Summary
Inhibition of TGFβ1 signaling in skin suppresses UVB-induced skin tumor formation, acute skin inflammation and T-cell activation. Genetic and pharmacological blockade of TGFβ1 signaling in DCs blocks UV and steady-state LN migration.
Abstract
Transforming growth factor beta 1 (TGFβ1) is a pleiotropic cytokine in the skin that can function both as a tumor promoter and suppressor in chemically induced skin carcinogenesis, but the function in ultraviolet B (UVB) carcinogenesis is not well understood. Treatment of SKH1 hairless mice with the activin-like kinase 5 (ALK5) inhibitor SB431542 to block UVB-induced activation of cutaneous TGFβ1 signaling suppressed skin tumor formation but did not alter tumor size or tumor cell proliferation. Tumors that arose in SB-treated mice after 30 weeks had significantly reduced percentage of IFNγ+ tumor-infiltrating lymphocytes compared with control mice. SB431542 blocked acute and chronic UVB-induced skin inflammation and T-cell activation in the skin-draining lymph node (SDLN) and skin but did not alter UVB-induced epidermal proliferation. We tested the effect of SB431542 on migration of skin dendritic cell (DC) populations because DCs are critical mediators of T-cell activation and cutaneous inflammation. SB431542 blocked (i) UVB-induced Smad2 phosphorylation in dermal DC (dDC) and (ii) SDLN and ear explant migration of CD103+ CD207+ and CD207− skin DC subsets but did not affect basal or UV-induced migration of Langerhans cells. Mice expressing a dominant-negative TGFβ type II receptor in CD11c+ cells had reduced basal and UVB-induced SDLN migration of CD103+ CD207+ and CD207− DC subsets and a reduced percentage of CD86high dDC following UVB irradiation. Together, these suggest that TGFβ1 signaling has a tumor-promoting role in UVB-induced skin carcinogenesis and this is mediated in part through its role in UVB-induced migration of dDC and cutaneous inflammation.
Introduction
Ultraviolet B (UVB) radiation is a key environmental mutagen, acting as both an initiator and promoter of skin cancer (1). Chronic inflammation is a hallmark of carcinogenesis and has been widely implicated to be a potent tumor promoter (2). High doses of UVB radiation lead to vasodilation, erythema and inflammation (3), whereas suberythemal doses cause local and systemic immunosuppression (4). Langerhans cells (LCs) in the epidermis and CD103+ CD207+ and CD103− CD207− dendritic cell (DC) subsets in the dermis are key mediators of the cutaneous inflammatory response (5,6). LCs and dermal DC (dDC) subsets can be differentially activated by inflammatory stimuli (7–9) including UV irradiation (10), and LCs are thought to mediate the tolerogenic response to suberythemal doses of UV. However, the mechanism of UV-induced DC activation and inflammation in the skin in response to erythemal doses of UV is not clear.
Transforming growth factor beta 1 (TGFβ1) is a pleiotropic cytokine that acts on multiple immune cell types including DCs to either promote or suppress inflammation. In vitro, TGFβ1 can suppress DC function (11–14), and transgenic models with DC-specific inactivation of TGFβ signaling exhibit exacerbated severity of several inflammatory diseases (15,16). However, under some conditions, TGFβ-treated DCs can promote Th17 polarization (17,18). In the skin, TGFβ1 signaling in LC is essential for epidermal residency and maintenance of an immature phenotype (19–22), but the role of TGFβ1 signaling in other DC subsets is poorly understood.
We previously reported that TGFβ had a proinflammatory and tumor-promoting role in chemically induced skin carcinogenesis (23,24) and that elevated TGFβ1 expression in the epidermis caused dDC migration to the skin-draining lymph node (SDLN) and enhanced contact hypersensitivity responses (25). Here, we investigate the effects of activin-like kinase 5 (ALK5) inhibition during UV carcinogenesis. Here, we show that inhibition of TGFβ1 signaling suppresses UVB-induced tumor formation but enhances malignant progression. Both responses are linked to reduced lymph node (LN) activation of T cells and interferon γ+ (IFNγ+) CD4 and CD8 T cells in UVB-irradiated skin and skin tumors that in turn is linked to reduced migration of dDCs in response to UVB. Our results implicate TGFβ signaling in dDCs as an important component of the response to UVB that regulates cutaneous inflammation and UVB-induced skin tumor formation.
Materials and methods
Mice
Age-matched (6–9 weeks) and sex-matched male skin hairless mice (SKH1) were used for the pharmacological inhibition studies of TGFβ1 signaling with SB431542. CD11c-dnTGFβRII transgenic mice expressing a dominant-negative human TGFβ receptor II gene under the control of a CD11c (Itgax) promoter were obtained from Jackson Laboratories and were genotyped for the transgene as described (26), and age-matched (6–9 weeks) non-transgenic [wild-type (WT)] and transgenic littermates were used for experiments. All animals were treated according to approved Institutional Animal Care and Use (IACUC) protocols.
UVB irradiation
SKH1 mice were irradiated with minimal erythemal dose (MED) of 2.4 kJ/m2 from UV bulbs (American Ultraviolet Light Co.) covered with cellulose triacetate (KODAK) to filter out UVC radiation and produce UV wavelengths between 280 and 320nm as described (27). Irradiance was measured using a UVX radiometer (UVP, Upland, CA). Mice were pretreated with 200 µl of 10 µM SB431542 (Sigma) or 200 µl acetone 1 h prior to the UV treatment. CD11c-DNR transgenic mice on C57Bl6 background were exposed to MED of 5.4 kJ/m2 from UV bulbs. Mice were shaved 48 h prior to UVB irradiation (UVB IR).
UVB carcinogenesis
Seven-week-old SKH1 mice (10–13 per group) were treated with UVB to induce tumors at the MED dose (described above) three times a week with pretreatment of 200 µl of acetone or 200 µl of 10 µM SB (Sigma) for 25 weeks and the tumors were harvested at the end of 30 weeks. The number of papillomas per mouse (>1mm3 in volume) was counted and measured using a Digital Vernier Calipers and a tumor profile was constructed. The harvested tumors were digested into a single-cell suspension and immunophenotyped for tumor-infiltrating lymphocytes and IFNγ secretion as described above.
Antibodies
The following antibodies were purchased from eBioscience (San Diego, CA): anti-CD16/32 (93), APC eFluor 750-anti-CD45 (30-F11), FITC- and eFluor 450-anti-major histocompatibility complex II (MHCII) (M5/114.15.2), PE- and Alexa Fluor 700-anti-CD11c (N418), FITC-anti-CD4 (GK1.5), PECy5-anti-CD8α (53–6.7), PE-anti-CD103 (2E7), PECy7-anti-B220 (RA3-6B2), PercpCy5.5-anti-CD11b (M1/70), PE-anti-CD62L (MEL-14), PECy5-anti-CD44 (IM7) and Foxp3 (FJK-16s). The following antibodies were purchased from BD Pharmingen (San Diego, CA): PE-anti-CD45 (30-F11), Alexa Fluor 700-anti-CD86 (GL1) and PECy7-anti-IFNγ (XMG1.2). The following antibodies were purchased from BioLegend: PercpCy5.5-anti-CD40 (3–23) and PECy5-anti-CD197 CCR7 (4B12). Alexa 568-anti-Epcam (G8.8) and Alexa 647-anti-CD207 (L31) antibody conjugates were generated as described previously (28). The following antibodies were purchased from Cell Signaling Technology: pSmad2 (#3101 for western blotting; #9510 for fluorescence-activated cell sorting analysis), Smad2/3-3102, p53-2524, GAPDH-2118, p21-6246 (Santa Cruz) and Actin-1501 (Millipore). Antibodies used for immunohistochemistry (IHC): CD45 (#550539; BD Pharmingen) and CD3-ε (M-20) (1127, Santa Cruz)
Flow cytometry
DCs were isolated from the inguinal LNs, the epidermis and dermis as described ( 29 ). Single-cell suspensions of DCs were incubated with CD16/32 followed by staining for extracellular surface antigens. For anti-CD207 staining, cells were fixed and permeabilized using fixation/permeabilization buffer (eBioscience) and incubated with anti-CD207 antibody in 0.2% saponin buffer. For phospho-Smad2 staining, the cells were fixed with 2% paraformaldehyde for 10 min followed by staining for surface antigens. Cells were then permeabilized by 90% methanol for 30 min and then stained for pSmad2. Cells were analyzed using a Fortessa LSRII (BD Biosciences, San Jose, CA). Single-cell suspensions were prepared from the inguinal LNs and the UV-exposed dorsal skin as described (Mohammed, 2010 13018 /id). Following incubation with phorbol 12-myristate 13-acetate/ionomycin and Brefeldin A (eBioscience) for 4.5 h at 37°C, the cells were stained for surface antigens, then fixed with 4% paraformaldehyde, permeabilized with 0.2% saponin buffer and stained for intracellular IFNγ. Cells were acquired on FC500 (Beckman Coulter, Indianapolis, IN) and analyzed using the FlowJo software (Tree Star, Ashland, OR).
Ear explant cultures
Ears of CD11c-DNR mice were excised immediately after a single MED dose of UVB and rinsed in 70% ethanol, and then in a solution containing 200 IU/ml penicillin and 200 µg/ml streptomycin for 5 min. Only the dorsal sides of the ear were considered exposed to UVB and were split and cultured in complete RPMI media with 10% fetal calf serum for 72 h at 37°C. The culture media was then harvested to analyze the cells that migrated from the explant, enumerated, stained and analyzed by flow cytometry. Ears of SKH1 mice with one ear treated with the vehicle and the other ear with SB were irradiated with UVB at 1 MED and excised and cultured as described above.
Analysis of protein and RNA
RNA and protein were isolated and analyzed by quantitative reverse transcription–PCR and immunoblotting as described (23).
Statistical analysis
All statistical analyses were performed using the GraphPad Prism software and the values are expressed as mean ± standard error of the mean (SEM) (GraphPad Software, La Jolla, CA). A two-tailed Student’s t-test was performed to compare the groups. P values of significance were represented as: * P < 0.05, **P < 0.01.
Results
ALK5 inhibition suppresses UVB-induced Smad phosphorylation in skin and reduces outgrowth of UVB-induced skin tumors
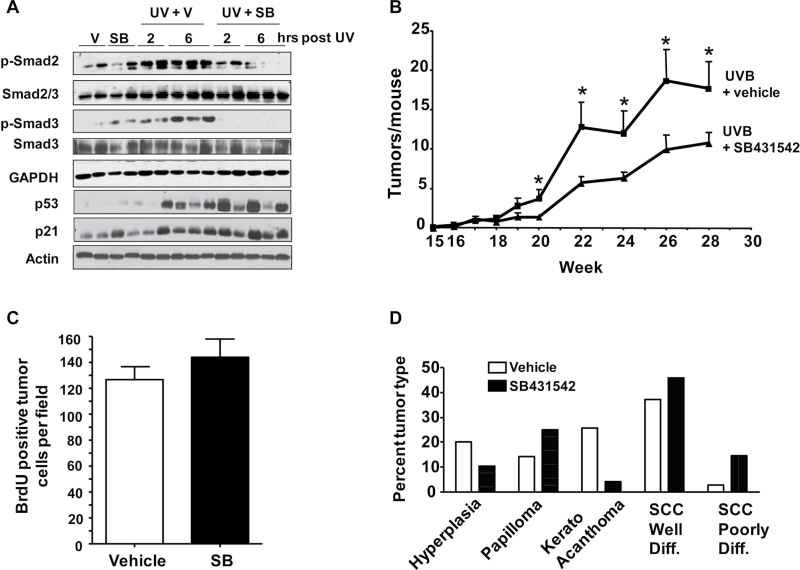
To test the effect of UV irradiation on the TGFβ1 pathway, we treated the skin of 7-week-old SKH1 mice with UVB at the minimum erythema dose (MED) of 2400 J/m2 (30). At both 2 and 6 h post-UVB, there was a rapid increase in the levels of phosphorylated Smad2 and phosphorylated Smad3, direct targets of ALK5 kinase, indicative of pathway activation. This increase was blocked with a 1 h pretreatment with 10 µM SB431542 (SB) (Figure 1A). In contrast, the characteristic UVB DNA damage response induction of p53 and p21 was unaffected with SB pretreatment, suggesting that the effects of SB inhibition are specific to the TGFβ signaling pathway, and that SB pretreatment was not acting as a non-specific sunblock. The increase in pSmad2 and pSmad3 in the skin was not associated with an increase in TGFβ1 message (Supplementary Figure S1, available at Carcinogenesis Online).
Fig. 1.
SB431542 suppresses UVB-induced Smad phosphorylation in skin and skin tumor formation. (A) Immunoblot of skin tissue at indicated times post-UVB IR from skin hairless (SKH1) mice pretreated with SB43152 (SB) or acetone (V) for 1h before UVB (2.4 kJ/m2). (B) Skin tumor number per week in SKH1 mice treated with 2.4 kJ/m2 UVB IR 3× per week for 25 weeks with 1h pretreatment with SB431542 (n = 13) or acetone vehicle (n = 10). Lesions ≥1mm3 in volume were counted. *Significantly different from acetone-treated group at indicated time points, P < 0.05. V = vehicle. (C) Bromodeoxyuridine-positive tumor cells per field. Tumor sections were stained with anti-bromodeoxyuridine by IHC and the number of positive cells per 40× field was determined and averaged from 6 to 10 fields per tumor, n = 17 tumors for vehicle and 10 for SB-treated tumors. (D) Tumor grade determined blindly from H&E stained sections, n = 35 and 48 tumors in control and SB-treated groups, respectively.
To determine if inhibition of TGFβ1 signaling with topical SB could block UVB-induced skin tumor formation similar to its effects in the two-stage chemical carcinogenesis model (23), we treated 7-week-old SKH1 mice in groups of 10–13 mice with 1 MED UVB 3× per week with or without SB. Mice were treated with this protocol for 25 weeks and tumors were harvested after an additional 5 weeks. Tumor development (lesions >1 mm3) in both acetone- and SB-treated mice was apparent at week 18 but the tumor number per mouse was reduced by 50% in the SB-treated mice at all subsequent time points (Figure 1B). However, there was no difference in overall tumor size or distribution at any time point or difference in tumor cell proliferation at study end (Figure 1C). Histopathology of tumors taken after 30 weeks showed that there were equivalent percentages of benign lesions (hyperplasias and papillomas) in both groups, but there was a trend toward less progressed malignancies in the vehicle-treated mice compared with SB-treated mice (Figure 1D).
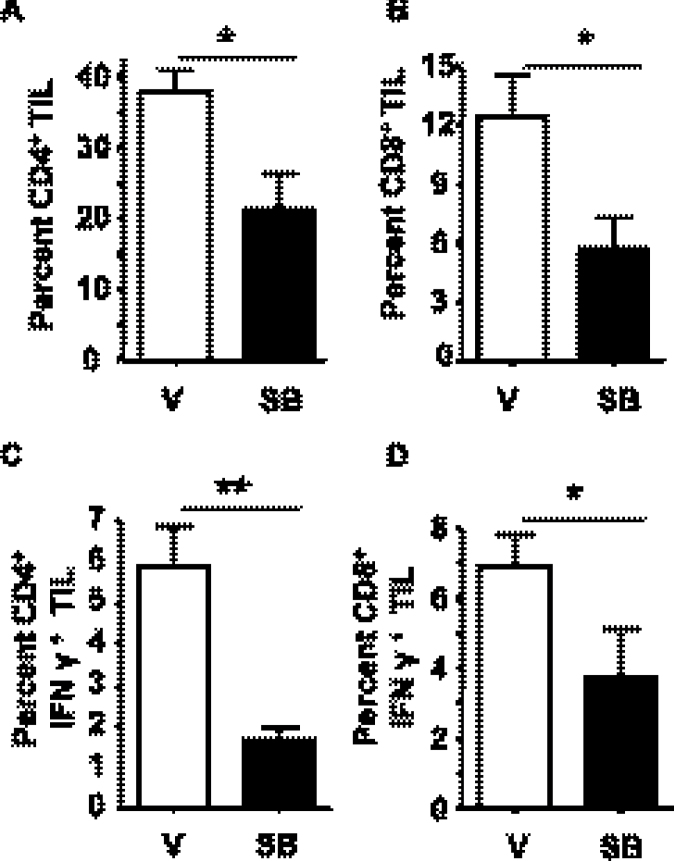
Because T-cell infiltration has been linked to both tumor suppression and progression (31), we isolated leukocytes from control and SB-treated tumors and analyzed frequencies of myeloid cells and tumor-infiltrating lymphocyte by flow cytometry. There was no significant difference in myeloid cells measured by flow cytometry (neutrophils: CD11b+ Ly6G+; macrophages: CD11b+ F4/80 Ly6G−) between tumors from vehicle- or SB-treated tumors (Supplementary Figure S2, available at Carcinogenesis Online). In contrast, the frequency of CD4+ as well as CD8+ T lymphocytes within the tumors that developed in SB-treated mice was reduced by 50% relative to vehicle control (Figure 2A and B). Additionally, compared with vehicle control tumors, the percentage of IFNγ+ CD4+ tumor-infiltrating lymphocyte was reduced from 6 to 1.5% and IFNγ+ CD8+ tumor-infiltrating lymphocyte reduced from 7 to 4% in SB-treated mice (Figure 2C and D).
Fig. 2.
Skin tumors arising in SB431542-treated mice have reduced frequency of IFNγ+ tumor infiltrating lymphocytes. Leukocytes were isolated from tumors arising in UVB-irradiated mice treated with vehicle or SB431542 at 30 weeks. The percentage of (A) CD4+ and (B) CD8+ T cells in total viable CD45+ leukocytes. The percentage of (C) IFNγ+ CD4+ and (D) CD8+ T cells of viable CD45+ leukocytes. N = at least seven tumors per group. Error bars = ±SEM. *P < 0.05 relative to indicated group; **P < 0.01 relative to indicated group.
ALK5 inhibition suppresses UVB-induced skin inflammation and UVB-induced T-cell activation in LN and skin
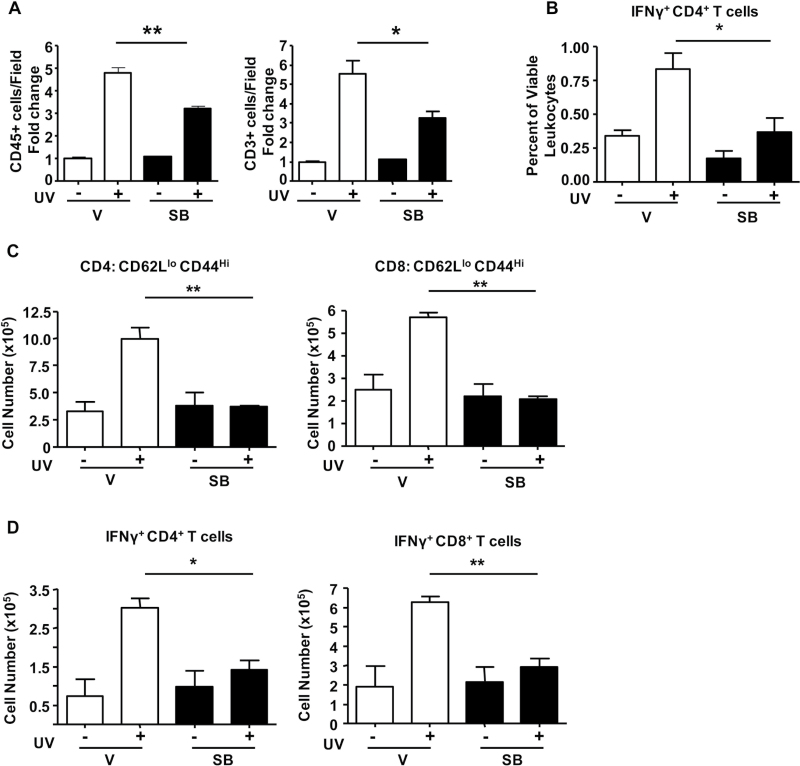
To understand how ALK5 inhibition could suppress tumor outgrowth, we examined the effects of SB on UVB-induced inflammation as this is critical for promotion of skin tumors. Seven-week-old SKH1 mice were irradiated with UVB 3× on alternate days at 2.4 kJ/m2 with or without a daily topical treatment of SB, and after 1 week, skin was analyzed by IHC and flow cytometry for changes in cutaneous responses to UVB. There was no significant difference between the groups in UVB-induced epidermal proliferation, as measured by anti-bromodeoxyuridine IHC (data not shown). UVB caused a 5-fold increase in total skin leukocytes (CD45+) and T (CD3+) cells and this was significantly suppressed by topical SB treatment, by 35 and 50%, respectively (Figure 3A; Supplementary Figure S3, available at Carcinogenesis Online). Although there was no significant difference in the percentage of myeloid cells (Supplementary Figure S2, available at Carcinogenesis Online), flow analysis showed that SB treatment significantly suppressed the UVB-induced increase in IFNγ+ CD4+ T-helper lymphocytes in the skin (Figure 3B). There was no significant increase in Th17 cells in UVB-irradiated skin or effect of SB on Th17 cells in the skin with or without UVB. Although UVB did not significantly increase IFNγ+ CD8+ T lymphocytes at this time point, SB by itself reduced the frequency of these cells in the skin (data not shown). Treg cells were <1% of CD45+ cells in the skin after UVB as measured by flow cytometry. Using IHC, we found a slight increase in rare FoxP3+ cells in the skin with UVB from 0.70 cells per 40× field to 1.2 cells per field and this was marginally increased to 1.97 cells per field with SB treatment plus UVB.
Fig. 3.
SB431542 suppresses UVB-induced skin inflammation and T-cell activation in LN and skin. SKH1 mice were irradiated 3× over 1 week with 1 MED UV radiation and treated daily with either acetone vehicle (V) or SB431542 (SB). Quantitation of (A) CD45+ and CD3+ cells in 7–10 random fields of view (FOV) from at least three stained sections per group. The average of the vehicle-treated control was 29.52 CD45+ cells per FOV and 29.62 CD3+ cells per FOV. n = 2 for controls and n = 3–4 for UVB IR groups. Error bars = ±SEM. *P < 0.05; **P < 0.01. Scale bar = 100 µm. (B) Percentage of CD4+ IFNγ+ T cells in viable CD45+ leukocytes isolated from SKH1 skin. (C) Cell number of effector CD4+ and CD8+ T cells (CD44Hi CD62LLo) from SDLN of mice. (D) Cell number of IFNγ+ CD4+ and CD8+ T cells in SDLN of mice treated as in (A). n = 2 for controls and n = 3–4 for UVB-irradiated groups.
Topical SB also suppressed UVB-induced T lymphocyte activation within the SDLNs. With UVB, there was a significant increase in CD44high CD62Llow central memory CD4+ and CD8+ T cells from 3 to 10 million and 2.5 to 6 million per LN, respectively. SB suppressed this increase in memory cells by 3- and 2-fold, respectively (Figure 3C). UVB also caused a 4-fold increase in IFNγ+ CD4+ T cells and 2-fold increase in IFNγ+ CD8+ T cells, which was reduced to control levels by SB (Figure 3D). Supplementary Figure S4, available at Carcinogenesis Online, shows that SB had the same effect on T-cell activation in the SDLN and skin with a 2 week chronic UVB IR protocol (6× on alternate days, with or without daily SB treatment). These results show that suppression of UVB-induced T-cell activation by topical SB431542 occurs early and is maintained in tumors even 5 weeks after removal from SB treatment.
SB431542 blocks UVB-induced migration of dDC subsets to the SDLNs and in ear explant culture
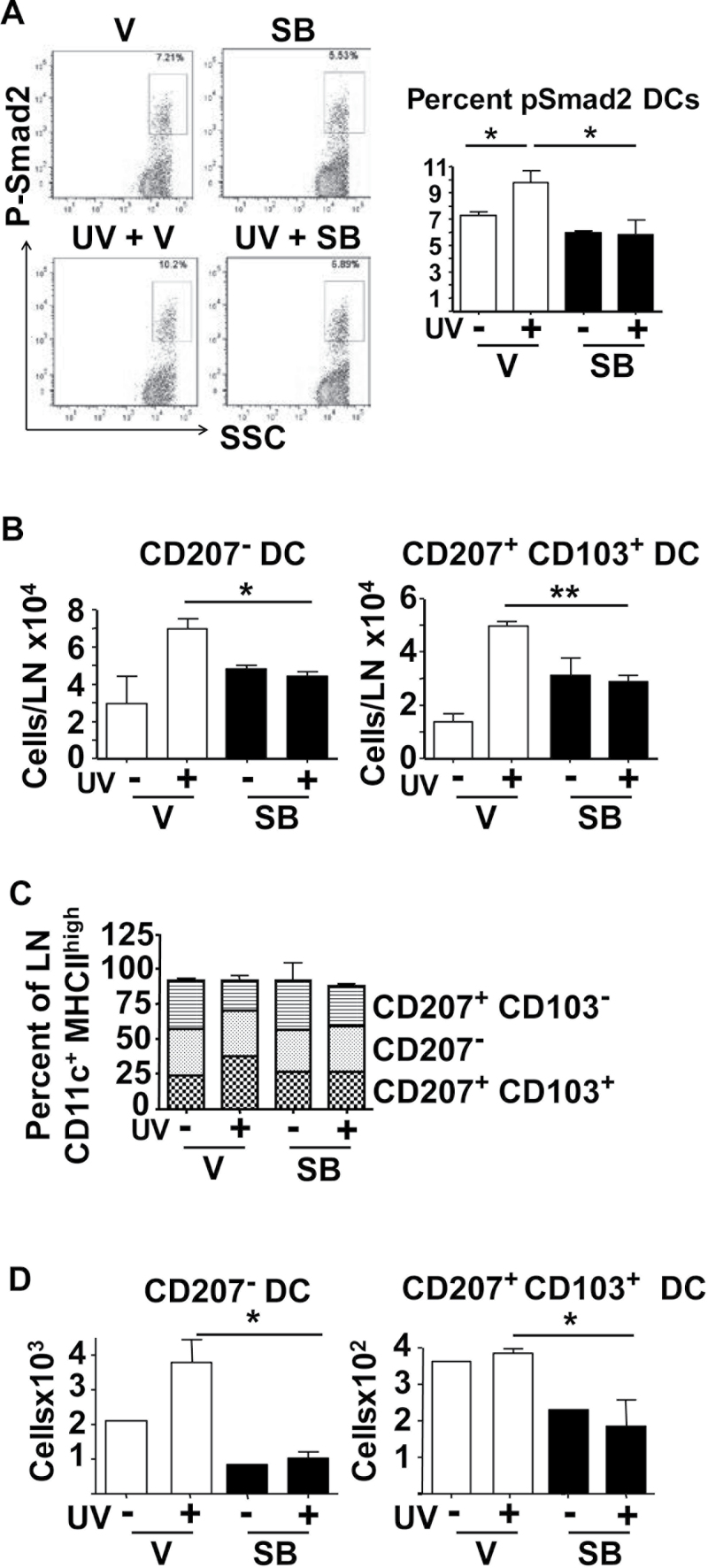
Because DCs are potential mediators of UVB-induced inflammatory responses and critical antigen-presenting cells in the skin, we next tested whether UVB increased Smad2 phosphorylation in cutaneous DC subsets, and if this could be inhibited by SB. At 2 h post-UVB IR, we analyzed the pSmad2 levels in different epidermal and dDC subsets by flow cytometry, gating DCs as CD45+ MHCIIhigh CD11c+ cells. UVB caused a small but statistically significant increase in percentage of pSmad2+ dDCs from 7 to 9.5% and this increase was blocked by topical SB treatment (Figure 4A). We did not observe a significant change in the mean fluorescence intensity of pSmad2+ dDCs. Importantly, there was no difference in the percentages of pSmad2+ LCs from the epidermis with UVB and SB did not decrease the pSmad2 levels below the baseline (Supplementary Figure S5, available at Carcinogenesis Online).
Fig. 4.
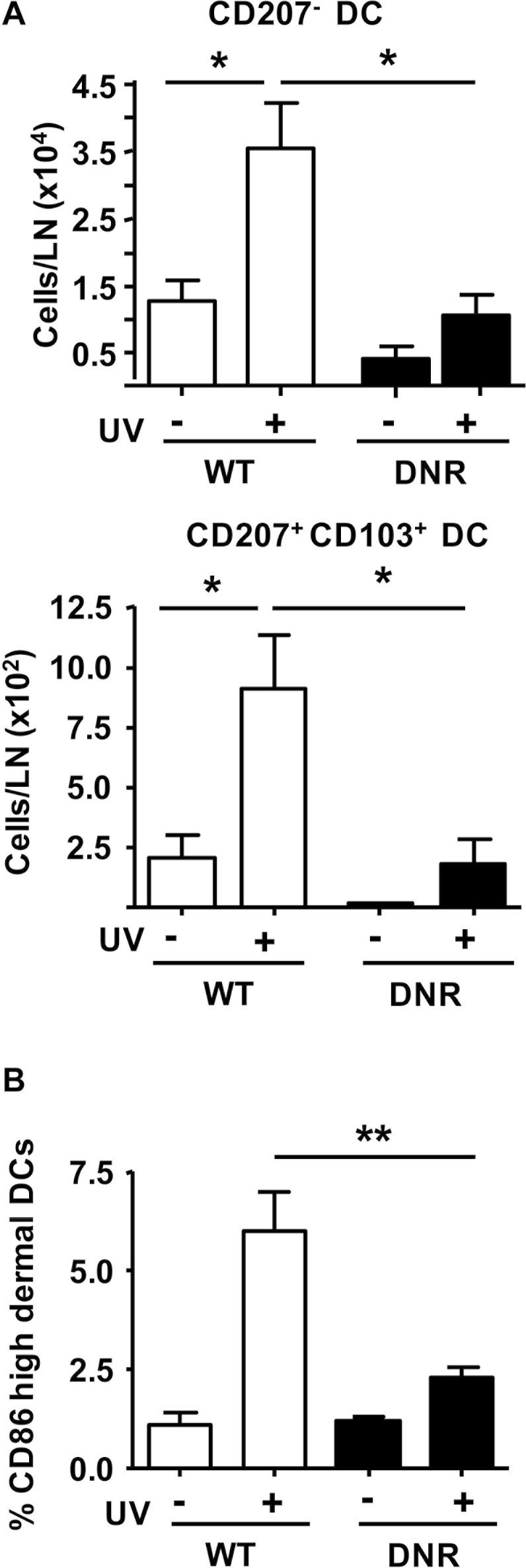
SB431542 blocks UVB-induced migration of dDC. (A) Representative fluorescence-activated cell sorting profile and quantitation of pSmad2+ cells in MHCIIHi CD11c+ DCs isolated from dermis of SKH1 mice 2h after UVB IR pretreated 1h with SB431542 (SB) or acetone (V). (B) Numbers of CD207− and CD207+ CD103+ DC subsets in SDLNs of SKH1 mice 72h post-UVB IR with 1h pretreatment with SB431542 or vehicle. (C) Percentages of indicated DC subsets in SDLN 72h after UVB IR with 1h pretreatment with SB431542 or acetone (V). (D) Effect of SB432541 on UVB IR-induced migration of DCs in ear explant culture 72h post-UVB. n = 2–3, controls; n = 3–4, UVB IR groups. Error bars = ±SEM. *P < 0.05 relative to indicated group; **P < 0.01 relative to control mice or indicated group.
To test the role of TGFβ1 pathway in UVB IR-induced activation and migration of skin resident DCs, we analyzed migratory DC subsets in SDLN 72 h post-UVB IR with or without SB pretreatment. After gating on MHCIIhigh CD11c+ cells and excluding LN resident MHCIIhigh CD8+ DCs and MHCIIhigh B220+ DCs, we defined three DC subsets in the LN for analysis that responded to UVB: CD207+ CD103+ (CD207+ dDCs), CD207+ CD103− and CD207−. UVB IR significantly increased the total numbers of MHCIIhigh CD11c+ cells in the SDLNs from 0.21 to 0.4 million but in the presence of SB, MHCIIhigh CD11c+ cells only increased to 0.27 million following UVB IR (data not shown). UVB increased the CD207− subset in the SDLN 2.3-fold, from 3 × 104 to 7 × 104 cells per LN, and CD207+ dDCs subset 3.5-fold, from 1.4 × 104 to 5 × 104 cells per LN (Figure 4B), and this increase was suppressed by SB. UVB also caused a selective increase in the proportion of the CD207+ dDC subset in the SDLN, which did not occur in SB-treated mice (Figure 4C). Similar results were obtained with chronic UVB, where mice were irradiated three times with UVB over 1 week and daily with SB or acetone. Again, SB suppressed UVB-induced migration of dDC to the SDLN, but in this case, chronic SB treatment also reduced the steady-state levels of dDC in the SDLN (Supplementary Figure S6, available at Carcinogenesis Online).
To further analyze the dependence of UVB-induced DC migration on TGFβ signaling, we prepared paired ear explant cultures from SKH1 mice treated with vehicle or SB 1 h prior to UVB IR and measured migration of DC subsets into the culture media after 72 h. Both baseline migration and UVB-induced migration of the CD207− DC subset was suppressed by SB and baseline migration of the CD207+ dDC subset was suppressed by 50% (Figure 4D). Finally, there was no effect of a single SB treatment on LC numbers in the SDLN with or without UVB or on migration in an ear explant culture assay (Supplementary Figure S7A and S7B, available at Carcinogenesis Online) but multiple SB treatments reduced the steady-state numbers of LC in the SDLN (Supplementary Figure S7C, available at Carcinogenesis Online).
Genetic blockade of TGFβ signaling in skin CD11c+ DCs suppresses UV-induced activation and migration
We used mice expressing a CD11c+ promoter-driven dominant-negative TGFβ type II receptor transgene (CD11c-DNR) (26) to block TGFβ1 signaling in DCs and determine effects on their UVB response. SDLNs were harvested 72 h after UVB IR and the numbers and percentages of CD207−, CD207+ CD103+ (CD207+ dDCs) and CD207+ CD103– skin DC subsets were analyzed in the SDLNs. In untreated steady-state conditions, the number of CD207+ dDC and CD207− DC subsets in the SDLN in DNR mice was reduced to 0.1- and 0.3-fold, respectively, compared with WT controls. Although the fold increase in CD207+ dDC and CD207− DC was similar in UVB-treated DNR mice, the total numbers of each subset in the SDLN after UVB IR was significantly reduced by 2- and 3.5-fold, respectively (Figure 5A).
Fig. 5.
Blockade of TGFβ signaling in dermal CD11c+ cells suppresses UV-induced activation and migration. (A) Numbers of CD207− and CD207+ CD103+ DCs in SDLNs of WT or CD11c-DNR transgenic mice (DNR) 72h after UVB IR. (B) Expression of activation marker CD86 in WT and CD11c-DNR dDCs 24h after UVB IR. Mean fluorescence intensity of CD86 was established by flow cytometry and the percentage of CD86High subset of dDCs was plotted for the different treatment groups. n = 2–3 for controls and n = 3–4 for UVB IR groups. Error bars = ±SEM. *P < 0.05 relative to indicated group; **P < 0.01 relative to indicated group.
Although there was no significant difference in steady-state or UVB-induced migration of the CD207+ CD103+ DC subset between DNR and WT mice in ear explants cultures (data not shown), the steady-state CD207− DC migration was decreased in DNR ear explants and UVB-induced migration was blocked (Supplementary Figure S8, available at Carcinogenesis Online). There was a reduction in steady-state numbers of LCs in the SDLNs in the CD11c-DNR mice but a similar fold increase in response to UVB IR (Supplementary Figure S9A, available at Carcinogenesis Online), but LC migration in ear explants cultures was not significantly different between CD11c-DNR and WT mice (Supplementary Figure S9B, available at Carcinogenesis Online).
To test whether genetic inactivation of TGFβ1 signaling prevented activation of dDC, we analyzed expression of CCR7 and CD86 24 h after UVB as increased expression of these molecules is associated with activation of skin DC in response to inflammatory stimuli (32–34). Although CCR7 was undetectable, the UVB IR-induced increase in CD86high DC in the dermis was significantly reduced in CD11c-DNR mice (Figure 5B). This suggests that UV-induced activation of dDC is impaired by blocking TGFβ1 signaling in these cells.
Discussion
Acute UVB IR is a potent activator of cutaneous immune responses. Low-dose UV is immunosuppressive in part through its effects on LCs and Tregs (35,36), whereas UVB IR doses equal to or greater than the MED cause an inflammatory response (3,37,38), that is linked to accelerated tumor development (30,39,40). Many studies have demonstrated that agents, which reduce inflammation in the skin through a number of different signaling pathways, significantly reduce UVB-induced skin tumors (41–43), pointing to a general tumor-promoting role for UVB-induced inflammation. Our previous studies showed that TGFβ1 has proinflammatory activities in skin carcinogenesis and immune responses (23–25). Similar to effects on chemical skin carcinogenesis, the ALK5 inhibitor SB431542 also suppressed UV-induced inflammation and total skin tumor numbers but enhanced the number of squamous cell carcinomas and poorly differentiated squamous cell carcinoma. We found no difference in initial p53 responses to UVB IR, UVB-induced epidermal proliferation, tumor volume at any time point or tumor cell proliferation or myeloid cells at study end, but rather a significant decrease in T-cell activation and IFNγ+ CD4 and CD8+ T cells, which was evident after 1 and 2 weeks UV exposure in skin and 5 weeks after SB treatment ceased in tumors, suggesting that this difference is linked to suppression of tumor formation and potentially to enhanced progression. Although it remains to be demonstrated directly, our results are consistent with previous studies demonstrating (i) a tumor-promoting role for IFNγ in the two-stage skin carcinogenesis model (44) and (ii) the well-established role of IFNγ in tumor surveillance (45).
While it is possible that SB could be acting directly on T cells in skin and there are reports that genetic blockade of TGFβ1 signaling in CD8+ T cells enhances Tc17 cells (46), we did not observe any significant change in IL17+ T cells in SB-treated mice. Instead our data support the idea that TGFβ1 signaling in DCs is critical for this T-cell response to UVB IR. There are multiple DC subsets in the skin but their role in responses to UV and effects of TGFβ1 are only partially understood. LCs have been implicated in UV-induced tolerance (35,47,48) possibly through UV inhibition of LC migration (48–50), although cyclopyrimidine dimer positive CD207+ LCs have been detected in the SDLNs after chronic UV exposure (51). The effect of UVB on dDC subsets is not clear as these earlier studies did not differentiate between migratory LCs and CD207+ dDCs, and the effects on CD207− dDC have not been reported (52–54). While in vitro studies have largely implicated TGFβ1 as an immunosuppressive cytokine for DCs (12–14) and mouse models have shown an absolute requirement for TGFβ1 signaling in LC for epidermal residency and maintenance of an immature phenotype (19–22), we recently showed that overexpression of TGFβ1 by keratinocytes causes rapid migration of dDC subsets and provokes an immunostimulatory response in a contact hypersensitivity assay (25). In contrast to some studies implying UVB-induced inactivation of TGFβ1 signaling in skin (55–57), our results show that UVB IR at a MED inflammatory dose activates TGFβ signaling as measured by increased pSmad2 and pSmad3 by immunoblotting of whole skin and pSmad2 levels by flow cytometry in dDCs. Because we did not observe an increase in TGFβ1 transcript levels, this suggests, at least at the level of whole skin, that pathway activation results from activation of latent TGFβ1, but this remains to be demonstrated conclusively.
Although there were differences in percentage of subsets in the SDLN between SKH1 and C57Bl/6, we consistently observed a UV-induced increase in SDLN migration of CD207− and CD207+ dDC, with CD207− forming the major subset. Similarly, in ear explant cultures of UV-irradiated mice, there was an increase in the migration of CD207+ dDC and CD207− subsets although, as expected with the inflammatory setting of these cultures (58), the controls by themselves showed enhanced migration. Within the total DC subset migrating to the SDLNs, there was a selective increase in CD207+ dDCs migration relative to the other DC subsets and this migration was significantly suppressed when TGFβ1 signaling was inhibited. Consistent with their reduced migration, CD86 upregulation on dDCs in response to UVB was suppressed in DNR mice. This suggests that TGFβ1 signaling is important in activation of dDC in response to UVB, but direct effects on migratory ability have not been ruled out.
Given the importance of TGFβ1 signaling in LC biology and epidermal residency as well as effects of low-dose UV on LC, it is surprising that UVB at 1 MED dose or short-term SB treatment had minimal effect on LC migration although this is consistent with the lack of change in pSmad2. As with the other DC subsets, repeated SB dosing did impact LC numbers in the SDLN in the absence of UVB. It is possible that the high UVB doses that we have used causes apoptosis in LCs (49,59), which masks any increase in migration over steady state. However, we did not observe significant differences in LC numbers in epidermal sheets at 24 or 48 h post-UVB between vehicle- and SB-treated skin (data not shown).
CD207+ dDCs are important in skin immunity with roles in antigen presentation (60), generating adaptive immune responses (8,61) and contact hypersensitivity (7). Previous studies have reported TGFβ signaling to be non-essential for the steady-state signaling and function of CD207+ dDCs (8,21). However, our results indicate that the steady-state migration of CD207+ dDCs as well as CD207− DCs was reduced with genetic inhibition of TGFβ in DCs. Coupled with the significant suppression of UV-induced T-cell activation in the SDLN and skin and suppression of cutaneous inflammation, these data strongly implicate TGFβ1 signaling in CD207+ dDC in UV-induced cutaneous inflammatory immune response.
A critical question is whether TGFβ1 signaling is important only for steady-state migration of dDC, and the effect on UVB-induced migration is superimposed on that migration defect, or if activation of TGFβ1 signaling is essential for UVB-induced migration. The observation that ~7% of dDCs were pSmad2+ in the steady state and CD11c-DNR mice had decreased steady-state numbers of CD207+ dDC and CD207− DC in the SDLN is consistent with the former hypothesis. In SKH1 mice, one application of SB431542 did not cause significant reduction in steady-state DC numbers in the SDLN even though UVB-induced migration was blocked, whereas multiple applications of SB431542 altered steady-state DC numbers. These results suggest that long-term inhibition of TGFβ1 signaling in dDC affects steady-state migration, whereas acute inhibition with SB431542 does not. Whether the defective DC migration we observe in CD11c-DNR mice reflects direct requirement of TGFβ1 signaling in dDC for activation or an indirect effect through synergy with other inflammatory pathways remains to be determined. It is also possible that migrated dDCs with blocked TGFβ1 signaling are also deficient in T-cell activation and this contributes to the reduced inflammatory response.
Taken together, these results suggest a model in which TGFβ1, rather than acting as an immunosuppressive cytokine, promotes dDC migration in response to UVB IR and, in conjunction with subsequent T-cell activation and cutaneous inflammation, promotes UV-induced skin tumor formation.
Supplementary material
Supplementary Figures S1–S9 can be found at http://carcin. oxfordjournals.org/
Funding
National Institutes of Health training grant in ‘ Animal Models of Inflammation’ (T32 AI074551-01A to A.R.); Dermal Toxicology-Society of Toxicology research grant and College of Agricultural Sciences research grant (to A.R.); grants from the Pardee Foundation and National Institutes of Health (R01 CA117957 to A.B.G.).
Supplementary Material
Acknowledgements
We thank Dr Mark C.Udey, NCI, for providing Alexa 568-anti-Epcam (G8.8) and Alexa 647-anti-CD207 (L31) antibody conjugates, the Huck Institute Flow Cytometry Core Facility for help with flow cytometry and Kyle Breech for help with genotyping and maintenance of mice.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- ALK5
activin-like kinase 5
- DCs
dendritic cells
- dDCs
dermal dendritic cells
- IFNγ
interferon γ
- IHC
immunohistochemistry
- LCs
Langerhans cells
- LN
lymph node
- MED
minimal erythemal dose
- MHC II
major histocompatibility complex II
- SDLN
skin-draining lymph node
- SEM
standard error of the mean
- SKH1
skin hairless mouse 1
- TGFβ1
transforming growth factor beta 1
- UVB IR
ultraviolet B irradiation
- WT
wild-type.
References
- 1. Matsumura Y., et al. (2004). Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol., 195, 298–308 [DOI] [PubMed] [Google Scholar]
- 2. Grivennikov S.I., et al. (2010). Inflammation and oncogenesis: a vicious connection. Curr. Opin. Genet. Dev., 20, 65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Terui T., et al. (2001). Occurrence of neutrophils and activated Th1 cells in UVB-induced erythema. Acta Derm. Venereol., 81, 8–13 [DOI] [PubMed] [Google Scholar]
- 4. Clydesdale G.J., et al. (2001). Ultraviolet light induced injury: immunological and inflammatory effects. Immunol. Cell Biol., 79, 547–568 [DOI] [PubMed] [Google Scholar]
- 5. Romani N., et al. (2010). Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol. Rev., 234, 120–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaplan D.H. (2010). In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol., 31, 446–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fukunaga A., et al. (2008). Dermal dendritic cells, and not Langerhans cells, play an essential role in inducing an immune response. J. Immunol., 180, 3057–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagao K., et al. (2009). Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc. Natl Acad. Sci. U S A, 106, 3312–3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Igyártó B.Z., et al. (2011). Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity, 35, 260–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakagawa S., et al. (1999). Differential modulation of human epidermal Langerhans cell maturation by ultraviolet B radiation. J. Immunol., 163, 5192–5200 [PubMed] [Google Scholar]
- 11. Geissmann F., et al. (1999). TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J. Immunol., 162, 4567–4575 [PubMed] [Google Scholar]
- 12. Fainaru O., et al. (2007). TGFbeta-dependent gene expression profile during maturation of dendritic cells. Genes Immun., 8, 239–244 [DOI] [PubMed] [Google Scholar]
- 13. Ohtani T., et al. (2009). TGF-beta1 dampens the susceptibility of dendritic cells to environmental stimulation, leading to the requirement for danger signals for activation. Immunology, 126, 485–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Torres-Aguilar H., et al. (2010). Tolerogenic dendritic cells generated with different immunosuppressive cytokines induce antigen-specific anergy and regulatory properties in memory CD4+ T cells. J. Immunol., 184, 1765–1775 [DOI] [PubMed] [Google Scholar]
- 15. Laouar Y., et al. (2008). TGF-beta signaling in dendritic cells is a prerequisite for the control of autoimmune encephalomyelitis. Proc. Natl Acad. Sci. U S A, 105, 10865–10870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ramalingam R., et al. (2012). Dendritic cell-specific disruption of TGF-β receptor II leads to altered regulatory T cell phenotype and spontaneous multiorgan autoimmunity. J. Immunol., 189, 3878–3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aliahmadi E., et al. (2009). TLR2-activated human Langerhans cells promote Th17 polarization via IL-1beta, TGF-beta and IL-23. Eur. J. Immunol., 39, 1221–1230 [DOI] [PubMed] [Google Scholar]
- 18. Bonnefoy F., et al. (2011). TGF-beta-exposed plasmacytoid dendritic cells participate in Th17 commitment. J. Immunol., 186, 6157–6164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borkowski T.A., et al. (1996). A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: the skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. J. Exp. Med., 184, 2417–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaplan D.H., et al. (2007). Autocrine/paracrine TGFbeta1 is required for the development of epidermal Langerhans cells. J. Exp. Med., 204, 2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kel J.M., et al. (2010). TGF-beta is required to maintain the pool of immature Langerhans cells in the epidermis. J. Immunol., 185, 3248–3255 [DOI] [PubMed] [Google Scholar]
- 22. Zahner S.P., et al. (2011). Conditional deletion of TGF-βR1 using Langerin-Cre mice results in Langerhans cell deficiency and reduced contact hypersensitivity. J. Immunol., 187, 5069–5076 [DOI] [PubMed] [Google Scholar]
- 23. Mordasky Markell L., et al. (2010). Use of a TGFbeta type I receptor inhibitor in mouse skin carcinogenesis reveals a dual role for TGFbeta signaling in tumor promotion and progression. Carcinogenesis, 31, 2127–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pérez-Lorenzo R., et al. (2010). Transforming growth factor beta1 enhances tumor promotion in mouse skin carcinogenesis. Carcinogenesis, 31, 1116–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mohammed J., et al. (2013). TGFβ1 overexpression by keratinocytes alters skin dendritic cell homeostasis and enhances contact hypersensitivity. J. Invest. Dermatol., 133, 135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laouar Y. et al. (2005) Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat. Immunol., 6, 600–607 [DOI] [PubMed] [Google Scholar]
- 27. Melnikova V.O. et al. (2005) Fate of UVB-induced p53 mutations in SKH-hr1 mouse skin after discontinuation of irradiation: relationship to skin cancer development. Oncogene, 24, 7055–7063 [DOI] [PubMed] [Google Scholar]
- 28. Gaiser M.R. et al. (2012) Cancer-associated epithelial cell adhesion molecule (EpCAM; CD326) enables epidermal Langerhans cell motility and migration in vivo. Proc. Natl Acad. Sci. U.S.A. [DOI] [PMC free article] [PubMed]
- 29. Mohammed J. et al. (2010) TGFbeta1-induced inflammation in premalignant epidermal squamous lesions requires IL-17. J. Invest Dermatol., 130, 2295–2303 [DOI] [PubMed] [Google Scholar]
- 30. Wilgus T.A., et al. (2003). Chemotherapeutic efficacy of topical celecoxib in a murine model of ultraviolet light B-induced skin cancer. Mol. Carcinog., 38, 33–39 [DOI] [PubMed] [Google Scholar]
- 31. de Visser K.E., et al. (2006). Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer, 6, 24–37 [DOI] [PubMed] [Google Scholar]
- 32. Banchereau J., et al. (1998). Dendritic cells and the control of immunity. Nature, 392, 245–252 [DOI] [PubMed] [Google Scholar]
- 33. Caux C., et al. (1994). Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med., 180, 1263–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ohl L., et al. (2004). CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity, 21, 279–288 [DOI] [PubMed] [Google Scholar]
- 35. Schwarz A., et al. (2010). Langerhans cells are required for UVR-induced immunosuppression. J. Invest. Dermatol., 130, 1419–1427 [DOI] [PubMed] [Google Scholar]
- 36. Schwarz T. (2008). 25 years of UV-induced immunosuppression mediated by T cells-from disregarded T suppressor cells to highly respected regulatory T cells. Photochem. Photobiol., 84, 10–18 [DOI] [PubMed] [Google Scholar]
- 37. Terui T., et al. (2000). Mediators of inflammation involved in UVB erythema. J. Dermatol. Sci., 23 (s uppl. 1), S1–S5 [DOI] [PubMed] [Google Scholar]
- 38. Halliday G.M., et al. (2008). Inflammatory doses of UV may not be necessary for skin carcinogenesis. Photochem. Photobiol., 84, 272–283 [DOI] [PubMed] [Google Scholar]
- 39. Rebel H., et al. (2005). Relationship between UV-induced mutant p53 patches and skin tumours, analysed by mutation spectra and by induction kinetics in various DNA-repair-deficient mice. Carcinogenesis, 26, 2123–2130 [DOI] [PubMed] [Google Scholar]
- 40. Fischer S.M., et al. (1999). Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol. Carcinog., 25, 231–240 [PubMed] [Google Scholar]
- 41. Khan N., et al. (2012). Pomegranate fruit extract inhibits UVB-induced inflammation and proliferation by modulating NF-κB and MAPK signaling pathways in mouse skin. Photochem. Photobiol., 88, 1126–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Burns E.M., et al. (2013). Preventative topical diclofenac treatment differentially decreases tumor burden in male and female Skh-1 mice in a model of UVB-induced cutaneous squamous cell carcinoma. Carcinogenesis, 34, 370–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mikulec C.D., et al. (2013). The chemopreventive efficacies of nonsteroidal anti-inflammatory drugs: the relationship of short-term biomarkers to long-term skin tumor outcome. Cancer Prev. Res. (Phila)., 6, 675–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xiao M., et al. (2009). IFNgamma promotes papilloma development by up-regulating Th17-associated inflammation. Cancer Res., 69, 2010–2017 [DOI] [PubMed] [Google Scholar]
- 45. Kaplan D.H., et al. (1998). Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc. Natl Acad. Sci. U S A, 95, 7556–7561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dwivedi V.P., et al. (2012). Transforming growth factor-β protein inversely regulates in vivo differentiation of interleukin-17 (IL-17)-producing CD4+ and CD8+ T cells. J. Biol. Chem., 287, 2943–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Timares L., et al. (2008). DNA damage, apoptosis and Langerhans cells–Activators of UV-induced immune tolerance. Photochem. Photobiol., 84, 422–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mizuno K., et al. (2004). Ultraviolet B radiation suppresses endocytosis, subsequent maturation, and migration activity of Langerhans cell-like dendritic cells. J. Invest. Dermatol., 122, 300–306 [DOI] [PubMed] [Google Scholar]
- 49. Kölgen W., et al. (2002). Epidermal Langerhans cell depletion after artificial ultraviolet B irradiation of human skin in vivo: apoptosis versus migration. J. Invest. Dermatol., 118, 812–817 [DOI] [PubMed] [Google Scholar]
- 50. Mittelbrunn M., et al. (2005). Solar-simulated ultraviolet radiation induces abnormal maturation and defective chemotaxis of dendritic cells. J. Invest. Dermatol., 125, 334–342 [DOI] [PubMed] [Google Scholar]
- 51. Vink A.A., et al. (1996). Localization of DNA damage and its role in altered antigen-presenting cell function in ultraviolet-irradiated mice. J. Exp. Med., 183, 1491–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gambichler T., et al. (2006). Alterations of TGF-beta/Smad mRNA expression in atopic dermatitis following narrow-band ultraviolet B phototherapy: results of a pilot study. J. Dermatol. Sci., 44, 56–58 [DOI] [PubMed] [Google Scholar]
- 53. Yin L., et al. (2003). The crucial role of TGF-beta in the age-related alterations induced by ultraviolet A irradiation. J. Invest. Dermatol., 120, 703–705 [DOI] [PubMed] [Google Scholar]
- 54. Ehrhart J.C., et al. (2003). UVB-induced mutations in human key gatekeeper genes governing signalling pathways and consequences for skin tumourigenesis. Photochem. Photobiol. Sci., 2, 825–834 [DOI] [PubMed] [Google Scholar]
- 55. Quan T., et al. (2004). Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. Am. J. Pathol., 165, 741–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Han K.H., et al. (2005). Alteration of the TGF-beta/SMAD pathway in intrinsically and UV-induced skin aging. Mech. Ageing Dev., 126, 560–567 [DOI] [PubMed] [Google Scholar]
- 57. Yang G., et al. (2008). Inhibition of PAX3 by TGF-beta modulates melanocyte viability. Mol. Cell, 32, 554–563 [DOI] [PubMed] [Google Scholar]
- 58. Stoitzner P., et al. (1999). Migration of Langerhans cells and dermal dendritic cells in skin organ cultures: augmentation by TNF-alpha and IL-1beta. J. Leukoc. Biol., 66, 462–470 [PubMed] [Google Scholar]
- 59. Rattis F.M., et al. (1998). Effects of ultraviolet B radiation on human Langerhans cells: functional alteration of CD86 upregulation and induction of apoptotic cell death. J. Invest. Dermatol., 111, 373–379 [DOI] [PubMed] [Google Scholar]
- 60. Henri S., et al. (2010). CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J. Exp. Med., 207, 189–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. King I.L., et al. (2010). GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in Th effector cell differentiation after subcutaneous immunization. J. Exp. Med., 207, 953–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.