Abstract
The risk factors, the optimal therapy and prognostic factors contributing to poor outcomes of neuroendocrine urinary bladder carcinoma are not fully elucidated because of its rarity. We reviewed the medical records of neuroendocrine bladder carcinoma patients treated at the University of Nebraska Medical Center between 1996 and 2011. Eighteen patients, 55% female with a median age of 77 years, had stage IV disease at diagnosis in 50% of cases. There was a high prevalence of smoking (78%), medical co-morbidities (94%), prior cancer history (22%) and family history of cancer (61%). Treatment modalities included surgery (72%), platinum-based chemotherapy (50%) and/or radiation (22%). Median overall survival was 18.5 months (95% confidence interval, 7-36 months). Patients with Stage II and III cancer who underwent radical surgery with or without neoadjuvant chemotherapy had a median survival of 37 months. In addition to smoking, for the first time, our study indicates that the personal or family history of cancer may increase risk to neuroendocrine bladder cancer. Advanced age and stage at diagnosis, and the presence of multiple co-morbidities contribute to poor overall survival. Patients with early-stage disease are likely to benefit from a combination of radical surgery and platinum-based neoadjuvant chemotherapy.
Key words: small cell urinary bladder carcinoma, large cell neuroendocrine carcinoma of urinary bladder, smoking, family history, radical surgery, chemotherapy
Introduction
Small cell carcinoma of the urinary bladder is a rare but distinct neuroendocrine neoplasm,1 that accounts for less than one percent of all urinary bladder cancers.2 Large cell neuroendocrine carcinoma of the urinary bladder is even rarer with just a few dozen reported cases in English literature.3 Although older age, and smoking are putative risk factors for this type of carcinoma,2 the role of familial or genetic predisposition is unknown. Similar on histological and immunohistochemical analysis, the management of small cell urinary bladder carcinoma is extrapolated from the knowledge of small cell lung carcinoma with few differences. In small cell lung carcinoma, surgery is not recommended for the vast majority of the patients,4 however, as for other bladder cancers, surgery is often utilized as a therapeutic modality in small cell bladder carcinoma, particularly in patients with concurrent transitional cell carcinoma.2 Although radical surgery alone has been shown to result in good outcomes in patients with limited disease in one study,5 other studies have shown the benefit of neoadjuvant chemotherapy prior to surgery,6,7 as well as no benefit from cystectomy.8 Thus, the optimal management of this rare disease is not clear. Being further rare, the therapy and outcomes of large cell neuroendocrine carcinoma of bladder are largely unknown and based on few reported cases. Our cases of large cell neuroendocrine carcinoma of bladder, although few, adds to the current scarce literature. Since both of these bladder neoplasms are neuroendocrine disease, we compared the outcomes of these two diseases to explore for any differences. Because of the rarity of these tumors, insights on the outcomes of these two neoplasms, when similar, may allow them to be studied together in the future. Additionally, in this case series, we explore the role of familial predisposition, personal history of cancer and comorbidities in this relatively older patient population. Our study also provides information on the prevalence of co-morbidities raising the possibility of its influence on overall prognosis. In most of the prior studies, co-morbidities have not been evaluated. We also describe the different therapies used in this cohort and its outcomes. The experience of our center confirms the current understanding and opinion about the disease. In the absence of any phase III trial, we feel that such confirmation is helpful in a rare disease entity like neuroendocrine bladder cancer, particularly in the presence of controversial results from prior studies.
Materials and Methods
We conducted a retrospective study of all patients with neuroendocrine urinary bladder cancers treated at the University of Nebraska Medical Center between January 1996 and December 2011. In addition to histological features, a panel of immunohistochemical stains were used to make the diagnosis, which included synaptophysin, chromogranin, neuron-specific enolase, thyroid transcription factor (TTF)-1, low molecular cytokeratin (CK), CK7, CK20, AE1/3, CAM5.2, CD 56, CD45, CD117, and others as clinically indicated. Electronic medical records were manually reviewed to obtain demographic features, patient characteristics, cancer characteristics and outcomes at the time of study analysis. Last known follow-up were obtained from medical records, while death were ascertained from the social security death index to obtain survival status and the date of death. This study was approved by the University of Nebraska Medical Center Institutional Review Board. Descriptive statistics were calculated using SAS version 9.3 for windows. Kaplan Meier curve was plotted for overall survival. Given the lack of any significant difference in overall survival between small cell bladder carcinoma and large cell neuroendocrine carcinoma of bladder, these were subsequently combined in the study to calculate overall survival for the entire population.
Results
Of 572 urinary bladder cancers diagnosed during the study period, 14 patients had small cell bladder carcinoma (2.4%) and 4 patients had large cell neuroendocrine bladder carcinoma (0.69%). One patient had small cell carcinoma on the primary site and large neuroendocrine carcinoma on the metastatic site; this was included under small cell carcinoma (Table 1). Median follow-up was 14.5 (range 2-108) months. There were 10 females (55%) and the median age of the cohort was 77.5 years (range 36-89). Common symptoms at diagnosis included hematuria (61%) or other urinary symptoms. Half of the patients presented with Stage IV disease, whereas the rest presented with Stage II (39%) or III (11%) disease. All patients, except one, had one or more major co-morbidities; the most common of which were hypertension (55%), coronary artery disease or myocardial infarction (28%), hypothyroidism (28%), depression (22%) and diabetes (17%). Four patients (22%) had prior history of cancer and 11 patients (61%) had history of cancer in one or more family members. Fourteen patients (78%) had a history of smoking whereas only two patients (11%) had significant history of alcohol consumption. In terms of histology, 5 patients had pure small cell carcinoma and 3 patients had pure large neuroendocrine carcinoma whereas rest had mixed histology (n=9), and concurrent small cell carcinoma and urothelial carcinoma in situ (n=1). Of the patients with mixed histology, one patient had concurrent small cell and large cell neuroendocrine carcinoma whereas the other patient had small cell carcinoma in the primary and large cell neuroendocrine carcinoma in the metastatic site.
Table 1.
Characteristics of the patients with small cell bladder carcinoma and large cell neuroendocrine carcinoma of bladder.
| ID | Age/Sex | Smoking/drinking* | Major co-morbidities | Presenting symptom(s) | Family history | Prior cancer | Stage at diagnosis | Histology | Primary treatment | OS (m) | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 67/M | Y/N | Coronary artery disease, myocardial infarction | Dysuria | Prostate cancer in brother and cousin | No | II | Pure small cell | Radical surgery | 84 | Alive |
| 2 | 80/F | Y/N | Hypertension | Frequency, dysuria, Hematuria | No | No | II after neoadjuvant therapy | Small cell and high grade urothelial carcinoma | Neoadjuvant 4 cycle of Ca/E, then radical surgery | 48 | Dead |
| 3 | 62/M | Y/Y | Depression | Hematuria | No | No | II | Small cell and high grade papillary urothelial carcinoma | NA | 36 | Alive |
| 4 | 85/F | N/N | Stroke, myocardial infarction, hypertension, dementia | Renal failure | Colon cancer in son | Breast cancer | II | Pure Lc NEc# | TURBT then concurrent chemoradiation | 36 | Dead |
| 5 | 80/M | Y/N | Coronary artery disease, diabetes, hypertension, hypothyroidism | Difficulty in urination | Lung cancer in mother | No | II | Primary Lc NEc,# papillary urothelial carcinoma | Radical surgery | 25 | Dead |
| 6 | 70/M | Y/Y | Depression | Diagnosed on surveillance cystoscopy | None | TCCb, prostate cancer | II | Pure Lc NEc | Neoadjuvant 3 cycles of Ci/G, then radical surgery then 1 cycle of Ci/G | 17 | Alive |
| 7° | 78/F | Y/N | Coronary artery disease, myocardial infarction, atrial fibrillation, hypertension, hypothyroidism, depression | Hematuria | Breast cancer in 2 daughters | No | II | Pure small cell | Concurrent chemoradiation | 7 | Dead |
| 8 | 84/F | N/N | Hypertension, hypothyroidism | Hematuria | Lung in father | Breast | III | Small cell and squamous differentiation | Radical surgery | 108 | Alive |
| 9 | 69/M | Y/N | Hypertension | Hematuria | Kidney in father | No | III | Small cell and high grade urothelial carcinoma | Radical surgery | 26 | Dead |
| 10 | 55/F | Y/N | None | Left neck mass | Cancer in brother | No | IV | Small cell in primary and large cell neuroendocrine carcinoma in metastasis | Neoadjuvant 5 cycles of Ci/E, then radical surgery, then adjuvant 2 cycles of Ci/E | 30 | Dead |
| 11 | 89/M | Y/N | Dementia | Hematuria | No | No | IV | Small cell and adenocarcinoma | Partial cystectomy | 12 | Dead |
| 12 | 69/M | Y/N | Diabetes | Back pain | Gastric in mother | No | IV | Small cell and Lc NEc | 4 cycle of Ca/E, then radiation | 10 | Dead |
| 13 | 67/F | N/N | Congestive heart failure, diabetes, hypothyroidism | Hematuria/frequency, then pain | Unknown Cancer in mother; prostate cancer in brother | No | IV | Pure small cell | Chemotherapy, then RT which was prematurely stopped | 8 | Dead |
| 14 | 36/F | Y/N | Asthma | Hematuria | Lung cancer in father and grandmother; leukemia in aunt | No | IV | Pure Lc NEc# | Radical surgery, then Ci/E | 8 | Dead |
| 15 | 77/M | Y/N | Coronary artery disease, atrial fibrillation, hypertension, aortic aneurysm | Hematuria | No | No | IV | Small cell bladder carcinoma and prostate adenocarcinoma | Radical surgery | 7 | Dead |
| 16 | 78/F | N/N | Hypertension | Urgency, frequency | No | No | IV | Small cell and non-papillary urothelial carcinoma | Radical surgery | 6 | Dead |
| 17 | 78/F | Y/N | Hypertension, hypothyroidism, transient ischemic attack | Hematuria | Gastric in mother | No | IV | Pure small cell | Radical surgery | 5 | Dead |
| 18 | 84/F | Y/N | Depression and hypertension | Hematuria | No | Rectal | IV | Small cell and urothelial carcinoma in situl cycle of chemo, then hospice | 2 | Dead | |
OS, overall survival; Ca/E, Carboplatin/Etoposide chemotherapy; yr, Year; m, Month; Lc NEc, Large cell neuroendocrine carcinoma; TURBT, Transurethral resection of bladder tumor; TCCb, transitional cell carcinoma of bladder; Ci/G, Cisplatin/Gemcitabine chemotherapy; Ci/E, Cisplatin/Etoposide chemotherapy; NA not available.
*Smoking status was defined as no for never-smoker and yes for ever-smoker. Social drinking was considered as no drinking.
°Cancer cells showed multiple copies of chromosomes 3, 7 and 17.
#The primary cancer in these patients was large cell neuroendocrine carcinoma.
Treatment modalities utilized included one or more of the following: surgery (72%), chemotherapy (50%) and radiation (22%). In the majority of the patients, surgery included cystectomy with or without prostatectomy (in men), hysterectomy and salphingo-oophorectomy (in women), pelvic lymph node dissection and resection of additional tumor, if any. Chemotherapy included platinum agent with etoposide or gemcitabine when used without radiation and single-agent platinum agent when used as radiation sensitizer. Three patients who received neoadjuvant platinum-based chemotherapy showed complete remission or pathological downstaging at resection. Median overall survival for the entire study population was 18.5 months (95% CI, 7-36 months) (Figure 1A). The survival probabilities at 1, 3 and 5 years were 50% (95% CI, 26-70%), 25% (95% CI, 8-47%) and 17% (95% CI, 3-39%) respectively. The median overall survival for pure histology was 8 months, compared to 25 months for mixed histology (Table 2; Figure 1B). The median overall survival for small cell urinary bladder carcinoma and large cell neuroendocrine carcinoma of the urinary bladder was 11 months and 25 months respectively (Figure 1D). The overall survival probability for small cell urinary bladder carcinoma and large cell neuroendocrine carcinoma of the urinary bladder were 43% (95%CI 17-66) and 75% (95% CI 13-96) respectively at 1 year and 19% (95%CI 4-44) and 0% respectively at 5 years. Patients who received radical surgery with or without chemotherapy had a median overall survival of 26 months compared to 10 months with other treatment (Figure 1C). Patients with Stage II and III cancer who underwent radical surgery with or without neoadjuvant chemotherapy (n=6) had a median survival of 37 months (17-108 months). In patients with Stage IV cancer, radical surgery resulted in a median survival of 7 months (5-30 months). One patient with Stage IV cancer (multiple enlarged mediastinal lymph nodes and biopsy-proven left supraclavicular lymph node involvement) with small cell carcinoma in primary cancer and non-small cell carcinoma in left supraclavicular lymph node metastasis survived for 30 months. This patient was initially treated with 5 cycles of neoadjuvant cisplatin and etoposide followed by radical local surgery in complete remission. After about 12-month disease-free period, the patient developed local recurrence of disease which was treated with 2 cycles of cisplatin and gemcitabine followed by 2 cycles of paclitaxel (nanoparticle albumin bound). Subsequently, the patient underwent second radical surgery for local recurrence. Repeat surgery showed adenocarcinoma as well as neuroendocrine carcinoma. This was followed with 3 cycles of cisplatin and etoposide for metastatic disease until the care was switched to supportive care only.
Figure 1.
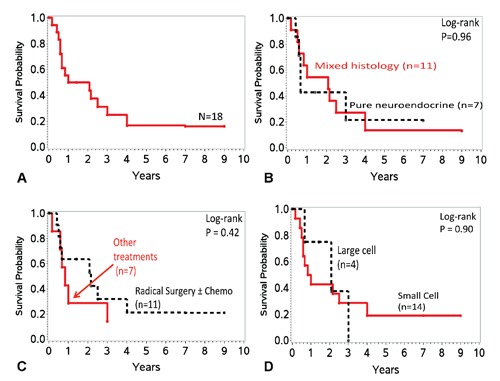
Overall survival of neuroendocrine urinary bladder cancer A) over time (n=18), B) over time based on histology (pure versus mixed histology) (n=18); C) over time based on histology (large cell versus small cell) (n=18); D) over time based on treatment (radical surgery with or without chemotherapy versus other treatment) (n=18).
Table 2.
Outcomes of neuroendocrine bladder cancer based on histology and therapy.
| Variable | 1-yr OS, % |
3-yr OS, % |
5-yr OS, % |
Median OS |
Log-rank P-value |
|---|---|---|---|---|---|
| Histology | |||||
| Pure | 43 (10-73) |
21 (1-58) |
21 (1-58) |
8 months | 0.96 |
| Mixed | 54 (23-78) |
27 (6-54) |
14 (1-42) |
25 months | |
| Therapy | |||||
| Radical surgery± chemo | 64 (30-84) |
32 (8-60) |
21 (3-49) |
26 months | 0.42 |
| Other treatment | 28 (4-61) |
14 (1-46) |
14 (1-46) |
10 months | |
OS, overall survival.
Discussion
This case series shows that neuroendocrine urinary bladder cancer is predominantly a disease of elderly patients, who present with stage IV cancer at the time of diagnosis in up to 50% cases. This is consistent with a large study based on Surveillance, Epidemiology and End Results (SEER) database of small cell bladder carcinoma (n=642) which showed the predominance of elderly Caucasian men with a median age of 73 years. Thirty-six percent of the patients presented with Stage IV disease at the time of diagnosis.9 Smoking, an established risk factor for urothelial bladder cancer and small cell lung carcinoma, was prevalent in more than two thirds of the patients with neuroendocrine bladder cancer. Prior studies have shown a 65-75% prevalence of smoking.2 Half of the patients had mixed pathology and one patient had small cell carcinoma on primary site and non-small cell neuroendocrine carcinoma on the metastasis. In the later patient, resection at local recurrence showed adenocarcinoma in addition to neuroendocrine carcinoma. These results support the hypothesis that the small cell bladder carcinoma originates from multipotent stem cell.2
Interestingly, we found an unusually high percentage of family history of cancer as well as prior personal history of cancer. In SEER study, more than one-third of the patients had more than one primary cancer but family history was not available.9 Prior studies have shown that bladder cancer can develop as a complication of radiation therapy for prostate cancer or following the use of cyclophosphamide.10,11 Overall, cancer survivors, compared to the general population, have 14% higher risk of developing second cancer.12 Similarly, patients with family history of cancer or bladder cancer are at an increased risk of bladder cancer.13-15 Although it needs further confirmation, for the first time, our study indicates that the personal or family history of cancer may increase risk of neuroendocrine bladder cancer. High prevalence of family history of cancer in this patient population might indicate the possibility of genetic predisposition or common environmental exposure (e.g. smoking) whereas a high prevalence of personal history of cancer might indicate the possibility of genetic predisposition, therapy related event (complication of chemotherapy or radiation) or shared risk factor (e.g. smoking).
The management of neuroendocrine bladder cancer is unclear. Small cell bladder carcinomas are often treated as small cell lung carcinomas with the exception that surgical therapies are often important part of management in small cell bladder carcinomas.2 A study did not reveal any difference in patients treated with or without cystectomy, however, the five-year disease-specific survival was only 16%.8 Another large retrospective analysis showed no difference in 5-year overall survival between trans-urethral resection of bladder tumor (TURBT), radiation and chemotherapy (19%) versus cystectomy and chemotherapy (26%).16 However, this study is criticized for the poor outcomes in both arms.17 In fact, surgery has been shown to be potentially curative for limited stage disease, and radical cystectomy has been recommended in the absence of metastasis.5 Other studies including a prospective trial have shown that for surgically resectable disease, neoadjuvant chemotherapy followed by surgery can result in a 5-year survival of 80% or more.6,7,18 The majority of patients in these studies received cisplatin/etoposide or ifosfamide/doxorubicin alternating with cisplatin/etoposide.6,7,18 The much better survival outcomes clearly suggests the beneficial roles of neoadjuvant chemotherapy and surgery. Neoadjuvant chemotherapy can also result in pathological down-staging, thus facilitating surgery.7 Conversely, adjuvant chemotherapy following cystectomy was not shown to be superior to cystectomy alone.6 In another study, chemotherapy was shown to improve outcomes in all stages of diseases among patients who were treated with TURBT, however, chemotherapy did not improve outcomes in addition to cystectomy.16 In our series, patients with stage II and III cancer who underwent radical surgery with or without neoadjuvant chemotherapy had a median survival of 37 months (17-108 months). Chemotherapy included platinum agent with etoposide or gemcitabine when used without radiation. Three patients who received neoadjuvant platinum-based chemotherapy showed excellent response. Although neuroendocrine bladder carcinomas are generally considered to have poor prognosis, a subset of patients with early-stage disease who can undergo radical surgery particularly with neoadjuvant chemotherapy may survive for several years. One patient with metastatic neuroendocrine cancer underwent neoadjuvant chemotherapy, followed by radical surgery in complete remission followed by adjuvant chemotherapy and survived for 30 months. This might indicate that carefully selected patients with metastatic disease might also be a candidate for radical surgery. In a study, patients with clinically node positive disease underwent cystectomy and extended lymph node dissection after complete response to neoadjuvant chemotherapy, which resulted in a median overall survival of 23 months,7 thus suggesting the feasibility of this approach in select patients. In our study, patients with stage IV cancer had a median survival of 7 months (5-30 months) with radical surgery. Hence, for stage IV disease or metastatic cancer, radical surgery alone does not result in good outcome. In one study, neoadjuvant chemotherapy (alternating ifosfamide/doxorubicin and etoposide/cisplatin) with an intent for surgical consolidation in responders resulted in 100% partial or complete response rate in patients with metastatic disease, however, most patients relapsed and the overall survival was 13.3 months.18 In our study, patients who were not candidate for radical surgery received chemotherapy with or without radiation in the majority of the cases. Single-agent platinum agent was used as radiation sensitizer. In a study among patients with localized small cell bladder carcinoma (n=17), sequential chemoradiation with platinum-based chemotherapy resulted in an overall survival of 32.5 months and a local recurrence of 23.5%.19 Although this is not the preferred option, the study suggests chemoradiation as a bladder sparing option in patients who refuse surgery or are high-risk for surgical complications. Even though the incidence of brain metastasis from small cell bladder carcinomas is much higher than urothelial cancers, it is much less than small cell lung carcinomas. The pooled analysis of available literature revealed a cumulative incidence of brain metastasis of approximately 11% among small cell bladder carcinomas. Based on this, some experts recommend against prophylactic cranial irradiation. Brain metastasis, as from other primary cancer, portends a poor prognosis and is often treated with whole brain radiation therapy.20 Despite initial response, the cancer often relapses and patients frequently die of progressive disease.2,5,21 There is a lack of data on the second-line chemotherapy options for patients who develop disease progression or recurrence. In a case series (n=3), single-agent weekly vinorelbine had shown promising safety and efficacy profile.22 In our study, one patient who had disease recurrence after neoadjuvant chemotherapy with cisplatin and etoposide followed by radical surgery was successfully treated with cisplatin and gemcitabine followed by paclitaxel (nanoparticle albumin bound), thus indicating the possible role of these agents. Since c-kit expression is common in small cell bladder carcinoma,23 studies should explore the therapeutic benefit of targeted agents such as imatinib.
In our study, median overall survival for the entire study population was 18.5 months and the survival probabilities at 1, 3 and 5 years were 50%, 25% and 17% respectively. Overall survival did not differ between small cell bladder carcinoma and large neuroendocrine carcinoma of bladder, however, the number of patients with large neuroendocrine carcinoma was small. The overall survival for small cell bladder carcinoma reported in the literature is variable depending on the stage at presentation, patient characteristics and treatment options.2,9 In SEER study, the median overall survival for the entire cohort was 11 months without any significant improvement from 1991 to 2005.9 Other studies show a five-year overall survival rate of about 19%.2 Age, race, marital status, TNM staging and histology have been shown to be the prognostic factors. Patients with T4, N2 or M1 disease or pure small cell histology had poor outcomes.2,9 Although not statistically significant, patients with pure cell histology had poor outcomes in our study as well. Our study further shows that the majority of the patients had one or more major co-morbidities, which likely contributes to poor overall survival. The rarity of large neuroendocrine carcinoma of bladder does not allow calculation of overall survival or prognostic factors, however, reported overall survival ranges from <1 year for advanced disease to >2 years for early disease.24-27
The major limitation of the study was retrospective single-center design. Although we combined small cell bladder carcinoma and large cell neuroendocrine bladder cancer for outcome studies because of lack of any difference, we do acknowledge that the numbers were not large enough to detect any differences. Univariate and multivariate analysis for prognostic factors was not done because of the small number of the patients, however, a patient population of 18 in such an unusual cancer is a reasonable size. Importantly, the patients were followed-up until their death and for up to several years among the survivors.
Conclusions
This study confirms that neuroendocrine bladder carcinomas present in elderly patients and at an advanced stage. In addition to smoking, for the first time, our study indicates that the personal or family history of cancer may increase risk to neuroendocrine bladder cancer. Advanced age and stage at diagnosis, and the presence of multiple co-morbidities contribute to poor overall survival. Fit patients with early-stage disease are likely to benefit from combination of radical surgery particularly with platinum-based neoadjuvant chemotherapy. However, given overall poor prognosis, further large multicenter trials are needed to determine the optimal and novel therapies, which are needed to improve outcomes.
References
- 1.Eble JN, Sauter G, Epstein JI, Sesterhenn IA.World Health Organization classification of tumors. Pathology and genetics of tumours of the urinary system and male genital organs. Lyon: IARC Press; 2004 [Google Scholar]
- 2.Ismaili N. A rare bladder cancer—small cell carcinoma: review and update. Orphanet J Rare Dis 2011; 6:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hata S, Tasaki Y. A case of the large cell neuroendocrine carcinoma of the urinary bladder. Case Rep Med 2013; 2013:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Downey RJ, Krug LM. Surgery in the management of small cell lung cancer. J Natl Compr Canc Netw 2004;2:159-62 [DOI] [PubMed] [Google Scholar]
- 5.Choong NW, Quevedo JF, Kaur JS. Small cell carcinoma of the urinary bladder. The Mayo Clinic experience. Cancer 2005;103:1172-8 [DOI] [PubMed] [Google Scholar]
- 6.Siefker-Radtke AO, Dinney CP, Abrahams NA, et al. Evidence supporting preoperative chemotherapy for small cell carcinoma of the bladder: a retrospective review of the M. D. Anderson cancer experience. J Urol 2004;172:481-4 [DOI] [PubMed] [Google Scholar]
- 7.Lynch S P, Shen Y, Kamat A, et al. Neoadjuvant chemotherapy in small cell urothelial cancer improves pathologic downstaging and long-term outcomes: results from a retrospective study at the MD Anderson Cancer Center. Eur Urol 2012;62:e65-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng L, Pan CX, Yang XJ, et al. Small cell carcinoma of the urinary bladder: a clinicopathologic analysis of 64 patients. Cancer 2004;101:957-62 [DOI] [PubMed] [Google Scholar]
- 9.Koay EJ, Teh BS, Paulino AC, Butler EB. A surveillance, epidemiology, and end results analysis of small cell carcinoma of the bladder: epidemiology, prognostic variables, and treatment trends. Cancer 2011;117:5325-33 [DOI] [PubMed] [Google Scholar]
- 10.Neugut AI, Ahsan H, Robinson E, Ennis RD. Bladder carcinoma and other second malignancies after radiotherapy for prostate carcinoma. Cancer 1997;79:1600-4 [DOI] [PubMed] [Google Scholar]
- 11.Travis LB, Curtis RE, Glimelius B, et al. Bladder and kidney cancer following cyclophosphamide therapy for non-Hodgkin’s lymphoma. J Natl Cancer Inst 1995;87:524-30 [DOI] [PubMed] [Google Scholar]
- 12.Curtis RE, Freedman DM, Ron E, et al. New malignancies among cancer survivors: SEER cancer registries, 1973-2000. National Cancer Institute. Bethesda: NIH; 2006 [Google Scholar]
- 13.Murta-Nascimento C, Silverman DT, Kogevinas M, et al. Risk of bladder cancer associated with family history of cancer: do low-penetrance polymorphisms account for the increase in risk? Canc Epidemiol Biomarkers Prev 2007;16:1595-600 [DOI] [PubMed] [Google Scholar]
- 14.Plna K, Hemminki K. Familial bladder cancer in the National Swedish Family Cancer Database. J Urol 2001;166:2129-33 [PubMed] [Google Scholar]
- 15.Aben KK, Witjes JA, Schoenberg MP, et al. Familial aggregation of urothelial cell carcinoma. Int J Cancer 2002;98:274-8 [DOI] [PubMed] [Google Scholar]
- 16.Koay EJ, Teh BS, Paulino AC, Butler EB. Treatment trends and outcomes of small-cell carcinoma of the bladder. Int J Radiat Oncol Biol Phys 2012;83:64-70 [DOI] [PubMed] [Google Scholar]
- 17.Ismaili N. Treatment trends and outcomes of small-cell carcinoma of the bladder: in regard to Koay et al. (Int J Radiat Oncol Biol Phys 2011 Oct 20). Int J Radiat Oncol Biol Phys 2012;82:1319-20 [DOI] [PubMed] [Google Scholar]
- 18.Siefker-Radtke AO, Kamat AM, Grossman HB, et al. Phase II clinical trial of neoadjuvant alternating doublet chemotherapy with ifosfamide/doxorubicin and etoposide/cisplatin in small-cell urothelial cancer. J Clin Oncol 2009;27:2592-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bex A, de Vries R, Pos F, et al. Long-term survival after sequential chemoradiation for limited disease small cell carcinoma of the bladder. World J Urol 2009;27:101-6 [DOI] [PubMed] [Google Scholar]
- 20.Bex A, Sonke GS, Pos FJ, et al. Symptomatic brain metastases from small-cell carcinoma of the urinary bladder: the Netherlands Cancer Institute experience and literature review. Ann Oncol 2010;21:2240-5 [DOI] [PubMed] [Google Scholar]
- 21.Ismaili N, Heudel PE, Elkarak F, et al. Outcome of recurrent and metastatic small cell carcinoma of the bladder. BMC Urol 2009; 9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.June RR, Dougherty DW, Reese CT, et al. Significant activity of single agent vinorelbine against small-cell cancer of the bladder as second line chemotherapy: a case series and review of the literature. Urol Oncol 2012;30:192-5 [DOI] [PubMed] [Google Scholar]
- 23.Pan CX, Yang XJ, Lopez-Beltran A, et al. c-kit expression in small cell carcinoma of the urinary bladder: prognostic and therapeutic implications. Mod Pathol 2005;18:320-3 [DOI] [PubMed] [Google Scholar]
- 24.Evans AJ, Al-Maghrabi J, Tsihlias J, et al. Primary large cell neuroendocrine carcinoma of the urinary bladder. Arch Pathol Lab Med 2002;126:1229-32 [DOI] [PubMed] [Google Scholar]
- 25.Colarossi C, Pino P, Giuffrida D, et al. Large cell neuroendocrine carcinoma (LCNEC) of the urinary bladder: a case report. Diagn Pathol 2013; 8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertaccini A, Marchiori D, Cricca A, et al. Neuroendocrine carcinoma of the urinary bladder: case report and review of the literature. Anticancer Res 2008;28:1369-72 [PubMed] [Google Scholar]
- 27.Serrano F Alijo, Sanchez-Mora N, Arranz J Angel, et al. Large cell and small cell neuroendocrine bladder carcinoma: immunohistochemical and outcome study in a single institution. Am J Clin Pathol 2007;128:733-9 [DOI] [PubMed] [Google Scholar]


