the spheres was smaller compared with cells treated with PRL alone. These results suggest that PRL signaling can potentially regulate colosphere formation.
Fig. 3.
PRL affects colosphere formation. (A) Colon cancer cells were grown in specific spheroid media in ultra-low binding plates and treated with increasing dose of PRL. After 5 days, the colonospheres were photographed and counted. (B) A dose-dependent increase in spheroid number was observed, with higher significance at 500 and 1000ng/ml of PRL (*P < 0.05). (C) Similar increase in diameter was noted at similar doses (*P < 0.05). (D and E) Pre-treatment with AG490 and PD98059 alone or in combination leads to significant decrease in spheroid formation and number compared with PRL treatment alone (*P < 0.05).
PRL induces expression of colon CSC marker genes
Since PRL stimulated colosphere formation, a marker for stem-cell-dependent growth, we next determined whether PRL affects stem cells. Stem cells were quantified using real-time PCR and western blot of specific markers, including DCLK1 (29–31), LGR5 (32–35), CD44 (36), CD133 (37) and ALDH1A1 (38). Real time PCR analysis demonstrated that PRL treatment induced expression of DCLK1, LGR5 and ALDH1 (Figure 4A–C). Further confirmation was obtained by western blot analyses, which showed that all three proteins along with CD44 and c-Myc, an oncogene that plays a predominant role in stemness (56), are upregulated compared with untreated controls (Figure 4D). This further demonstrates that biological relevance of STAT3 activation by PRL. We also determined whether signaling through the JAK-STAT and ERK1/2 pathways affects stem cell marker expression. Pre-treatment with AG490 or PD98059 alone caused a decrease in expression of DCLK1 and LGR5, which were partially rescued upon PRL treatment (Figure 4E). These data further suggest that PRL signaling can modulate the expression of colon CSC maker protein expression.
Fig. 4.
PRL induces stem cell marker protein expression. Colon cancer cells were treated with 500ng/ml PRL for various time points up to 12h. Total RNA and cell lysates were generated for real time PCR and western blot analyses. Real-time PCR shows increased levels of (A) DCLK1 (B) LGR5 and (C) ALDH1A1 mRNA after PRL treatment (*P < 0.05). (D) Lysates from these cells indicate significant increase in expression of DCLK1, LGR5, ALDH1A1, CD44 and c-MYC (MYC) in the presence of PRL. (E) Cells treated with either AG490 or PD98059 had decreased DCLK1 and LGR5 induction compared with PRL treatment. PRL was able to rescue this activation; however, the combination of both AG490 and PD98059 leads to complete abrogation of DCLK1 and LGR5 expression.
PRL affects CSCs and progenitor cells by inducing Notch signaling
Notch signaling plays a significant role in stem cells and is a pathway active in colon CSCs (42,44). Phosphorylated ERK1/2 induces JAG1, a Notch receptor ligand (49). Binding of JAG1 to the Notch receptor causes a conformational change and sequential cleavage by ADAM and γ-secretase complex proteins to release the Notch intracellular domain (NICD), which translocates into the nucleus and activates the expression of target genes (57). We examined whether PRL treatment affects Notch signaling in colon cancer cells by modulating JAG1 expression. Quantitative real-time PCR analyses demonstrated increased expression of JAG1 and the Notch signaling target gene HEY1 (Figure 5A and B). Western blot analyses further confirmed the upregulation of JAG1 and HEY1 (Figure 5C). In addition, there was an increase in NICD protein levels, along with increased levels of γ-secretase complex protein anterior pharynx defective 1, presenilin 1 (PSEN1) and presenilin enhancer (Figure 5C). To further confirm that Notch signaling is activated upon PRL treatment, we transfected HCT116 and HT29 cells with a plasmid encoding the luciferase reporter gene under the control of the Hes-1 promoter. Following 500ng/ml PRL treatment, a robust induction in luciferase activity was observed in both the cells (Figure 5D).
Fig. 5.
PRL treatment activates Notch signaling. (A) Real time PCR analysis on cells treated with PRL show a time-dependent increase in expression of JAG1 (*P < 0.05). (B) Similar increase in expression of Notch target gene HEY1 was also observed in real-time PCR analysis (*P < 0.05). (C) Lysates of PRL-treated cells show increased JAG1 and HEY1 expression, NICD accumulation and induction of γ-secretase complex proteins anterior pharynx defective 1, PSEN1 and presenilin enhancer expression compared with controls. (D) Colon cancer cells, transfected with HES1 responsive luciferase plasmid, showed a PRL dependent induction of luciferase activity. (E) AG490 and PD98059 pre-treatment caused a decrease in NICD accumulation and JAG1, HEY1, HES1 and PSEN1 expression compared with PRL treatment. PRL was able to rescue this activation; however, combination of both AG490 and PD98059 leads to complete abrogation of NICD accumulation and HEY1, HES1 and PSEN1 expression to levels similar to control.
These results were also confirmed using the specific JAK2 and ERK inhibitors. Inhibiting either JAK2 or ERK1/2 signaling alone using AG490 or PD98059 showed decreased JAG1 expression, NICD cleavage and expression of HEY1, HES1 and PSEN1 genes (Figure 5E). This was partially rescued by PRL. Combined inhibition of both the inhibitors leads to a further reduction in JAG1 expression, Notch-1 cleavage (NICD) and expression of HEY1, HES1 and PSEN1 (Figure 5E) even in the presence of PRL, suggesting that PRL can regulate Notch signaling through either JAK2-STAT3 or JAK2-ERK1/2 pathway.
Notch signaling is necessary to mediate PRL-induced changes
Based on the above findings, we hypothesized that PRL induces JAK2-STAT3 and JAK2-ERK1/2 cascades that in turn activate JAG1-mediated Notch signaling. To evaluate this, we overexpressed NICD in the three colon cancer cell lines. NICD overexpression significantly induced colosphere formation, similar to that observed when cells were treated with PRL (Figure 6A). There was an increase in the number and size of the spheroids (Figure 6B and C). Furthermore, treatment with the inhibitors alone did not affect the number or size of spheroids in the presence of NICD overexpression. However, a small decrease was observed in secondary spheroids when treated with the combination of the two inhibitors (Figure 6D). Similarly, protein levels of DCLK1, LGR5 or CD44 increased in NICD overexpressing cells, to levels comparable to PRL-treated cells (Figure 6E). The two inhibitors, either alone or in combination, did not affect the expression of stem cell markers in the NICD overexpressing cells, suggesting further that PRL-induced activation of Notch signaling is sufficient to enhance stem cell activity.
Fig. 6.
NICD overexpression recapitulates PRL-induced changes in colosphere formation and stem cell marker protein expression. (A) Colon cancer cells were transfected with NICD overexpressing plasmid, grown in specific spheroid media in low adherent plates for 5 days in the presence of PRL and inhibitors. Neither AG490 nor PD98059 treatment alone or in combination cause any significant decrease in colosphere formation in NICD expressing cells. (B) Significant increase in spheroid number is observed in NICD expressing cells compared with untreated controls. The inhibitors did not affect the NICD overexpressing cells. (*P < 0.005). (C) Increased colosphere diameter was also observed in NICD overexpressing cells compared with control (*P < 0.05). (D) Secondary spheroids. The primary spheroids were collected, trypsinised and replated without PRL or the inhibitors. PRL primed and NICD expressing cells treated with either inhibitor maintained high colosphere formation compared with control. Combination of the inhibitors had comparable decrease in spheroid number compared with PRL-treated or NICD-expressing cells. (E) Lysates from NICD overexpressing cells either alone or treated with the inhibitors alone or in combination had increased expression of CSC markers DCLK1, LGR5 and CD44. However, expression of PSEN1 and presenilin enhancer was not affected in NICD expressing cells, compared with PRL-treated cells.
Discussion
PRL, a peptide hormone produced by the lactotroph cells of the anterior pituitary and other non-pituitary tissues. In lactating rats, PRL is known to modulate water and electrolyte transport in the intestine (58). It can also stimulate proliferation of the mucosal cells of the gastrointestinal tract during pregnancy (59). PRL has a mitogenic role in mammary (6), lung (60), bladder (61), prostate and ovarian (62) tumorigenesis. Elevated serum levels of PRL has been shown to be associated with increased incidence of mammary(63,64) and prostate(19) cancers. High serum levels of PRL (27) and PRLR expression (65) were also observed in CRC patients. Our findings also implicate an increase in PRLR transcript in CRC samples compared with adjacent normal tissue. Similarly, CRC cell lines had increased PRLR levels compared with normal FHC cells. In line with earlier observation (65), SW480 cells expressed relatively lower PRLR levels compared with other cells. No significant change in mRNA expression or robust secretion of PRL into the media was noted in CRC cell lines compared with FHC. These findings clearly show a preferential upregulation of PRLR in CRC cells suggesting active role for PRL–PRLR signaling in colorectal tumors.
Binding of PRL to PRL receptor (PRLR) activates JAK2/ STAT and/ or RAS-RAF-ERK1/2 pathway (66,67). In fact, we observed a rapid and robust increase in STAT3 phosphorylation. We also observed an increase in STAT3 phosphorylation when treated with PD98059; a similar finding was reported earlier (68). In their studies, the authors demonstrated that treating melanoma cell line LU1205 with only PD98059 induces robust phosphorylation of STAT3 and STAT5 (68). Previous studies in breast cancers have also demonstrated STAT5 activation, and not STAT3 activation, in the presence of PRL. More importantly, in breast cancers, it was determined that STAT5 and STAT3 mediate opposing effects on several key target genes such as BCL6, with STAT5 exerting a dominant role. When both STAT3 and STAT5 are activated at the same time, there is, in fact, a reduction in the proliferation of the breast cancer cells. Moreover, there was an increased sensitivity to chemotherapeutic drugs (69,70). In our studies, we observed that PRL did not affect the proliferation of CRC cells. Given our observation that STAT3 is predominantly activated in CRC following PRL treatment, suggests a differential activation of intracellular signaling modules by PRL, which may depend on the type of cancer. It would be interesting to see if overexpressing STAT5 in CRC and subsequent treatment with PRL would lead to an increase in chemotherapy-induced cell death, as observed in breast cancer cells.
Spheroid formation assay helps us determine the presence of cancer-initiating cells in a cancer cell population (54,55). Our results show a dose-dependent increase in spheroid formation, number and diameter in CRC cells following PRL treatment. This is in agreement with previous studies with prostate cancers, where PRL expression in mouse prostate led to increase in stem cell/basal cell population (17,41). Similarly, neurosphere formation along with the expansion of hippocampal precursor cell population (40) has been observed in a PRL-dependent manner. We also observed increased expression of DCLK1, LGR5, ALDH1 and CD44. However, there were differences seen in the stemness based on cell lines. HT29 cells expressed only moderately higher levels of the marker proteins compared with HCT116. This is also in line with studies on PRL effects on neural stem cells. It would be interesting to examine whether expression of stem-cell-related proteins is affected in neural stem cells and whether this expression is affected in brain tumors.
PRL also induced Notch signaling via the JAK2-ERK1/2 pathway by inducing JAG1 expression, leading to NICD accumulation along with the increase in expression of Notch target genes. This is of high significance because, clinically, increased ERK1/2 activation was noted in patients with familial adenomatous polyposis (53). Moreover, previous studies have also demonstrated that ERK1/2 can modulate Notch signaling by regulating the expression of its ligand JAG1 (49). Notch signaling is active in intestinal crypts (43) and helps regulate stem cell hierarchy and determine cell fate (42). Deregulation in this pathway can lead in colorectal cancer (45,71). It would be interesting to determine whether PRL upregulation is essential for the tumorigenesis process.
Based on our observation, we put forward a model where the presence of PRL in the tumor microenvironment of CRC cells would activate JAK2 after binding to PRLR, which would in turn induce ERK1/2 phosphorylation. Activated ERK1/2 would induce JAG1 expression in the cells, which would translocate to the cell membrane. Binding of JAG1 extracellular domain to the single-pass transmembrane Notch-1 receptor would lead to intracellular conformational changes and cleavage by the γ-secretase complex proteins, leading to separation of the NICD from the transmembrane domain. The cleaved NICD would then translocate to the nucleus and complex with mastermind-like and recombining binding protein suppressor of hairless to induce respective gene expression (Figure 7). Our findings further indicate an increase in expression of PRLR but not PRL in CRC cells. However, previous studies have demonstrated increased levels of PRL in the blood stream of patients (23,24). This suggests that PRL expression is induced at other sites. This is also different from what has been observed in breast cancers, where the cancer tissue itself induces PRL expression (6,17). It would be interesting to determine how and where PRL expression is induced in colon tumorigenesis. In this regard, it should be noted that PRL is believed to be a hormone whose expression is responsive to stress. PRL does increase in response to psychosocial stress, although women may have higher magnitude of increase than men, and this might be dependent on estradiol levels (62). Moreover, dietary fat was shown to induce circulating PRL under conditions of ether stress, and dietary fat can also affect tumorigenesis (63). It would be interesting to determine whether these conditions affect PRLR expression and PRL-mediated signaling—a future direction of our research program.
Fig. 7.
Working model. PRL, present in the tumor microenvironment, would bind to PRLR and induce Jak2-ERK1/2 phosphorylation. This activates ERK1/2 and STAT3 proteins, which in turn would induce expression of Notch ligand, JAG1. JAG1 would translocate to the cell membrane and bind to the transmembrane Notch receptor in the neighboring cell. This binding would induce a conformational change in the receptor leading to sequential cleavages by various enzymes including the γ-secretase complex. This results in the release of the NICD that then translocates into the nucleus, complexes with mastermind-like and recombining binding protein suppressor of hairless to activate target gene expression.
Funding
National Institutes of Health (CA109269, CA135559); National Cancer Institute Cancer Center (CA168524 to The Biospecimen Repository of the University of Kansas Cancer Center).
Acknowledgements
We thank Paul Terranova, Ph.D. and John Wood, Ph.D. for providing their insights during the course of these studies. We thank Anant lab members for their help and advice during the course of this work and manuscript preparation. S. Anant is an Eminent Scientist of the Kansas Biosciences Authority.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- CRC
colorectal cancer
- CSC
cancer stem cells
- DCLK1
doublecortin like kinase 1
- ERK1/2
extracellular signal regulated kinase 1/2
- JAG1
jagged 1
- Jak2
janus kinase 2
- LGR5
leucine-rich repeat-containing G-protein coupled receptor 5
- NICD
Notch intracellular domain
- PRL
prolactin
- PRLR
prolactin receptor.
References
- 1. Siegel R., et al. (2013). Cancer statistics, 2013. CA. Cancer J. Clin., 63, 11–30 [DOI] [PubMed] [Google Scholar]
- 2. Majek O., et al. ; GEKID Cancer Survival Working Group (2013). Sex differences in colorectal cancer survival: population-based analysis of 164,996 colorectal cancer patients in Germany. PLoS One, 8, e68077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson T.R., et al. (1984). Prolactin’s mitogenic action on the pigeon crop-sac mucosal epithelium involves direct and indirect mechanisms. Gen. Comp. Endocrinol., 54, 236–246 [DOI] [PubMed] [Google Scholar]
- 4. Charles V., et al. (2003). The role of prolactin in mammary carcinoma. Endocr. Rev., 24,1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Das R., et al. (1997). Prolactin as a mitogen in mammary cells. J. Mammary Gland Biol. Neoplasia, 2, 29–39 [DOI] [PubMed] [Google Scholar]
- 6. Ginsburg E., et al. (1995). Prolactin synthesis and secretion by human breast cancer cells. Cancer Res., 55, 2591–2595 [PubMed] [Google Scholar]
- 7. Dagvadorj A., et al. (2007). Autocrine prolactin promotes prostate cancer cell growth via Janus kinase-2-signal transducer and activator of transcription-5a/b signaling pathway. Endocrinology, 148, 3089–3101 [DOI] [PubMed] [Google Scholar]
- 8. Cataldo L., et al. (2000). Inhibition of oncogene STAT3 phosphorylation by a prolactin antagonist, hPRL-G129R, in T-47D human breast cancer cells. Int. J. Oncol., 17, 1179–1185 [DOI] [PubMed] [Google Scholar]
- 9. Gubbay O., et al. (2002). Prolactin induces ERK phosphorylation in epithelial and CD56(+) natural killer cells of the human endometrium. J. Clin. Endocrinol. Metab., 87, 2329–2335 [DOI] [PubMed] [Google Scholar]
- 10. Maus M.V., et al. (1999). Prolactin as a chemoattractant for human breast carcinoma. Endocrinology, 140, 5447–5450 [DOI] [PubMed] [Google Scholar]
- 11. Perks C.M., et al. (2004). Prolactin acts as a potent survival factor for human breast cancer cell lines. Br. J. Cancer, 91, 305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buckley A.R., et al. (1985). Prolactin is a tumor promoter in rat liver. Life Sci., 37, 2569–2575 [DOI] [PubMed] [Google Scholar]
- 13. Wang W.W., et al. (2013). Identification of serum monocyte chemoattractant protein-1 and prolactin as potential tumor markers in hepatocellular carcinoma. PLoS One, 8, e68904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clendenen T.V., et al. (2013). Circulating prolactin levels and risk of epithelial ovarian cancer. Cancer Causes Control, 24, 741–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levina V.V., et al. (2009). Biological significance of prolactin in gynecologic cancers. Cancer Res., 69, 5226–5233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mendoza-Romo M.A., et al. (2010). The role of prolactin in prostate cancer. Rev. Mex. Urol., 70, 55–60 [Google Scholar]
- 17. Rouet V., et al. (2010). Local prolactin is a target to prevent expansion of basal/stem cells in prostate tumors. Proc. Natl. Acad. Sci. U. S. A., 107, 15199–15204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruffion A., et al. (2003). The survival effect of prolactin on PC3 prostate cancer cells. Eur. Urol., 43, 301–308 [DOI] [PubMed] [Google Scholar]
- 19. Van Coppenolle F., et al. (2001). Effects of hyperprolactinemia on rat prostate growth: evidence of androgeno-dependence. Am. J. Physiol. Endocrinol. Metab., 280, E120–E129 [DOI] [PubMed] [Google Scholar]
- 20. Nagano M., et al. (1995). Expression of prolactin and growth hormone receptor genes and their isoforms in the gastrointestinal tract. Am. J. Physiol., 268(3 Pt 1), G431–G442 [DOI] [PubMed] [Google Scholar]
- 21. Deachapunya C., et al. (2012). Site-specific regulation of ion transport by prolactin in rat colon epithelium. Am. J. Physiol. Gastrointest. Liver Physiol., 302, G1199–G1206 [DOI] [PubMed] [Google Scholar]
- 22. Teerapornpuntakit J., et al. (2012). Proliferation and mRNA expression of absorptive villous cell markers and mineral transporters in prolactin-exposed IEC-6 intestinal crypt cells. Cell Biochem. Funct., 30, 320–327 [DOI] [PubMed] [Google Scholar]
- 23. Bhatavdekar J.M., et al. (1992). Comparison of plasma prolactin and CEA in monitoring patients with adenocarcinoma of colon and rectum. Br. J. Cancer, 66, 977–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhatavdekar J.M., et al. (2001). Ectopic production of prolactin by colorectal adenocarcinoma. Dis. Colon Rectum, 44, 119–127 [DOI] [PubMed] [Google Scholar]
- 25. Soroush A.R., et al. (2004). Plasma prolactin in patients with colorectal cancer. BMC Cancer, 4, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ilan Y., et al. (1995). Plasma and tumor prolactin in colorectal cancer patients. Dig. Dis. Sci., 40, 2010–2015 [DOI] [PubMed] [Google Scholar]
- 27. Otte J.M., et al. (2003). Expression of functional prolactin and its receptor in human colorectal cancer. Int. J. Colorectal Dis., 18, 86–94 [DOI] [PubMed] [Google Scholar]
- 28. Bonnet D., et al. (1997). Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med., 3, 730–737 [DOI] [PubMed] [Google Scholar]
- 29. Nakanishi Y., et al. (2013). Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat. Genet., 45, 98–103 [DOI] [PubMed] [Google Scholar]
- 30. Gagliardi G., et al. (2012). Immunolocalization of DCAMKL-1, a putative intestinal stem cell marker, in normal colonic tissue. Pathol. Res. Pract., 208, 475–479 [DOI] [PubMed] [Google Scholar]
- 31. May R., et al. (2009). Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells, 27, 2571–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barker N., et al. (2012). Lgr5(+ve) stem/progenitor cells contribute to nephron formation during kidney development. Cell Rep., 2, 540–552 [DOI] [PubMed] [Google Scholar]
- 33. Barker N., et al. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature, 449, 1003–1007 [DOI] [PubMed] [Google Scholar]
- 34. Muñoz J., et al. (2012). The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. EMBO J., 31, 3079–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sato T., et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature, 459, 262–265 [DOI] [PubMed] [Google Scholar]
- 36. Chaffer C.L., et al. (2011). Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc. Natl. Acad. Sci. U. S. A., 108, 7950–7955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Montgomery R.K., et al. (2008). Small intestinal stem cell markers. J. Anat., 213, 52–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Januchowski R., et al. (2013). The role of aldehyde dehydrogenase (ALDH) in cancer drug resistance. Biomed. Pharmacother., 67, 669–680 [DOI] [PubMed] [Google Scholar]
- 39. Kvinlaug B.T., et al. (2007). Targeting cancer stem cells. Expert Opin. Ther. Targets, 11, 915–927 [DOI] [PubMed] [Google Scholar]
- 40. Walker T.L., et al. (2012). Prolactin stimulates precursor cells in the adult mouse hippocampus. PLoS One, 7, e44371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dillner K., et al. (2003). Gene expression analysis of prostate hyperplasia in mice overexpressing the prolactin gene specifically in the prostate. Endocrinology, 144, 4955–4966 [DOI] [PubMed] [Google Scholar]
- 42. Kopan R., et al. (1996). The Notch pathway: democracy and aristocracy in the selection of cell fate. Curr. Opin. Neurobiol., 6, 594–601 [DOI] [PubMed] [Google Scholar]
- 43. Sander G.R., et al. (2004). Expression of notch receptors and ligands in the adult gut. J. Histochem. Cytochem., 52, 509–516 [DOI] [PubMed] [Google Scholar]
- 44. Fre S., et al. (2005). Notch signals control the fate of immature progenitor cells in the intestine. Nature, 435, 964–968 [DOI] [PubMed] [Google Scholar]
- 45. Nickoloff B.J., et al. (2003). Notch signaling as a therapeutic target in cancer: a new approach to the development of cell fate modifying agents. Oncogene, 22, 6598–6608 [DOI] [PubMed] [Google Scholar]
- 46. Schroeter E.H., et al. (1998). Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature, 393, 382–386 [DOI] [PubMed] [Google Scholar]
- 47. De Strooper B., et al. (1999). A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature, 398, 518–522 [DOI] [PubMed] [Google Scholar]
- 48. Lai E.C. (2004). Notch signaling: control of cell communication and cell fate. Development, 131, 965–973 [DOI] [PubMed] [Google Scholar]
- 49. Goh F., et al. (2009). Selective induction of the Notch ligand Jagged-1 in macrophages by soluble egg antigen from Schistosoma mansoni involves ERK signalling. Immunology, 127, 326–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Subramaniam D., et al. (2012). Curcumin induces cell death in esophageal cancer cells through modulating Notch signaling. PLoS One, 7, e30590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Besser D., et al. (1999). A single amino acid substitution in the v-Eyk intracellular domain results in activation of Stat3 and enhances cellular transformation. Mol. Cell. Biol., 19, 1401–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Curry C.L., et al. (2005). Gamma secretase inhibitor blocks Notch activation and induces apoptosis in Kaposi’s sarcoma tumor cells. Oncogene, 24, 6333–6344 [DOI] [PubMed] [Google Scholar]
- 53. Wang J., et al. (2012). Expression of EGFR, HER2, phosphorylated ERK and phosphorylated MEK in colonic neoplasms of familial adenomatous polyposis patients. J. Gastrointest. Cancer, 43, 444–455 [DOI] [PubMed] [Google Scholar]
- 54. Singh S.K., et al. (2003). Identification of a cancer stem cell in human brain tumors. Cancer Res., 63, 5821–5828 [PubMed] [Google Scholar]
- 55. Liu J., et al. (2013). Spheroid body-forming cells in the human gastric cancer cell line MKN-45 possess cancer stem cell properties. Int. J. Oncol., 42, 453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nie Z., et al. (2012). c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell, 151, 68–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fortini M.E. (2009). Notch signaling: the core pathway and its posttranslational regulation. Dev. Cell, 16, 633–647 [DOI] [PubMed] [Google Scholar]
- 58. Dusanter-Fourt I., et al. (1992). Expression of prolactin (PRL) receptor gene and PRL-binding sites in rabbit intestinal epithelial cells. Endocrinology, 130, 2877–2882 [DOI] [PubMed] [Google Scholar]
- 59. Kagnoff M.F. (1993). Immunology of the intestinal tract. Gastroenterology, 105, 1275–1280 [DOI] [PubMed] [Google Scholar]
- 60. Ozarda A.T. (1983). Prolactin-secreting tumors. J. Surg. Oncol., 22, 9–10 [DOI] [PubMed] [Google Scholar]
- 61. Stanisic T.H., et al. (1986). Prolactin secreting renal cell carcinoma. J. Urol., 136, 85–86 [DOI] [PubMed] [Google Scholar]
- 62. Hoffmann W.H., et al. (1987). Ectopic prolactin production from gonadoblastoma. Cancer., 60, 2690–2695 [DOI] [PubMed] [Google Scholar]
- 63. MUHLBOCK O., et al. (1959). Induction of mammary cancer in mice without the mammary tumor agent by isografts of hypophyses. Cancer Res., 19, 402–412 [PubMed] [Google Scholar]
- 64. Welsch C.W., et al. (1977). Prolactin and murine mammary tumorigenesis: a review. Cancer Res., 37, 951–963 [PubMed] [Google Scholar]
- 65. Harbaum L., et al. (2010). Clinicopathological significance of prolactin receptor expression in colorectal carcinoma and corresponding metastases. Mod. Pathol., 23, 961–971 [DOI] [PubMed] [Google Scholar]
- 66. Bole-Feysot C., et al. (1998). Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev., 19, 225–268 [DOI] [PubMed] [Google Scholar]
- 67. Yu-Lee L.Y., et al. (1998). Lactogenic hormone signal transduction. Biol. Reprod., 58, 295–301 [DOI] [PubMed] [Google Scholar]
- 68. Krasilnikov M., et al. (2003). ERK and PI3K negatively regulate STAT-transcriptional activities in human melanoma cells: implications towards sensitization to apoptosis. Oncogene, 22, 4092–4101 [DOI] [PubMed] [Google Scholar]
- 69. Sarah R., et al. (2009). Reciprocal effects of STAT5 and STAT3 in breast cancer. Mol. Cancer Res., 7, 966–976 [DOI] [PubMed] [Google Scholar]
- 70. Sarah R., et al. (2013). STAT5 Outcompetes STAT3 to regulate the expression of the oncogenic transcriptional modulator BCL6. Mol. Cell Biol., 33, 2879–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sureban S.M., et al. (2008). Knockdown of RNA binding protein musashi-1 leads to tumor regression in vivo . Gastroenterology, 134, 1448–1458 [DOI] [PubMed] [Google Scholar]