Abstract
Renal cell carcinoma (RCC) may metastasize anywhere in the body and sometimes the primary tumor is missing and necessitates extensive investigations to detect. In this report, we describe a case of RCC metastasizing to the thigh in a 70 year old male with a highly pleomorphic morphology suggesting a high grade sarcoma that showed unequivocal positivity for desmin directing the diagnosis for pleomorphic rhabdomyosarcoma. After completion of 33 cycles of radiotherapy, the patient developed large intraabdominal mass that showed conventional areas of RCC with immunoreactivity for CD10, CK, EMA, carbonic anhydrase IX and vimentin. The tumor cells in other areas resembled that of thigh mass which raised suspicions whether the two masses represented the same tumor or not. Surprisingly, the tumor cells of thigh mass showed diffuse positivity for CD10 and focal expression for CK, EMA and carbonic anhydrase IX. Extensive investigations failed to detect any primary renal lesions. The present case demonstrated that RCC can metastasize to virtually any body site and can have significant morphologic overlap with other non-renal neoplasms. Absence of primary origin of RCC according to radiological and operative data should not hinder the diagnosis of metastatic RCC. RCC with sarcomatoid and rhabdoid features carries aggressive behavior manifested by great metastatic potential and short survival time.
Key words: RCC, rhabdoid variant, sarcomatoid variant, desmin
Introduction
RCC similar to other malignant tumors my undergo dedifferentiation to more aggressive histologic types. It may acquire spindle cell morphology, which is designated as sarcomatoid differentiation and it may acquire rhabdoid features, which is designated as rhabdoid differentiation.1 Rhabdoid features was described in other malignancies including carcinomas,2 melanoma,3 and sarcomas.4,5Cohesive epithelioid cells with eccentric vesicular nuclei, prominent nucleoli and intracytoplasmic hyaline esinophilic globules are the main features of rhabdoid cells.6 These cells resembled rhabdomyoblasts in morphology but they differed immunohistochemically and ultrastructurally.1 The proportion of rhabdoid components in RCC ranges from 1 to 90% of the total tumor volume,6-8 however occasional cases were composed entirely of rhabdoid cells without detection of conventional RCC.9,10
Sarcomatoid RCC is a rare renal malignancy with a reported incidence ranging between 0.7 and 13.2% of all renal parenchymal carcinomas.11 Five percent of patients with RCC had sarcomatoid component especially with chromophobe (8.7%) and conventional types of RCC (5%).12 Regions of rhabdoid differentiation are reported in 15% of sarcomatoid RCC,13 whereas in 39%, the two components are intimately admixed.14 The present study demonstrates some of the unusual features of RCC such as its presentation as a metastatic thigh mass with absence of primary tumor, combined aggressive histologic features such as sarcomatoid and rhabdoid dedifferentiation, expression of desmin as a type of mesenchymal metamorphosis.
Acquiring sarcomatoid morphology, unusual immunophenotyping and presentation as thigh mass have directed the diagnosis towards sarcoma more than carcinoma.
Case Report
Male patient 70 years old presented with a thigh mass measured 35×24×9 cm with grayish white lobulated cut section. Histopathological examination revealed epithelioid sheets of cells exhibiting great pleomorphism and frequent mitoses (1A-C). Most of these malignant cells showed abundant eosinophilic cytoplasm with occasional eccentric nuclei (Figure 1A, B). Some areas showed clear cell changes (Figure 1C). Malignant multinucleated giant cells and areas of necrosis were also seen. The possibility for sarcoma was highly thought.
Figure 1.
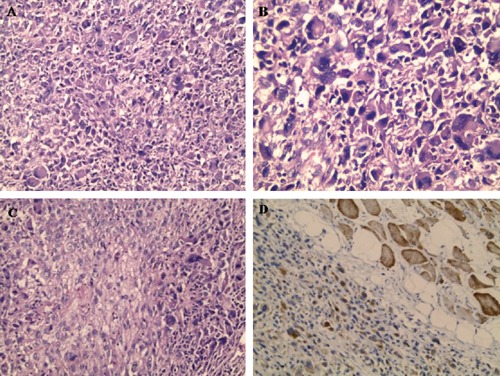
Histomorphology of thigh mass showing sheets of epithelioid cells with pleomorphic eccentric nuclei (A, B and C) that showed immunoreactivity for desmin (D). Skeletal muscle bundles served as internal positive control for desmin (Haematoxylin and Eosin staining ×200 for A and C and ×400 for B, Immunohistochemical staining ×200 for D).
Immunohistochemical panel of soft tissue sarcoma was performed including SMA, desmin, vimentin, S100 and pan cytokeratin. The neoplastic cells showed positivity for desmin (Figure 1D) and vimentin and the mass was designated at that time as pleomorphic rhabdomyosarcoma. At this moment, the patient has not submitted to further investigations. The patient received 33 cycles of radiotherapy, during which, he suffered from bad wound healing of the thigh wound.
Unfortunately, the patient suffered seven months later from abdominal pain. Abdominal CT revealed huge intra-abdominal mass that was located pararenal and extended to psoas muscle replacing and destroying it and both kidneys were free.
The patient underwent abdominal exploration where the intra-abdominal mass was resected. The mass measured 30×24×10 cm with whitish lobulated cut section (Figure 2A, B).
Figure 2.
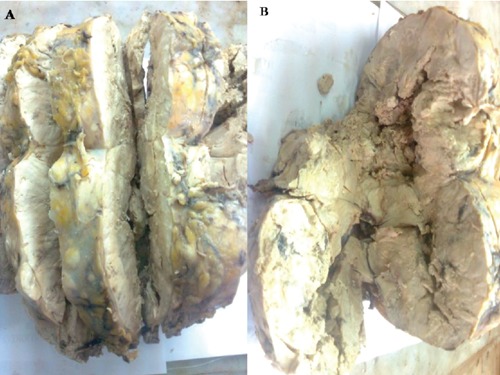
Gross picture of abdominal mass showed grayish white discoulration with friability and necrosis.
Microscopic evaluation of the abdominal mass revealed malignant epithelial cells characterized by clear cytoplasm with variable degrees of pleomorphism, where there were areas of moderate pleomorphiam to anaplastic areas (Figure 3). The first impression of this mass is RCC versus adrenocortical carcinoma.
Figure 3.
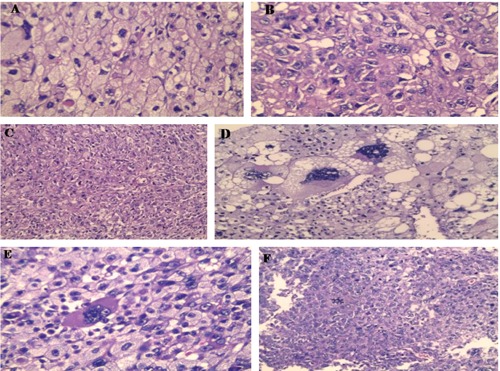
Histopthological picture of abdominal mass showing malignant neoplasm with conventional clear areas (A), granular esinophilic cytoplasm (B and C), anaplastic areas with monster cells (D and E) and abnormal mitosis (F) (Haematoxylin and Eosin staining ×400 for B, D and E, ×200 for C and F).
Stepping sections revealed areas simulating the morphology of thigh mass. Three iliac lymph nodes were dissected and revealed infiltration by the same neoplasm (Figure 4A). The tumor cells were diffusely positive for CD10 (Figure 4B) and vimentin and focally positive for pan cytokeratin (Figure 4C) and EMA (Figure 4D).
Figure 4.
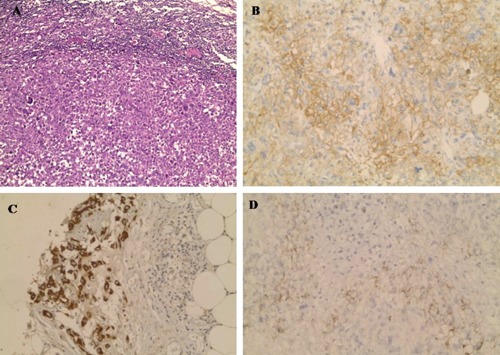
One of metastatic lymph node (A) (Haematoxylin and Eosin staining ×200). The malignant cells of thigh mass showed diffuse positivity for CD10 (B) and focal positivity for CK (C) and EMA (D) (Immunohistochemical staining ×200).
The tumor cells were completely negative for inhibin and other sarcoma markers such as SMA, desmin, myogenin and S100. Therefore, the morphological and immunohistochemical pictures indicated the diagnosis of RCC. But investigations failed to prove the presence of any renal masses. Paraffin blocks of thigh mass were retrieved and were submitted to immunostaining for CD10, EMA, vimentin, pancytokeratin, desmin, myogenin and carbonic anhydrase IX.
For our surprise, the tumor cells of thigh mass were diffusely positive for CD10 (Figure 5A), and vimentin and focally positive for pan cytokeratin (Figure 5B), EMA (Figure 5C) and carbonic anhydrase (Figure 5D) and still showed immunoreactivity for desmin. However, the tumor cells of thigh mass were negative for myogenin.
Figure 5.
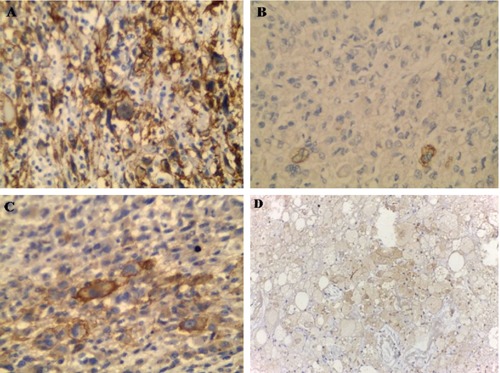
CD10, CK, EMA and carbonic anhydrase IX expression in malignant cells of thigh mass, respectively (Immunohistochemical staining ×400).
Therefore, we are now dealing with the same tumor which is RCC metastasizing to the thigh. Patient died within two months after the resection of intra-abdominal mass and receiving one cycle of Doxorubicin.
Discussion
The present case demonstrated several issues, such as the presence of metastatic RCC with absence of primary tumor. Some studies explained this finding by spontaneous regression of the primary cancer, delayed development of the primary tumor or the presence of ectopic tissue giving rise to the neoplasm.15 According to Johnson et al.,15 RCC was found to be metastasizing to adrenal gland with absence of primary and according to Wayne et al.,16 RCC was metastasizing to three sites (the subcutaneous tissue of the arm, the pancreas and the parotid gland) without detection of primary renal origin.17
The second issue presented in this case is metastasis to unusual sites such as thigh. Some authors reported the atypical presentation of RCC and its metastatic potential to rare sites including thigh.18 Skeletal muscle represented a rare site for carcinoma metastasis that reached less than 1% of all carcinomas metastases.19 Metastases from the lung and gastrointestinal tract are the most common sources of skeletal muscle metastases.20,21 Renal cell carcinoma most commonly metastasizes to the lungs, lymph nodes, bone, and liver.22 Grossly, RCC at metastatic site was reported to be grayish white and multinodular.18
Skeletal muscle is not a preferred site for metastasis in spite of its rich blood supply and huge body mass because of several reported factors, such as resistance to angiogenesis by the produced lactic acid. Also, frequent contractions of the muscle may prevent cancer seeding and metastatic growth through wide variation in blood flow. In addition, high concentrations of free radicals and local temperature fluctuations may also hinder development of metastases in skeletal muscle.23 Sarcomatoid RCC can metastasize to unusual sites including breast,24 colon and oropharynx.25,26
The third issue in the context of the present case, is why the tumor cells of the thigh mass expressed desmin? This mislead to the diagnosis of rhabdomyosarcoma, which was not confirmed later by using more specific markers such as myogenin. According to Koga et al.,27 sometimes sarcomatoid renal cell carcinoma not only takes the morphology of sarcoma but it may express sarcoma marker and in their described case, it expressed myoglobin as one of skeletal muscle markers and they concluded that their tumor was in process of metamorphosis to rhabdomyosarcoma.
RCC may gain true molecular features of mesenchymal neoplasms with preservation of their epithelial features.28,29 Spindle cell carcinoma from different body areas such as larynx, skin and esophagus was reported to express mesenchymal markers such as smooth muscle actin, desmin and vimentin.30 Epithelial-mesenchymal transition has been reported to be an etiological factor in metaplastic carcinoma.31 The expression of desmin in the current case especially with absence of myogenin expression suggested the possibility of leiomyosarcoma. However, at histological level, leiomyosarcoma is very rarely to show great pleomorphism and anaplastic areas. Leiomyosarcoma may express epithelial markers focally but still could not show CD10 or carbonic anhydrase expression. Renal leiomyosarcoma is a rare entity that sometimes causes a diagnostic challenge with sarcomatoid RCC,32 necessitating extensive sampling of the tumor and applying the immunohistochemical panel for differentiation.
The present case at the level of morphology showed definite sarcomatoid areas, but still we have seen areas with rhabdoid features manifested by abundant esinophilic cytoplasm and eccentric nuclei. Rhabdoid variant of RCC or what is called adult RCC with rhabdoid morphology has been described as an entity representing dedifferentiation of RCC similar to the sarcomatoid variant.1 Both rhabdoid and sarcomatoid areas may represent clonal evolution of definite carcinomatous areas toward biologically aggressive tumors. Unfortunately, our patient died within short period after the intraabdominal operation. Median survival durations of sarcomatoid RCC without treatment (from the time of diagnosis) are 3.8 to 6.8 months.33
According to Chivelle et al.12 cancer specific survival rates at 2 and 5 years after nephrectomy were 33.3% and 14.5%, respectively. Therefore, small amounts of sarcomatoid differentiation in RCC may be clinically relevant and should be included in the pathology report. Some authors considered 5-10% of sarcomatoid areas are associated with progressive course.14
Regarding response to treatment, metastatic sarcomatoid RCC showed partial response to immunotherapy in 33% of patients.34 Similar results with combination chemotherapy regimens such as doxorubicin plus gemcitabine were reported to give complete or partial responses in 40% of cases.35 Unfortunately, the current case under discussion received one cycle of doxorubicin and then the patient died.
Conclusions
Finally, our case represents conventional renal cell carcinoma with sarcomatoid and rhabdoid features metastasizing to abdomen and thigh without evidence of primary tumor. RCC can metastasize to virtually any body site and can have significant morphologic overlap with other non-renal neoplasms. Absence of primary origin of RCC according to radiological and operative data should not hinder the diagnosis of metastatic RCC. RCC with sarcomatoid and rhabdoid features carries aggressive behavior manifested by great metastatic potential and short survival time.
References
- 1.Chapman-Fredricks JR, Herrera L, Bracho J, et al. Adult renal cell carcinoma with rhabdoid morphology represents a neoplastic dedifferentiation analogous to sarcomatoid carcinoma. Ann Diagn Pathol 2011;15:333-7 [DOI] [PubMed] [Google Scholar]
- 2.Fukumura Y, Fujii H, Mitani K, et al. Urothelial carcinoma of the renal pelvis with rhabdoid features. Pathol Int 2009;59:322-5 [DOI] [PubMed] [Google Scholar]
- 3.Chung BY, Ahn IS, Cho SI, et al. Primary malignant rhabdoid melanoma. Ann Dermatol 2011;23:S155-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yorulmaz G, Erdogan G, Pestereli HE, et al. Epithelioid leiomyosarcoma with rhabdoid features. Wien Klin Wochenschr 2007;119:557-60 [DOI] [PubMed] [Google Scholar]
- 5.Paláu L MA, Thu Pham T, Barnard N, et al. Primary synovial sarcoma of the kidney with rhabdoid features. Int J Surg Pathol 2007;15:421-8 [DOI] [PubMed] [Google Scholar]
- 6.Gökden N, Nappi O, Swanson PE, et al. Renal cell carcinoma with rhabdoid features. Am J Surg Pathol 2000;24:1329-38 [DOI] [PubMed] [Google Scholar]
- 7.Leroy X, Zini L, Buob D, et al. Renal cell carcinoma with rhabdoid features: an aggressive neoplasm with overexpression of p53. Arch Pathol Lab Med 2007;131:102-6 [DOI] [PubMed] [Google Scholar]
- 8.Peng HQ, Stanek AE, Teichberg S, et al. Malignant rhabdoid tumor of the kidney in an adult: a case report and review of the literature. Arch Pathol Lab Med 2003;127:e371-3 [DOI] [PubMed] [Google Scholar]
- 9.Kuroiwa K, Kinoshita Y, Shiratsuchi H, et al. Renal cell carcinoma with rhabdoid features: an aggressive neoplasm. Histopathology 2002;41:538-48 [DOI] [PubMed] [Google Scholar]
- 10.Schofield DE, Beckwith JB, Sklar J. Loss of heterozygosity at chromosome regions 22q11-12 and 11p15.5 in renal rhabdoid tumors. Genes Chromosomes Cancer 1996;15:10-7 [DOI] [PubMed] [Google Scholar]
- 11.Bennington J. Proceedings: Cancer of the kidney - Etiology, epidemiology, and pathology. Cancer 1973;32:1017-29 [DOI] [PubMed] [Google Scholar]
- 12.Cheville JC, Lohse CM, Zincke H, et al. Sarcomatoid renal cell carcinoma: an examination of underlying histologic subtype and an analysis of associations with patient outcome. Am J Surg Pathol 2004; 28:435-41 [DOI] [PubMed] [Google Scholar]
- 13.Kwak C, Park YH, Jeong CW, et al. Sarcomatoid differentiation as a prognostic factor for immunotherapy in metastatic renal cell carcinoma. J Surg Oncol 2007; 95:317-23 [DOI] [PubMed] [Google Scholar]
- 14.de Peralta-Venturina M, Moch H, Amin M, et al. Sarcomatoid differentiation in renal cell carcinoma: a study of 101 cases. Am J Surg Pathol 2001;25:275-84 [DOI] [PubMed] [Google Scholar]
- 15.Johnson MT, Bahnson RR, Zynger DL. Metastatic clear cell renal cell carcinoma to the adrenal gland without an identifiable primary tumor. Int J Urol 2012;19:92-3 [DOI] [PubMed] [Google Scholar]
- 16.Wayne M, Wang W, Bratcher J, et al. Renal cell cancer without a renal primary. World J Surg Oncol 2010; 8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sountoulides P, Metaxa L, Cindolo L. Atypical presentations and rare metastatic sites of renal cell carcinoma: a review of case reports. J Med Case Rep 2011; 5:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pompo F, King JJ, Iwenofu OH, et al. Thigh mass in a 73-year-old man. Clin Orthop Relat Res 2008;466:1764-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menard O, Parache R. [Muscle metastasis of cancers]. Ann Med Interne (Paris) 1991;142:423-8 [Article in French] [PubMed] [Google Scholar]
- 20.Damron TA, Heiner J. Distant soft tissue metastases: a series of 30 new patients and 91 cases from the literature. Ann Surg Oncol 2000;7:526-34 [DOI] [PubMed] [Google Scholar]
- 21.Herring CL, Jr, Harrelson JM, Scully SP. Metastatic carcinoma to skeletal muscle: a report of 15 patients. Clin Orthop Relat Res 1998;355:272-81 [DOI] [PubMed] [Google Scholar]
- 22.Saitoh H. Distant metastasis of renal adenocarcinoma. Cancer 1981;48:1487-91 [DOI] [PubMed] [Google Scholar]
- 23.Judd CD, Sundaram M. Radiologic case study: metastatic renal cell carcinoma. Orthopedics 2000;23:1123-24 [DOI] [PubMed] [Google Scholar]
- 24.Ding GT, Hwang JS, Tan PH. Sarcomatoid renal cell carcinoma metastatic to the breast: report of a case with diagnosis on fine needle aspiration cytology. Acta Cytol 2007;51:451-5 [DOI] [PubMed] [Google Scholar]
- 25.Zhao W P, Yu YL, Chen Z, et al. Colon metastasis of chromophobe renal cell carcinoma with sarcomatoid change. Chin Med J 2012;125:2352-4 [PubMed] [Google Scholar]
- 26.Gammon BL, Gleason BC, Thomas AB, et al. Sarcomatoid renal cell carcinoma presenting in oropharynx. J Cutan Pathol 2010;37:1255-8 [DOI] [PubMed] [Google Scholar]
- 27.Koga F, Kawano K, Honda M, et al. Sarcomatoid renal cell carcinoma with scant carcinomatous components. Int J Urol 2000;7:58-60 [DOI] [PubMed] [Google Scholar]
- 28.Khosand J, Ro JY, Mackay B, et al. Sarcomatoid renal cell carcinoma: An immunocytochemical and ultrastructural study of 26 cases. J Urol 1986;54:31A [Google Scholar]
- 29.Bonsib SM, Fisher J, Plattner S, et al. Sarcomatoid renal tumors: clinicopathologic correlation of three cases. Cancer 1987;59:527-32 [DOI] [PubMed] [Google Scholar]
- 30.Avagnina A, Juárez MA, Elsner B. [Spindle cell carcinomas. Immunohistochemical analysis of 15 cases]. Medicina (B Aires) 1990;50:325-9 [Article in Spanish] [PubMed] [Google Scholar]
- 31.Lien HC, Hsiao YH, Lin YS, et al. Molecular signature of metaplastc carcinoma of the breast by large-scale transcriptional profiling: identification of genes potentially related to epithelial-mesenchymal transition. Oncogene 2007;26:7859-71 [DOI] [PubMed] [Google Scholar]
- 32.Dhawan SP, Dhawan S. Primary renal leiomyosarcoma: a diagnostic challenge. Urol Ann 2012;4:48-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ro JY, Ayala AG, Sella A, et al. Sarcomatoid renal cell carcinoma: clinicopathologic study of 42 cases. Cancer 1987;59:516-26 [DOI] [PubMed] [Google Scholar]
- 34.Mian BM, Bhadkamkar N, Slaton JW, et al. Prognostic factors and survival of patients with sarcomatoid renal cell carcinoma. J Urol 2002;167:65-70 [PubMed] [Google Scholar]
- 35.Golshayan AR, George S, Heng DY, et al. Metastatic sarcomatoid renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. J Clin Oncol 2009;27:235-41 [DOI] [PubMed] [Google Scholar]


