Abstract
WNT5A has been identified as an important ligand in the malignant progression of a number of tumours. Although WNT5A signalling is often altered in cancer, the ligand’s role as either a tumour suppressor or oncogene varies between tumour types and is a contemporary issue for investigators of β-catenin-independent WNT signalling in oncology. Here, we report that one of the initial effects of active WNT5A signalling in malignant melanoma cells is an alteration in cellular energy metabolism and specifically an increase in aerobic glycolysis. This was found to be at least in part due to an increase in active Akt signalling and lactate dehydrogenase (LDH) activity. The clinical relevance of these findings was strengthened by a strong correlation (P < 0.001) between the expression of WNT5A and LDH isoform V in a cohort of melanocytic neoplasms. We also found effects of WNT5A on energy metabolism in breast cancer cells, but rather than promoting aerobic glycolysis as it does in melanoma, WNT5A signalling increased oxidative phosphorylation rates in breast cancer cells. These findings support a new role for WNT5A in the metabolic reprogramming of cancer cells that is a context- dependent event.
Introduction
It has been known for over 30 years that aberrant intracellular signalling mediated by the WNT family of secreted glycoproteins leads to tumour progression (1). Initially, WNT signalling was found to stabilize free pools of cytoplasmic β-catenin, leading to changes in gene transcription (2), but it is now realized that WNT proteins also signal via β-catenin-independent pathways as well, although complex interplay between the two exists. The archetypal WNT-β-catenin-independent signalling ligand is WNT5A, which is known to have both tumour-promoting and tumour-suppressive roles in cancer (3). For example, lower expression of WNT5A in breast cancer patients correlates with increased risk of death and aggressive disease (4,5), whereas in melanoma, the opposite is true and high WNT5A expression correlates with poor patient prognosis (6). Complexity of the WNT5A ligand’s role in cancer has previously been reviewed (3). WNT ligands that signal in a β-catenin-dependent manner result in the inactivation of a β-catenin degradation complex, leading to an increase in a cytosolic pool of β-catenin. Stabilization of β-catenin coincides with its nuclear translocation, where it acts as a transcriptional co-activator of T-cell factor (TCF)/lymphoid-enhanced binding factor (LEF)-responsive promoters. Overall, cross-talk between WNT and other pathways results in highly context-dependent cellular responses in tumour cells.
Cancer cells undergo metabolic reprogramming as one of their hallmark behavioural changes during the tumorigenic process (7). A common reprogramming mechanism is that of switching the mitochondrial tricarboxylic acid cycle away from ATP synthesis and towards the synthesis of lipids, proteins and nucleic acid precursors that serve the increased synthetic demands of tumour cells (8). This is associated with increased glucose-dependent production of lactic acid by cancer cells relative to normal cells in the process of aerobic glycolysis, which has been known for over five decades (9). Lactate dehydrogenase (LDH) is the critical enzyme for lactate production in cells as it controls the inter-conversion of lactate and pyruvate compounds. Specifically, there are five LDH isoforms (LDH I–V), where isoforms IV and V are predominantly involved in the production of lactate from pyruvate (10). All isoforms are generated from two gene products that encode M and H protein subunits encoded by the LDHA and LDHB genes, respectively. In addition to enhanced aerobic glycolysis, other atypical metabolic profiles of cancer cells include enhanced fatty acid synthesis and increased glutamine metabolism (8). Identification of the signalling mechanisms that control metabolic reprogramming in cancer cells has been an intensely investigated area of research in recent years and a number of pathways have been identified as regulators, which include key oncogenic signalling molecules such as Myc and Akt (8).
For a number of years now, the WNT-β-catenin-dependent signalling pathway has been linked to the control of cellular metabolism (11). For example, in hepatocytes, activation of β-catenin signalling results in the up-regulation of genes involved in glutamine metabolism (12), and a large number of metabolism genes contain TCF/LEF response elements within their promoter regions (13). Furthermore, WNT3A (an archetypal WNT-β-catenin-dependent signalling ligand) increases oxygen consumption and mitochondrial gene expression in adipocytes (14) and fibroblasts (15). Indeed in the C2C12 murine muscle cell line, WNT3A-β-catenin signalling enhanced mitochondrial proliferation, mediated at least in part through enhanced Myc expression leading to increased mitochondrial biogenesis (15). Taken together, these findings suggest that the WNT-β-catenin-dependent signalling pathway is a key regulator of cellular bioenergetics. However, it is currently unknown if WNT-β-catenin-independent signalling can also control cell metabolism.
Cutaneous melanoma is a malignancy of the pigment producing melanocytes in the skin. Alarmingly, incidence rates have been rising faster than any other cancer and have been steadily increasing for over 40 years (16). Despite a number of novel treatments developed in recent years that provide improved survival rates in patients (17–19), the success of these treatments is ultimately limited by the development of drug-resistant tumours and patient-restricted response to the drugs. There is, therefore, a distinct need for the discovery of novel anti-melanoma treatments that target a number of cancer hallmarks. By understanding key signalling pathways that are essential for melanoma cell survival, it is hoped that novel melanoma treatments can be found in the future.
In melanoma, WNT5A signals through the G protein-coupled receptor, Frizzled (FZD)-5 (20), to elicit a β-catenin-independent WNT-Ca2 + signalling pathway (21), but it also signals via the receptor tyrosine kinase, Ror-2, to mediate lamellipodia formation, increase cell migration and cell invasion and promote metastasis (21–23). Indeed, WNT5A-mediated Ror-2 signalling results in enhanced Src kinase activity in melanoma cells, which is essential for WNT5A-mediated cell migration (22). In a recent paper, WNT5A was suggested to promote melanoma cell migration/invasion via a FZD-4-β-catenin-dependent mechanism (24). Regardless of the signalling pathway involved, it is this ability of WNT5A signalling to promote melanoma cell migration that is attributed to advanced stage tumour progression in patients. However, the possibility remains that additional behavioural changes could occur in melanoma cells following WNT5A stimulation, which also contribute to enhanced disease progression.
Here, we used a quantitative proteomics approach to investigate early WNT5A-mediated events in melanoma cells. In particular, those signalling events that occur prior to enhanced cell migration, to assess what precursors might be necessary to elicit the behavioural changes that lead to WNT5A-mediated increases in melanoma cell motility. Interestingly, we have found that a large number of changes in the proteome involve metabolic enzymes, suggesting that WNT5A can reprogramme melanoma cell bioenergetics. In accordance with this, biochemical and molecular analysis revealed that WNT5A could enhance aerobic glycolysis in melanoma cells. However, in breast cancer cells, active WNT5A signalling increased mitochondrial-mediated oxidative phosphorylation instead, suggesting that WNT-β-catenin-independent signalling is a previously unrecognized, context-dependent, key regulator of cancer cell metabolism.
Materials and methods
Cell lines and treatments
Cell details, culture conditions, materials used and cell treatments are provided in Supplementary Material, along with other Supplementary Methods, available at Carcinogenesis Online. Details of statistical analysis for all experiments are also provided in Supplementary Methods, available at Carcinogenesis Online.
Cell migration assay
Time-lapse microscopy was used to track migration in the melanoma cells, as described previously (25). Briefly, A2058 cells were treated as indicated and pictures taken every 5–15 min over a 12 or 24 h period in a humidified 37°C chamber with 5% CO2. Track velocity was calculated using the Volocity Software (PerkinElmer).
Cell adhesion assay
A2058 cells were pre-treated with recombinant WNT5A (rWNT5A; and in some cases, also with the WNT5A inhibitory peptide, Box5) for the indicated times, either in the presence or absence of 5mg/ml cycloheximide, and assayed as described previously (21).
Mass spectrometry
Proteins were isolated from A2058 cells in DIGE lysis buffer (30mM Tris–HCl, 5mM Mg acetate, 8M Urea, 4% CHAPS). For each sample, 100 µg of whole cell protein extracts were prepared in accordance to the Tandem Mass Tags (TMT) Isobaric Mass Tagging Kit (Thermo Scientific) protocol. The labelled peptide samples were combined and fractionated off-line into 20 fractions by strong cation exchange chromatography (ICAT strong cation exchange cartridge; Applied Biosystems) at a flow rate of 50 µl/min. Fractions were eluted by injecting KCl at increasing concentrations (ranging from 0 to 600mM) in 5mM KH2PO4, 25% acetonitrile, pH 2.7. The collected fractions were desalted using UltraMicrospin columns (The Nest Group).
Reverse phase liquid chromatography (LC)–tandem mass spectrometry analysis was conducted using an Orbitrap-LTQ XL mass spectrometer (ThermoFisher, Bremen, Germany), which was coupled online to a split-less Eksigent 2D NanoLC system (Eksigent Technologies, Dublin, CA). The peptides were loaded with a constant flow rate of 10 µl/min onto a pre-column (Zorbax 300SB-C18 5 × 0.3mm, 5 µm; Agilent Technologies) and subsequently separated on a RP-LC analytical column (Zorbax 300SB-C18 150mm × 75 µm, 3.5 µm; Agilent Technologies) at a flow rate of 350 nl/min. The peptides were eluted with a linear gradient from 95% solvent A (0.1% formic acid in water) and 5% solvent B (0.1% formic acid in acetonitrile) to 40% solvent B over 55 min. Fragmentation was carried out using DDA and top 7 with alternate HCD/CID scans for all peptides. The raw data files were transformed to the mzData format using the Proteome Discoverer (ThermoFisher) tool and peptide identifications were performed using the MASCOT database (Matrix Science, London, UK). Positive identifications were accepted based on confidence scores of at least 95% with a false discovery rate of 0.01 (at both protein and peptide levels) using the human section UniProt (release 17 August 2011) together with a reverse decoy database. Relative quantification of proteins using the TMT labels was calculated using the tandem mass spectrometry scans as the ratio of the areas under the peaks at 126, 127, 128, 129, 130 and 131Da, which correspond to the representative masses of the TMT reagents.
Western blotting
Details are provided in Supplementary Methods, available at Carcinogenesis Online.
LDH activity assay
Measurement of LDH activity from total cell lysates was done using the CytoTox96™ LDH assay kit (Promega Corporation), according to the manufacturer’s instructions. The levels of LDH activity were expressed as normalized values against total protein.
Lactate determination
Secreted lactate in the cell media was measured using a Lactate Assay Kit (BioVision) according to the manufacturer’s instructions. Samples were stored at −80°C prior to analysis to ensure secreted LDH could not degrade the lactate, and all samples normalized against total protein. For inhibitor experiments, MK-2206 (0.5 µM) and rapamycin (80nM) were used to inhibit Akt and mTOR activity, respectively.
Metabolic analysis
Oxygen consumption rate (OCR) and pH changes (indicating carbon-based metabolism) were measured in real-time using a XF24 flux analyzer (Seahorse Bioscience). Cells were seeded on collagen I-coated 24-well microplates. All measurements were normalized against total protein content from cell lysates. Cells were analyzed in a modified Seahorse buffer (114mM NaCl, 4.7mM KCl, 1.2mM KH2PO4, 1.16mM MgSO4, 250mM CaCl2, 0.2g bovine serum albumin, pH 7.2) and incubated in low glucose (5mM) for 30 min prior to analysis. Where indicated, cells were treated with high glucose (28mM), 4 µg/ml oligomycin, 40 µM 2,4-dinitrophenol or 1 µM rotenone.
Glucose consumption assay
A2058 cells were treated with rWNT5A or carrier for 16 h as indicated above, and then media/total protein taken and assayed using a Glucose Assay Kit (Abcam), according to the manufacturer’s instructions. Glucose consumption was then calculated for each treatment.
Immunohistochemical analysis and patient cohort
Anti-WNT5A (26) or anti-LDHV (Abcam) antibodies and an EnVision™ FLEX, high pH kit (Dako) with a Dako Autostainer Plus instrument were used for immunostaining of tumour tissue samples. All reagents were used according to the manufacturer’s instructions and slides were counterstained with EnVision™ FLEX hematoxylin (Dako) as standard. Samples were scored by two independent researchers and any disagreements between the two observations made were reviewed by a pathologist for final classification. Melan-A staining was used in parallel in all tissue sections to ensure that only melanocytic lesions were scored.
Biopsy material included in this study was collected from a cohort of patients diagnosed with cutaneous melanoma in the Uppsala region of Sweden between 1982 and 2004 (27). All patients were followed up by the Department of Oncology, Uppsala University Hospital (Uppsala, Sweden). Ethical permission for the analysis was obtained from the Regional Ethical Review Board in Uppsala (EPN 2005; 232). Patients diagnosed with melanoma in situ and disseminated diseases at the time of diagnosis were excluded from the study as well as patients where only autopsy material was available. Patients where clinical information or a signed consent was missing were also excluded. In total, 152 patients fulfilled these inclusion criteria. All patients had their disease staged using the Union for International Cancer Control (UICC) 2002 TNM system. The fact that this tissue microarray has been used previously meant that 38 of the tumour samples in the original tissue microarray could not be further analyzed. Consequently, 1–3 tumour cores per tumour from 114 patient samples were analyzed in this study.
Results
Dynamics of WNT5A-mediated melanoma cell migration
Previously, we have shown that the human A2058 melanoma cell line is an excellent model to study WNT5A-mediated increases in cell migration and invasion (21,25). These cells have low endogenous WNT5A expression and strongly respond to rWNT5A signalling with an increased cell invasion phenotype and, therefore, we used these cells as a model to further investigate how WNT5A signalling increases cell motility in melanoma. In agreement with our own and other published data (20,21,25,28), we showed that rWNT5A stimulation of melanoma cells could increase cell migration as analysed by time-lapse imaging, which could be detected after 24 h of stimulation, but not 12 h (Figure 1A and B).
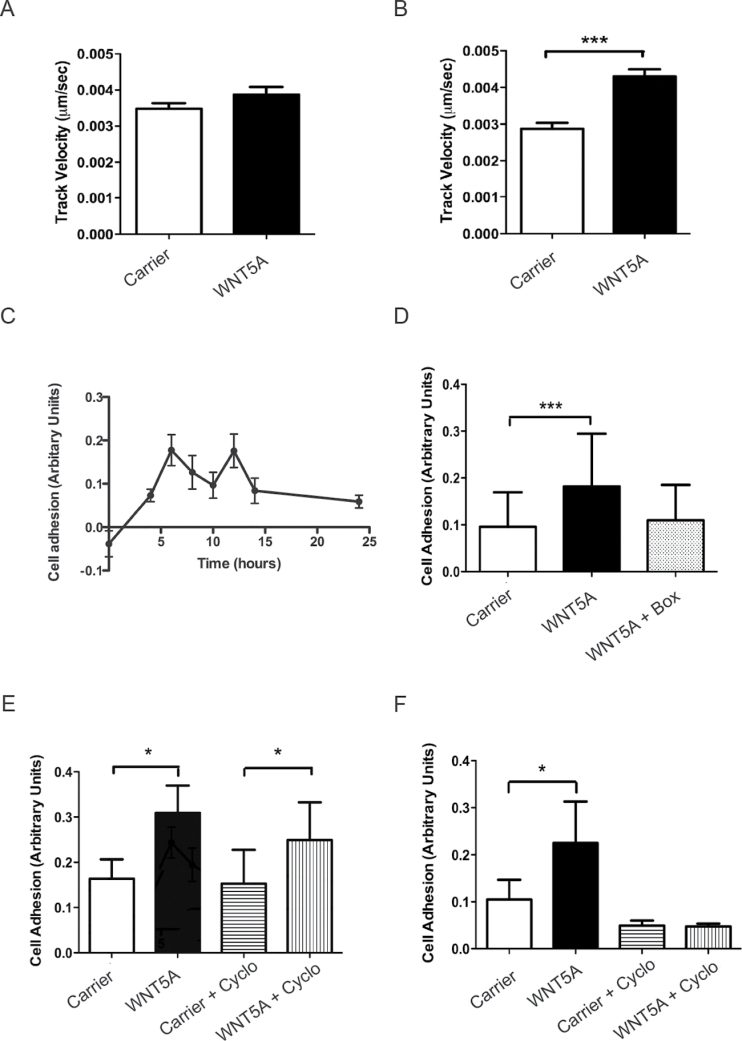
Fig. 1.
WNT5A increases melanoma cell migration and adhesion. (A) Time-lapse migration assay in A2058 cells following 12h of rWNT5A treatment or carrier. N = 4. (B) Time-lapse migration assay in A2058 cells following 24h of rWNT5A or carrier pre-treatment. N = 4. (C) Time course of rWNT5A-mediated cell adhesion of A2058 cells pre-treated prior to analysis at 4, 6, 8, 10, 12, 14 and 24h and normalized against background adhesion (carrier-treated cells). For clarity, error bars represent standard error of the mean. N = 3. (D) Adhesion assay in A2058 cells following 24h of rWNT5A, rWNT5A plus Box5 or carrier pre-treatment. N = 3. (E) Adhesion assay in A2058 cells following 6h of rWNT5A pre-treatment in the absence or presence of cycloheximide (Cyclo in the figure). N = 3. (F) Adhesion assay in A2058 cells following 12h of rWNT5A or carrier pre-treatment in the absence or presence of cycloheximide. N = 3. *P < 0.05; ***P < 0.001.
This increase in WNT5A-mediated migration is preceded by changes in cell adhesion properties of the melanoma cells (Figure 1C), which are also increased by 24 h, effects that can be inhibited by a WNT5A-specific antagonist peptide called Box5 (21) (Figure 1D). Further analysis of WNT5A-mediated increases in A2058 cell adhesion prior to changes in cell migration (up to 12 h of treatment; Figure 1A) revealed increases in cell adhesion even at these early time points following rWNT5A stimulation (Figure 1C, E and F). However, experiments using the translational inhibitor, cycloheximide, suggest that de novo protein synthesis is not required for this effect on cell adhesion up until 6 h post-stimulation but is essential at the 12 h time point (Figure 1E and F). This suggests that at 12 h post-rWNT5A stimulation in A2058 cells, altered protein expression levels of key mediators are required for changes to cell adhesion. These changes precede WNT5A-mediated increases in cell motility and are likely to be essential to the promotion of a WNT5A-induced aggressive phenotype in melanoma cells.
Global proteomics profiling reveals changes in LDH activity due to WNT5A signalling
The effect of active WNT5A signalling for 12 h on changes to the global proteome of melanoma cells was assessed by proteomics screening. As an internal control for WNT5A signalling in the screen, we used the Box5 inhibitory peptide in the experimental design (Figure 2A). Determination of cytosolic free calcium levels was used to ensure active WNT5A signalling prior to mass spectrometry analysis (Supplementary Figure 1, available at Carcinogenesis Online). The relative abundance of labelled peptides from the samples was easily detected using the isobaric TMT labels (Supplementary Figure 2, available at Carcinogenesis Online) and overall, 174 differentially expressed proteins were identified upon rWNT5A treatment by TMT-coupled LC–tandem mass spectrometry analysis (Supplementary Table 1, available at Carcinogenesis Online). Box5 was capable of inhibiting expression changes in most of these proteins, thereby providing an internal validation of the screen (Supplementary Table 1, available at Carcinogenesis Online).
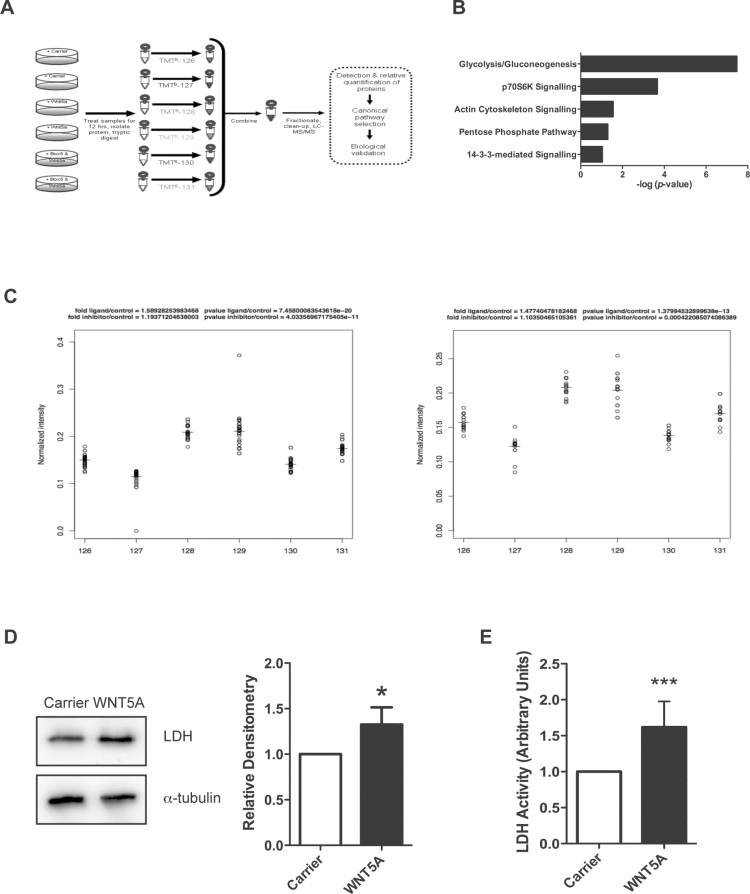
Fig. 2.
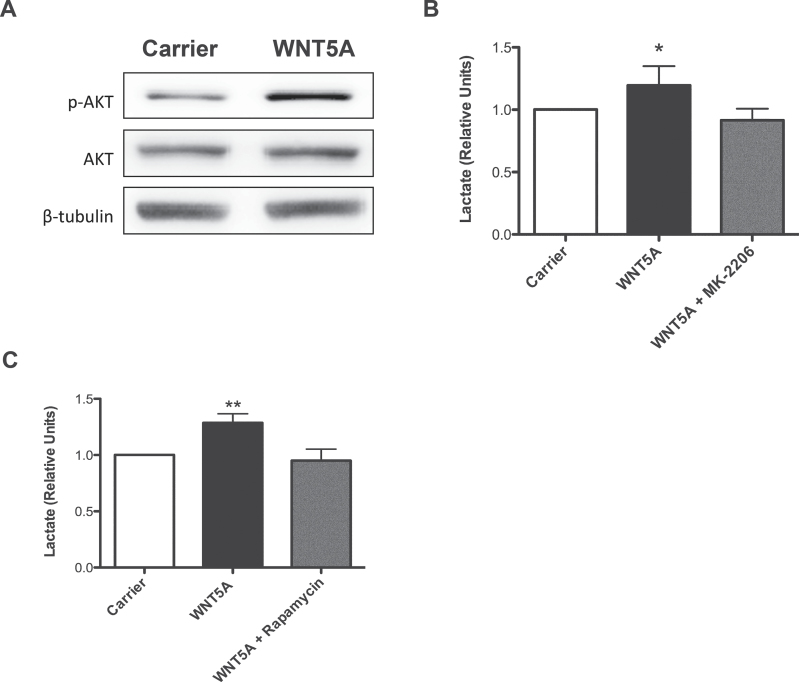
Proteomics analysis of WNT5A signalling in A2058 melanoma cells reveals changes to cellular metabolism machinery. (A) Schematic diagram of the experimental design for the proteomics screen. (B) WNT5A-regulated proteins were categorized into top canonical-affected pathways using the IPA Ingenuity® Knowledge Base dataset (Ingenuity Systems; bar chart; P value generated by a Fisher’s exact test). (C) Peptide spread plots of the LDH gene products from the proteomics data set. Each peptide identified for either the product from the LDHA or LDHB gene is represented in the plot by a circle and was labelled with the corresponding TMT labels shown on the x-axis: 126/127: carrier-treated samples; 128/129: rWNT5A-treated samples and 130/131: rWNT5A- and Box5-treated samples. Left panel: normalized intensity of peptide spreads from the LDHA gene product. Right panel: normalized intensity of peptide spreads from the LDHB gene product. The horizontal lines represent the mean values of the peptide spreads. (D) Left panel: LDH protein levels were measured by immunoblotting in rWNT5A-treated A2058 cells, compared to carrier treated (12h). Right panel: a representative of five independent experiments is shown, with relative densitometry. (E) LDH activity in lysates from A2058 cells treated as described for D. N = 3. *P < 0.05; ***P < 0.001.
Molecular functions of the differentially expressed proteins were classified using a variety of gene ontology tools (Figure 2B and Supplementary Figure 3, available at Carcinogenesis Online). In all analyses, cellular metabolism was defined as the top category for the most affected cellular process. IPA Ingenuity® Knowledge Base (Ingenuity® Systems) dataset analysis highlighted glycolysis or potentially the reciprocal gluconeogenetic metabolic pathway as the most affected functional pathway in the rWNT5A-treated A2058 cells (Figure 2B). As gluconeogenesis is a metabolic process normally confined to the kidneys and liver in vertebrates, we hypothesized that WNT5A could alter glycolysis in melanoma cells, which suggests a novel role for WNT5A in the control of energy metabolism. The proteomics analysis detected WNT5A-mediated increased levels in peptides generated from the parental gene products of both the LDH-M and LDH-H subunit genes (LDHA and LDHB), which were blocked by Box5 treatment (Figure 2C). Increased LDH levels following rWNT5A stimulation in melanoma cells were further confirmed by western analysis (Figure 2D). Interestingly, this increase in LDH expression is not dependent on transcriptional control of the genes that encode the enzyme subunits (Supplementary Figure 4, available at Carcinogenesis Online), so is therefore a post-transcriptional event. Importantly, this post-transcriptional increase in LDH levels can lead to markedly enhanced LDH enzyme activity (Figure 2E). Taken together, this suggests that initial events in WNT5A-mediated changes to melanoma cell behaviour result in their metabolic reprogramming, which includes increased LDH expression and activity.
WNT5A signalling increases glycolysis in melanoma cells
Such large increases in LDH expression and activity in the melanoma cells stimulated by WNT5A signalling probably maintain glycolysis at a high rate particularly when availability of oxygen is limited. The formation of lactate provides the NAD+ required for the action of glyceraldehyde-3-phosphate dehydrogenase, thus supporting glycolysis. To confirm this, we measured extracellular lactate, which is derived from cells producing the acid and will reflect glycolytic rate in the cells. rWNT5A stimulation of A2058 cells results in increased lactate levels in the cell media (Figure 3A), suggesting enhanced aerobic glycolysis, which is inhibited by Box5 (Figure 3B). Conversely, transient knockdown of the low endogenous WNT5A levels in A2058s and indeed in a line with higher endogenous WNT5A expression, HTB63 (21) (Supplementary Figure 5, available at Carcinogenesis Online), resulted in a marked decrease in lactate secretion (Figure 3C and D). Further analysis revealed the same effects of WNT5A-induced increases in LDH expression and lactate secretion in SK-MEL28 and M229 human melanoma cell lines (Supplementary Figure 6, available at Carcinogenesis Online).
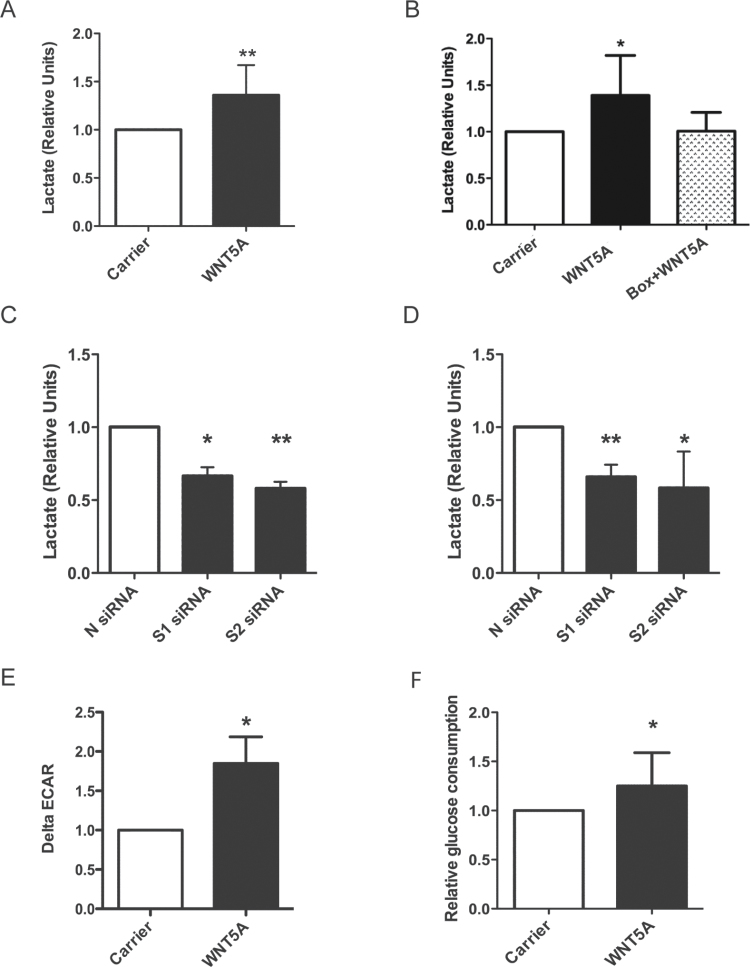
Fig. 3.
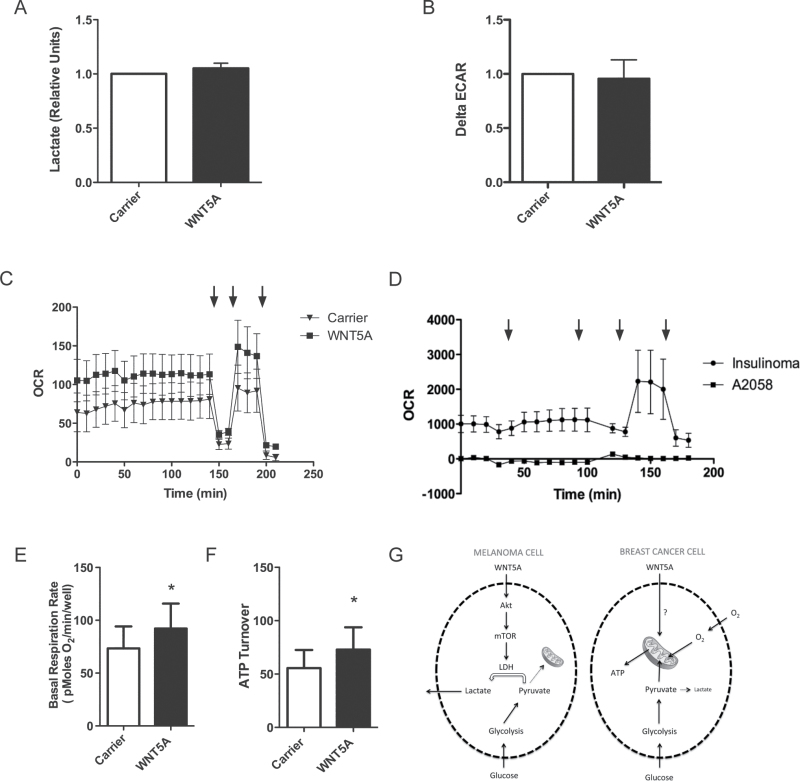
WNT5A promotes aerobic glycolysis in melanoma cells. (A) Extracellular lactate concentration in A2058 cells treated with rWNT5A or carrier overnight. N = 4. (B) Extracellular lactate concentration in A2058 cells treated with carrier, rWNT5A or rWNT5A + Box5 peptide for 12h. N = 8. (C) Extracellular lactate concentration in A2058 cells following 48h of transfection with two WNT5A targeting siRNAs (S1 and S2) compared to a control scrambled siRNA (N). N = 4. (D) Extracellular lactate concentration in HTB63 cells following 72h of transfection with siRNAs as described for B. N = 6. (E) ECAR was measured in A2058 cells using an XF24 bioanalyser following 12h of rWNT5A treatment. ΔECAR was calculated following a change to high glucose added after 40min of low glucose treatment. N = 5. (F) Glucose consumption in A2058 cells following rWNT5A or carrier treatment, as for A. N = 10. *P < 0.05; **P < 0.01.
To corroborate these findings, we used a Seahorse XF24 extracellular flux analyser to provide real-time analysis of extracellular acidification rates (ECARs) in the melanoma cells following rWNT5A stimulation. In cells where tricarboxylic acid cycle activity and hence formation of CO2 is abrogated, ECAR can be used as a surrogate readout for glycolysis. ΔECAR calculated upon high glucose addition to the melanoma cells is markedly increased in those pre-treated with rWNT5A (Figure 3E). Although lactic acid is not the only potential source of acidification from cancer cells (29), it is highly likely that the increased WNT5A-mediated lactate secretion (Figure 3A–C) contributes to the increase in ΔECAR observed. Furthermore, increased glycolysis in rWNT5A-treated melanoma cells is confirmed by increased glucose consumption (Figure 3F). These findings show that active WNT5A signalling in melanoma cells stimulates aerobic glycolysis.
WNT5A and LDH expression positively correlate in melanoma
LDH-M has a lower Km and higher V max for pyruvate reduction than LDH-H (30). Hence, of the five tetrameric LDH isoforms, lactic dehydrogenase V (LDHV) with four LDH-M subunits preferentially catalyses pyruvate reduction to lactate and, therefore, has a huge capacity for promoting glycolytic flux. Although these findings do not necessarily directly confer significant physiological relevance for the capacity of individual LDH isoforms to produce lactate in tissues (31), LDHV can reduce pyruvate to lactate and is associated with poor prognosis in melanoma patients (32). For this reason and since it has been shown to correlate to tumour progression (30), we chose to investigate the expression levels of LDHV in melanoma patient tissues and check if there is any correlation with WNT5A expression. We conducted immunostaining of biopsy samples of melanocytic neoplasms from 114 melanoma patients. Relative expression of LDH and WNT5A were scored based on staining intensity as: 0: negative; 1: weak; 2: moderate or 3: strong (Figure 4A and Supplementary Figure 7, available at Carcinogenesis Online).
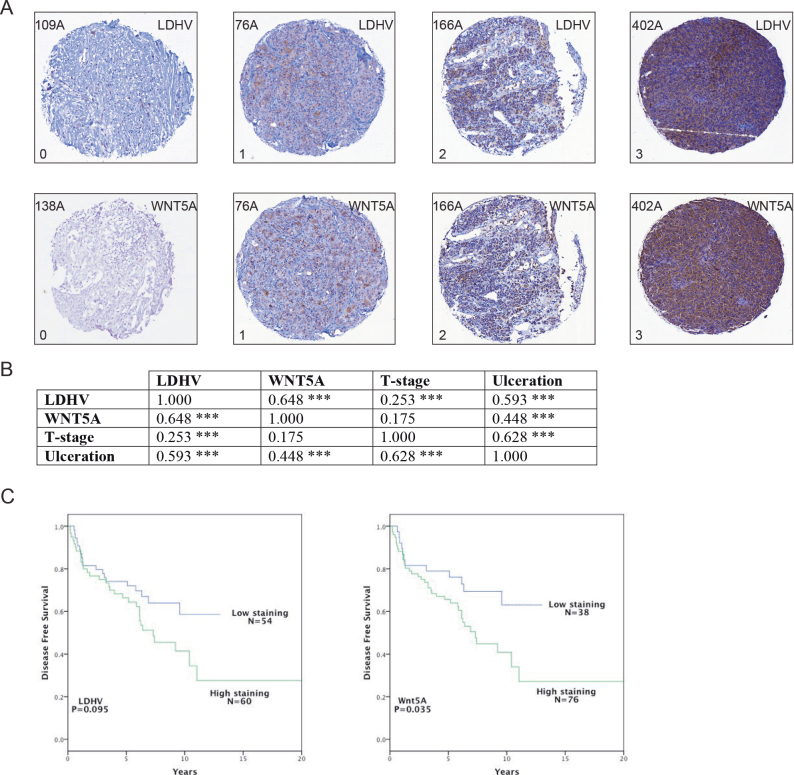
Fig. 4.
There is a positive association between LDHV and WNT5A expression in melanoma. (A) Example LDHV and WNT5A expression in a tissue array of melanoma patient tumour samples. Five example patient tumours are shown: 109A, 138A, 76A, 166A and 402A, where each tumour core is ~600 µm across; 0 and 1 indicate no to low expression, respectively, whereas 2 and 3 indicate moderate to high expression, respectively. (B) Correlations of WNT5A and LDHV expression in patient tissues (n = 114), and matched against the prognostic markers: tumour ulceration and T-stage. Correlations determined by Spearman’s Rho and cognate P value are shown. (C) Kaplan–Meier survival curves depicting disease-free survival versus the LDHV (left panel) or WNT5A (right panel) intensity of staining in the tumour cells. The intensity of staining was defined as outlined in A. *P < 0.05; **P < 0.01; ***P < 0.001.
Interestingly, we found a positive correlation between LDHV and WNT5A expression in melanoma patient tissue (correlation coefficient, ρ = 0.648; Figure 4B). T-stage and ulceration are two critical criteria used in the TNM staging system for cutaneous melanoma, where high levels predict poor patient prognosis (33). Expectedly, these two criteria correlated with one another (ρ = 0.628), where LDHV expression also correlates with ulceration (ρ = 0.593) and weakly correlates with T-stage (ρ = 0.253; Figure 4B). WNT5A expression, however, moderately correlates with ulceration (ρ = 0.448), but only very weakly, with T-stage (ρ = 0.175). Nonetheless, high intensity staining levels of both LDHV (32) and WNT5A (6) are associated with reduced disease-free survival in melanoma patients (Figure 4C). This analysis shows that WNT5A and LDHV expression positively correlate in cutaneous melanoma.
WNT5A promotes aerobic glycolysis in melanoma by activating Akt
To determine key downstream effector molecules of WNT5A signalling in melanoma cells that promote increased aerobic glycolysis, we used human melanoma cell lines to investigate Akt signalling. Stimulation of the cells with rWNT5A markedly increased Akt phosphorylation, indicating activated Akt signalling in response to WNT5A stimulation in A2058 melanoma cells (Figure 5A) and in SK-MEL28 and M229 melanoma cells (Supplementary Figure 8, available at Carcinogenesis Online). We next investigated the effect of Akt and its downstream effector, mTOR on the glycolytic pathway using chemical inhibitors. Using an Akt inhibitor (MK-2206; Selleckbio) and an mTOR inhibitor (rapamycin), we found that WNT5A-mediated increases in aerobic glycolysis can be blocked in melanoma cells (Figure 5B and C). Indeed, both MK-2206 and rapamycin treatment blocked rWNT5A-induced lactate production in melanoma cells. These results show that WNT5A β-catenin-independent signalling in melanoma cells stimulates glycolytic flux by activating Akt-mTOR signalling.
Fig. 5.
WNT5A stimulates aerobic glycolysis via the activation of Akt signalling. (A) A2058 cells treated with rWNT5A for 30–90min have increased phospho-Akt (p-Akt) levels compared with carrier-treated cells, as determined by immunoblotting. Example experiment of 5 is shown. (B) Inhibition of rWNT5A-mediated increase in lactate secretion in A2058 cells by MK-2206 (an allosteric Akt-1, -2 and -3 inhibitor) treatment. N = 3. (C) Inhibition of rWNT5A-mediated increase in lactate secretion in A2058 cells by rapamycin (mTOR complex-1 inhibitor) treatment. N = 3.
WNT5A controls breast cancer cell bioenergetics
WNT5A signalling is misregulated in a variety of tumour types and not always with a predictable outcome, as it has been proposed to have both oncogenic and tumour-suppressing roles depending on the cancer in question. Down-regulation of WNT5A expression has been associated with a number of different cancers, one of which is breast cancer (4,26,34). Given that WNT5A signalling can promote aerobic glycolysis in melanoma, a cancer where WNT5A promotes tumour progression, it raises the question of whether WNT5A signalling can also affect energy metabolism in cancers where it possesses a tumour suppressor role. To test this, we investigated if WNT5A can alter cellular bioenergetics in breast cancer cells.
We found that unlike in melanoma cells (Figure 3), WNT5A signalling does not promote lactate secretion in MDA-MB-468 breast cancer cells (Figure 6A). Moreover, WNT5A does not affect ΔECAR in breast cancer cells (Figure 6B). These data suggest that in breast cancer cells, WNT5A does not promote aerobic glycolysis. However, further analysis using the XF24 instrument revealed that OCR is elevated in MDA-MB-468 cells upon rWNT5A stimulation (Figure 6C). This is not observed in A2058 melanoma cells, where OCR is extremely low (Figure 6D). We used well-characterized metabolism inhibitors to assess cell respiratory control (35) in the breast cancer cells (Figure 6C) in order to calculate basal respiration rate and ATP turnover. Active WNT5A signalling in the breast cancer cells led to significant increases in both of these (Figure 6E and F). In A2058 cells, the low OCR rates (Figure 6C) make analysis with mitochondrial respiratory inhibitors impossible and, therefore, changes in OCR due to WNT5A signalling in A2058 cells could not be calculated. Taken together, these data suggest that in breast cancer cells, rather than affecting glycolytic flux, WNT5A signalling promotes increased mitochondrial respiration, which is in contrast to what was observed in melanoma cells as schematically outlined in Figure 6G.
Fig. 6.
WNT5A increases mitochondrial-dependent respiration in breast cancer cells. (A) Extracellular lactate concentration in MDA-MB-468 cells treated with rWNT5A or carrier for 24h. N = 3. (B) ECAR was measured in MDA-MB-468 cells using an XF24 bioanalyser following 48h of rWNT5A treatment or with carrier. ΔECAR was calculated following a change to high glucose added after 40min of low glucose treatment. N = 4. (C) OCR of MDA-MB-468 cells was measured on the XF24 bioanalyser following 48h of rWNT5A treatment or with carrier. Drugs were added at the indicated times (arrows), from left to right: oligomycin, 2,4-dinitrophenol and rotenone. A representative example of a cell respiratory control experiment is shown. N = 4. (D) OCR in A2058 cells compared with other cancer cells. The XF24 bioanalyser instrument was used to monitor OCR in INS-1 and A2058 cells. Drugs were added at the indicated times (arrows) from left to right: high glucose, oligomycin, 2,4-dinitrophenol and rotenone. N = 3. (E) Basal respiration as calculated from the cell respiratory control data (an example experiment is shown in C). OCR levels prior to the addition of oligomycin represent the basal respiration rate of the cells. N = 4. (F) ATP turnover as calculated from the cell respiratory control data (an example experiment is shown in C). ATP turnover is calculated from the oligomycin-sensitive respiration. N = 4. (G) A schematic model for WNT5A-mediated reprogramming of glucose metabolism in melanoma and breast cancer cells. *P < 0.05.
Discussion
Active WNT5A signalling alters cancer cell bioenergetics in the model systems tested. Using a combination of proteomics, molecular and biochemical analyses, we have shown that WNT5A can promote aerobic glycolysis in melanoma and shown that in patients, its expression positively correlates with that of the LDH isoform most commonly associated with glycolytic flux. In melanoma, there is a considerable amount of evidence showing that WNT5A signalling promotes cell motility (Figure 1) (20–23,25,28,36–38), but to the best of our knowledge, it has not before been found to control melanoma cell metabolism by increasing aerobic glycolysis (Figure 3). Overall, WNT5A activity can advance melanoma progression but is unlikely to be an initiating event in the development of the disease. This highlights a new role for WNT-β-catenin-independent signalling in melanoma cell biology and suggests that WNT5A exhibits several cancer-promoting functions, all of which could explain why high WNT5A expression is associated with increased tumour grade (6). Indeed, melanoma cells primarily use glycolysis as an energy source during motility (39), so it is logical that WNT5A signalling can promote both migration and aerobic glycolysis in these cells. Collectively, these findings further support the idea of developing WNT5A-specific inhibitors as novel therapeutics for malignant melanoma treatment (21).
LDH plays a central role in cellular metabolism and in cancer, where high serum levels of this enzyme in melanoma patients correlates with poor prognosis (40,41). Furthermore, high LDHV expression in melanoma has been shown to correlate with reduced disease-free survival rates (32), which is in agreement with our findings. Recently, it was also shown in advanced stage melanoma that patients with elevated serum LDH levels had tumours strongly associated with metabolic shifts towards glycolysis (42). Given that we have found that WNT5A increases LDH expression (Figure 2), promotes aerobic glycolysis (Figure 3) and correlates with LDHV expression in melanoma tissue (Figure 4), it would also be expected to positively correlate with high serum LDH levels in patients, although this remains to be tested. We found that LDH expression was controlled in a post-transcriptional manner in the melanoma cells. Regulation of LDH gene expression at the transcriptional level is well documented and involves the activity of transcription factors such as the hypoxia-inducible factor-1α and c-Myc (43). We were unable to detect elevated expression of either of these transcription factors in melanoma cells following rWNT5A stimulation (data not shown). This and the evidence that increases in LDH activity are only found at the protein level, suggests that WNT5A signalling regulates LDH expression by a currently unresolved post-translational mechanism.
The PI3K-Akt-mTOR signalling pathway is known to promote glycolysis in cancer cells, at least in part by increasing LDH levels (44). WNT5A has previously been found to be upstream of this pathway in a variety of cell types, including fibroblasts (45) and platelets (46). Interestingly, we found that WNT5A was able to increase active Akt levels in melanoma cells and that WNT5A-mediated increases in glycolysis could be blocked through inhibition of the Akt-mTOR signalling pathway (Figure 5), suggesting that WNT5A can increase the glycolytic capacity of melanoma cells via activation of PI3K/Akt signalling. Active mTOR signalling mediates increases in translational machinery (47,48) including ribosomal proteins and translational elongation factors (many of which we detected in the proteomics data set; Supplementary Table 1, available at Carcinogenesis Online), which could offer a plausible explanation as to why we detect higher LDH expression at the protein, but not the mRNA level. A recent study showed that WNT5A enhanced gastric cancer cell migration via the PI3K-Akt signalling pathway (49). This raises the distinct possibility that active WNT5A signalling in gastric cancer could also lead to glycolytic shifts in these cells. Indeed further studies should also investigate the role of WNT-β-catenin-independent signalling in controlling the metabolic phenotypes of other cancer types, particularly those where this signalling pathway is already known to have either tumour-suppressing or oncogenic function.
We found that the effect of WNT5A on reprogramming cancer cell metabolism is highly context dependent, as it alters energy metabolism in very distinct ways between melanoma and breast cancer cells. Rather than promoting aerobic glycolysis, in breast cancer cells, WNT5A signalling increased oxidative phosphorylation (mitochondrial respiration). Specifically, using flux analysis to measure cellular respiration rates, we determined that WNT5A could increase basal respiration and ATP turnover in breast cancer cells (Figure 6). These two findings are related, as basal respiration is controlled in the most part by ATP turnover (50) and, together, these findings show that mitochondrial function is increased upon WNT5A signalling in breast cancer cells. In contrast, we found that in the melanoma cells tested, there was a glycolytic shift, where aerobic glycolysis is commonly associated with rapidly growing tumours and high metastatic capacity. Therefore, given that WNT5A is a marker of good patient prognosis in breast cancer (26), which is opposite in melanoma (6), it is tempting to speculate that distinct changes in energy metabolism between cell types may underpin differing aggressive alterations noted for WNT5A in a range of cancers. Taken together, we have presented solid support for a new role of WNT-β-catenin-independent signalling in the metabolic reprogramming of cancer cells, which functions in a highly context-dependent fashion.
Our findings raise the question of how the other arm of WNT signalling (the WNT-β-catenin-dependent pathway) affects tumour cell metabolism. In melanoma at least, high nuclear β-catenin levels (which is indicative of active WNT-β-catenin-dependent signalling) is associated with good patient prognosis (51). However, there are a number of cancer types where WNT-β-catenin-dependent signalling plays an oncogenic role, including colorectal, breast and oesophageal cancer, where this context dependency has been recently discussed (52). A previous study found that Akt-mediated increases in nuclear β-catenin signalling results in increased glycolysis and mitochondrial dysfunction in oesophageal squamous cell carcinoma (53). Furthermore, recent work has shown that in breast cancer cell lines, WNT-β-catenin signalling can elicit a glycolytic switch mediated at least in part through suppressed expression of the mitochondrial respiration enzyme, cytochrome C oxidase (54). Taken together with our findings, this work suggests that both of the WNT signalling arms (β-catenin dependent and independent) have the potential to modulate cellular bioenergetics in cancer. Indeed in a recent study, Grossmann et al. (24) have discovered that WNT5A can induce β-catenin-dependent signalling through activation of the small GTPase, ARF6, in a subset of melanoma cells. These studies, coupled with ours, further highlight the context-dependent nature of WNT signalling in cancer and, moreover, the need for detailed investigations into the role of WNT signalling in reprogramming cancer cell metabolism in specific cellular contexts.
A variety of signalling cascades are activated in response to β-catenin-independent WNT signalling in different cell types. Thus, there is the distinct possibility that specific ligand–receptor interactions provide specificity for alternate WNT signalling responses. Indeed a large number of receptors and co-receptors have been proposed to bind and elicit WNT5A signalling in a variety of cellular contexts, including a large number of FZD receptors (55–57), Ror-1 (58) and Ror-2 (22,23,55), and the receptor-like tyrosine kinase, Ryk (59). It will be important to determine if alternate receptor–WNT5A interactions could be responsible for eliciting differing signalling pathways that evoke alternate metabolic reprogramming events, such as those we have found between melanoma and breast cancer cells. Likewise cross-talk with other signalling pathways in specific tumour cell contexts is also likely to influence WNT5A-mediated metabolic reprogramming in cancer.
Overall, our results support the role of WNT ligands as eliciting signals that can metabolically reprogramme tumour cells. The effects detected are diverse and likely to be highly context dependent. Indeed, distinct changes found in this present study with WNT5A suggest that changes in energy metabolism could well underpin differing aggressive behaviours noted for WNT5A in cancer. It is now clear that metabolism within tumours is not uniform, but that cancer cells exhibit metabolically flexibility to allow them thrive in dynamic environments of nutrient availability, oxygen tension and pH changes (60). Altered WNT signalling might be able to regulate this metabolic flexibility within tumours and investigations into the mechanisms that afford this regulation represent a vital area of future research into the role of WNT signalling in tumorigenesis.
Supplementary material
Supplementary methods, Table 1 and Figures 1–8 can be found at http://carcin.oxfordjournals.org/
Funding
Swedish Cancer Foundation; Swedish Research Council; Söderberg Foundations; Skåne University Hospital Research Foundations; Gunner Nilsson’s Cancer Foundation; Malmö Allmänna Sjukhus Cancer Foundation (all to T.A.); Royal Physiographic Society (to V.S. and E.J.E.); Knut and Alice Wallenberg Foundation (to F.P.); Research Fund ‘Stiftelsen för onkologiska klinikens forskningsfond’ at the Department of Oncology, Uppsala University (to M.B.); Swedish Strategic Research Council to CREATE Health (to P.J.).
Supplementary Material
Acknowledgements
The authors wish to thank Sofie Mattson, Lena Axelsson and Elise Nilsson for their technical assistance and Professor Hindrik Mulder, Lund University, for many useful discussions.
Conflict of Interest Statement: T.A. and V.S. are shareholders of WntResearch. T.A. is also part-time CSO of the company. This does not alter the authors’ adherence to all the ethical policies stated for Carcinogenesis.
Glossary
Abbreviations:
- ECAR
extracellular acidification rate
- LC
liquid chromatography
- LDH
lactate dehydrogenase
- LDHV
lactic dehydrogenase V
- OCR
oxygen consumption rate
- TMT
Tandem Mass Tags.
References
- 1. Nusse R., et al. (1982). Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell, 31, 99–109 [DOI] [PubMed] [Google Scholar]
- 2. Papkoff J., et al. (1996). Wnt-1 regulates free pools of catenins and stabilizes APC-catenin complexes. Mol. Cell. Biol., 16, 2128–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McDonald S.L., et al. (2009). The opposing roles of Wnt-5a in cancer. Br. J. Cancer, 101, 209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dejmek J., et al. (2005). Expression and signaling activity of Wnt-5a/discoidin domain receptor-1 and Syk plays distinct but decisive roles in breast cancer patient survival. Clin. Cancer Res., 11(2 Pt 1), 520–528 [PubMed] [Google Scholar]
- 5. Leris A.C., et al. (2005). WNT5A expression in human breast cancer. Anticancer Res., 25(2A), 731–734 [PubMed] [Google Scholar]
- 6. Da Forno P.D., et al. (2008). WNT5A expression increases during melanoma progression and correlates with outcome. Clin. Cancer Res., 14, 5825–5832 [DOI] [PubMed] [Google Scholar]
- 7. Hanahan D., et al. (2011). Hallmarks of cancer: the next generation. Cell, 144, 646–674 [DOI] [PubMed] [Google Scholar]
- 8. Vander Heiden M.G., et al. (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science, 324, 1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Warburg O. (1956). On the origin of cancer cells. Science, 123, 309–314 [DOI] [PubMed] [Google Scholar]
- 10. Porporato P.E., et al. (2011). Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front. Pharmacol., 2, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sethi J.K., et al. (2010). Wnt signalling and the control of cellular metabolism. Biochem. J., 427, 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cadoret A., et al. (2002). New targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene, 21, 8293–8301 [DOI] [PubMed] [Google Scholar]
- 13. Schwartz D.R., et al. (2003). Novel candidate targets of beta-catenin/T-cell factor signaling identified by gene expression profiling of ovarian endometrioid adenocarcinomas. Cancer Res., 63, 2913–2922 [PubMed] [Google Scholar]
- 14. Mori H., et al. (2012). Secreted frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibition. J. Clin. Invest., 122, 2405–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoon J.C., et al. (2010). Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev., 24, 1507–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lens M.B., et al. (2004). Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br. J. Dermatol., 150, 179–185 [DOI] [PubMed] [Google Scholar]
- 17. Hodi F.S., et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med., 363, 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chapman P.B., et al. ; BRIM-3 Study Group (2011). Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med., 364, 2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robert C., et al. (2011). Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med., 364, 2517–2526 [DOI] [PubMed] [Google Scholar]
- 20. Weeraratna A.T., et al. (2002). Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell, 1, 279–288 [DOI] [PubMed] [Google Scholar]
- 21. Jenei V., et al. (2009). A t-butyloxycarbonyl-modified Wnt5a-derived hexapeptide functions as a potent antagonist of Wnt5a-dependent melanoma cell invasion. Proc. Natl Acad. Sci. USA, 106, 19473–19478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lai S.S., et al. (2012). Ror2-Src signaling in metastasis of mouse melanoma cells is inhibited by NRAGE. Cancer Genet., 205, 552–562 [DOI] [PubMed] [Google Scholar]
- 23. O’Connell M.P., et al. (2010). The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma. Oncogene, 29, 34–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grossmann A.H., et al. (2013). The small GTPase ARF6 stimulates ß-catenin transcriptional activity during WNT5A-mediated melanoma invasion and metastasis. Sci. Signal., 6, ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ekström E.J., et al. (2011). Methylation and loss of Secreted Frizzled-Related Protein 3 enhances melanoma cell migration and invasion. PLoS ONE, 6, e18674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jönsson M., et al. (2002). Loss of Wnt-5a protein is associated with early relapse in invasive ductal breast carcinomas. Cancer Res., 62, 409–416 [PubMed] [Google Scholar]
- 27. Strömberg S., et al. (2009). Selective expression of Syntaxin-7 protein in benign melanocytes and malignant melanoma. J. Proteome Res., 8, 1639–1646 [DOI] [PubMed] [Google Scholar]
- 28. O’Connell M.P., et al. (2009). Wnt5A activates the calpain-mediated cleavage of filamin A. J. Invest. Dermatol., 129, 1782–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Helmlinger G., et al. (2002). Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin. Cancer Res., 8, 1284–1291 [PubMed] [Google Scholar]
- 30. Markert C.L., et al. (1975). Evolution of a gene. Multiple genes for LDH isozymes provide a model of the evolution of gene structure, function and regulation. Science, 189, 102–114 [DOI] [PubMed] [Google Scholar]
- 31. Quistorff B., et al. (2011). The isoenzyme pattern of LDH does not play a physiological role; except perhaps during fast transitions in energy metabolism. Aging (Albany. NY)., 3, 457–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhuang L., et al. (2010). Lactate dehydrogenase 5 expression in melanoma increases with disease progression and is associated with expression of Bcl-XL and Mcl-1, but not Bcl-2 proteins. Mod. Pathol., 23, 45–53 [DOI] [PubMed] [Google Scholar]
- 33. Balch C.M., et al. (2009). Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol., 27, 6199–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Säfholm A., et al. (2008). The Wnt-5a-derived hexapeptide Foxy-5 inhibits breast cancer metastasis in vivo by targeting cell motility. Clin. Cancer Res., 14, 6556–6563 [DOI] [PubMed] [Google Scholar]
- 35. Brand M.D., et al. (2011). Assessing mitochondrial dysfunction in cells. Biochem. J., 435, 297–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Camilli T.C., et al. (2011). Loss of Klotho during melanoma progression leads to increased filamin cleavage, increased Wnt5A expression, and enhanced melanoma cell motility. Pigment Cell Melanoma Res., 24, 175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Witze E.S., et al. (2008). Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science, 320, 365–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O’Connell M.P., et al. (2008). Assaying Wnt5A-mediated invasion in melanoma cells. Methods Mol. Biol., 468, 243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beckner M.E., et al. (1990). Glycolysis as primary energy source in tumor cell chemotaxis. J. Natl Cancer Inst., 82, 1836–1840 [DOI] [PubMed] [Google Scholar]
- 40. Manola J., et al. (2000). Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J. Clin. Oncol., 18, 3782–3793 [DOI] [PubMed] [Google Scholar]
- 41. Finck S.J., et al. (1983). LDH and melanoma. Cancer, 51, 840–843 [DOI] [PubMed] [Google Scholar]
- 42. Ho J., et al. (2012). Importance of glycolysis and oxidative phosphorylation in advanced melanoma. Mol. Cancer, 11, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gordan J.D., et al. (2007). HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell, 12, 108–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Robey R.B., et al. (2009). Is Akt the “Warburg kinase”?-Akt-energy metabolism interactions and oncogenesis. Semin. Cancer Biol., 19, 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kawasaki A., et al. (2007). Wnt5a promotes adhesion of human dermal fibroblasts by triggering a phosphatidylinositol-3 kinase/Akt signal. Cell. Signal., 19, 2498–2506 [DOI] [PubMed] [Google Scholar]
- 46. Kim S.Y., et al. (2011). Wnt5a potentiates U46619-induced platelet aggregation via the PI3K/Akt pathway. Mol. Cells, 32, 333–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reiter A.K., et al. (2004). The mTOR signaling pathway mediates control of ribosomal protein mRNA translation in rat liver. Int. J. Biochem. Cell Biol., 36, 2169–2179 [DOI] [PubMed] [Google Scholar]
- 48. Thoreen C.C., et al. (2012). A unifying model for mTORC1-mediated regulation of mRNA translation. Nature, 485, 109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu J., et al. (2013). PI3K/Akt-dependent phosphorylation of GSK3ß and activation of RhoA regulate Wnt5a-induced gastric cancer cell migration. Cell. Signal., 25, 447–456 [DOI] [PubMed] [Google Scholar]
- 50. Ainscow E.K., et al. (1999). Top-down control analysis of ATP turnover, glycolysis and oxidative phosphorylation in rat hepatocytes. Eur. J. Biochem., 263, 671–685 [DOI] [PubMed] [Google Scholar]
- 51. Chien A.J., et al. (2009). Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc. Natl Acad. Sci. USA, 106, 1193–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Anastas J.N., et al. (2013). WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer, 13, 11–26 [DOI] [PubMed] [Google Scholar]
- 53. Kim M.S., et al. (2010). Neurofilament heavy polypeptide regulates the Akt-beta-catenin pathway in human esophageal squamous cell carcinoma. PLoS ONE, 5, e9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee S.Y., et al. (2012). Wnt/Snail signaling regulates cytochrome C oxidase and glucose metabolism. Cancer Res., 72, 3607–3617 [DOI] [PubMed] [Google Scholar]
- 55. Nishita M., et al. (2010). Ror2/Frizzled complex mediates Wnt5a-induced AP-1 activation by regulating Dishevelled polymerization. Mol. Cell. Biol., 30, 3610–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sato A., et al. (2010). Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J., 29, 41–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Takada R., et al. (2005). Analysis of combinatorial effects of Wnts and Frizzleds on beta-catenin/armadillo stabilization and Dishevelled phosphorylation. Genes Cells, 10, 919–928 [DOI] [PubMed] [Google Scholar]
- 58. Fukuda T., et al. (2008). Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proc. Natl Acad. Sci. USA, 105, 3047–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li L., et al. (2009). Wnt5a induces simultaneous cortical axon outgrowth and repulsive axon guidance through distinct signaling mechanisms. J. Neurosci., 29, 5873–5883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nakajima E.C., et al. (2013). Metabolic symbiosis in cancer: refocusing the Warburg lens. Mol. Carcinog., 52, 329–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.