Abstract
Rhabdomyosarcoma is a soft tissue malignant tumor affecting 1% of children from 0 to 14 years. Preoperative imaging may not always be diagnostic for hepatobiliary rhabdomyosarcoma and differential diagnosis with choledochal cyst (CC) could be difficult. We report a case of 2-years-old girl with a strange CC pattern of presentation. A grapelike lesion involving the choledochal and biliary ducts was easily and completely resected by robotic assisted surgery. Since no previous reports were available about oncologic safety of robotic approach, the porto-enterostomy was performed in open surgery. On histologic examination, the specimen revealed a botryoidembryonal rhabdomyosarcoma affecting both the common bile duct and the common hepatic duct. One year postoperatively the child is safe of tumor relapse. Robotic approach seems to be safe and advantageous to obtain a radical excision of the tumor at the porta hepatis, even in case of misdiagnosed malignant lesion mimicking a CC.
Key words: biliary botryoid rhabdomyosarcoma, choledochal cyst, robotic surgery, porta hepatis
Introduction
The biliary tract botryoid embryonal rhabdomyosarcoma (RMS) is a very rare malignancy of childhood and accounts for 0.04% of childhood neoplasm. It was first described by Wilks and Moxon in 1875 on the basis of typical location and gross description of the tumor. Median age at presentation is 3.5 years; with a slight male preponderance.1-4 Preoperative radiological studies may be insufficient to define the exact nature of the lesion, due to the nature of the tumor being both solid and cystic. Embryonal rhabdomyosarcoma (ERMS) has been already known to mimic radiologic and clinical features of choledochal cyst (CC).5-9 We report a case of 2-years-old girl with ERMS of the common bile duct, which was considered to be a CC preoperatively. Initial dissection of the cyst was done in robotic assisted surgery. This revealed the presence of intra choledocal grapelike mass, extending to the porta hepatis.
Case Report
A 2 years-old girl was admitted for loss of appetite and slight fever. Laboratory studies showed: AST 537 mU/mL, ALT 589 mU/mL, GGT 738 mU/mL, alkaline phosphatase 718 mU/L, total bilirubin 1.8 mg/dL. Hepatic infection profile and tumor markers were negative. On physical examination there was no tenderness, hepato-spleenomegaly or abdominal mass. Abdominal ultrasound showed hypoechoic dilatation of the common hepatic duct (CHD) and common bile duct (CBD). Thickening of the CHD walls with dilatation of the intrahepatic bile ducts were suggestive of CC. Abdominal CT scan showed an ovoid, heterogeneously non enhancing mass localized at the liver hilum of 18×30 mm with hyperdense contents. Magnetic resonance imaging (MRI) confirmed the presence of marked dilatation with thickened wall of CHD and CBD consistent with congenital CC type IV (Figure 1). Laparoscopic intraoperative cholangiography, prior to choledochal cyst excision, is usually valuable for detection of biliary variants. Liver biopsies are routinely done for grading of liver fibrosis in obstructive cholangiopathy (Figure 2). Unfortunately cholangiography in our case was not conclusive and CHD and intrahepatic biliary ducts were not visualized. The biopsies showed fibrosis of the septum at the portal space associated with signs of chronic inflammation.
Figure 1.
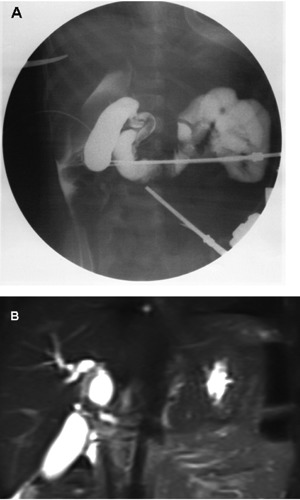
Choledochal cyst imaging. A) cholangiography showing the choledochal fusiform dilatation; B) magnetic resonance imaging aspect with evidence of dilatation of biliary ducts.
Figure 2.
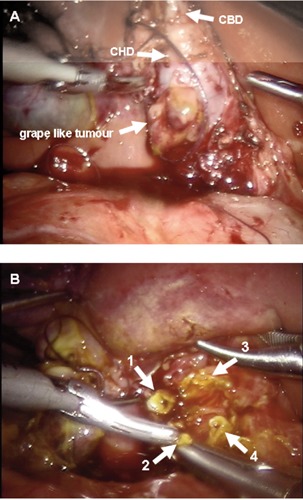
Intraoperative features of biliary three A) extrahepatic ducts: common hepatic duct (CHD) showing grape like tumour and common bile duct (CBD); B) intrahepatic biliary ducts: right anterior and posterior (1-2) and left anterior and posterior (3-4).
The child underwent mini-invasive robotic surgery for CC resection and Roux-en-Y choledochojejunostomy. Open umbilical insertion of the 8.5 mm stereoscope was used.
Two 5 mm working ports were placed at the left and right hypocondrium on either side of the umbilicus under direct vision, at 3 cm from the costal margins along the hemi-clavicular line bilaterally. A 3 mm servicing trocar was inserted in the right inferior iliac space. Transabdominal stitches were fixed on the gallbladder and on the falciform ligament for liver retraction. Pressure insufflations were around 4 mmHg. Circumferential dissection of the CC was achieved to the duodenum and ligated. Proximally dissection extended beyond the CHD to the left and right hepatic biliary ducts that were also involved in a grapelike tumor. The high degree of magnification allowed the resection to be carried out in the parenchyma to free the right posterior and anterior ducts and evidenced another supernumerary duct. On the left side, the lesion was freed from the grapelike cysts and healthy tissue was found at the two segmental tributaries. Although feasible by robotic approach, but still controversial in the literature, the anastomosis at the porta hepatis was carried out in open surgery. A retrocolic Roux-en-Y portoenterostomy was performed using 5/0 PDS. Histologic examination revealed a botryoidembryonal rhabdomyosarcoma of the bile ducts affecting both the CBD and CHD. The patient underwent chemotherapy after diagnosis. Twelve months later, the patient is relapse free, with normal bilirubin and liver profile.
Discussion and Conclusions
Rhabdomyosarcoma is a soft tissue malignant tumor. Approximately 1% of children from 0 to 14 years are affected. About 2% of RMSs are present at birth.1-2
Rhabdomyosarcoma is divided into 5 major histological categories: embryonal, alveolar, botryoid embryonal, spindle cell embryonal and anaplastic.1-2
ERMS is the most common subtype observed in children, accounting for approximately 60% of all cases.10 Botryoid type accounts for 6% of all cases of RMS. Desmin and muscle specific actin are the typical stains used to identify RMS.4
Biliary tract botryoid rhabdomyosarcoma is a very rare malignancy of childhood with a
Grape-like presentation accounting for 1% of all ERMSs.4 Intermittent jaundice, fever and anorexia is its typical presentation.
Nearly 90 cases of rhabdomyosarcoma of the liver and biliary tree in children have been reported in the literature.1-10 Diagnosis of the biliary tree RMS is difficult for its radiologic appearance. A delay in diagnosis can influence the prognosis.3 Few reports have mentioned cases of ERMS mimicking a CC, whether they were primary ERMS or ERMS with CC is questionable.11,12 In 6 papers the authors described biliary rhabdomyosarcoma mimicking choledochal cyst, as our case.5-10
Preoperative radiologic studies may reveal biliary cystic dilatation, leading to a diagnosis of congenital CC.5 Because of the combined cystic and solid areas within an ERMS mass, due to bile stasis and the filling of the lumen with soft, inspissated debris, an ERMS can mimic the radiologic appearance of a CC. In particular, the appearance of the mass could be similar to CC, if it is well demarcated, and if there is no local invasion to the adjacent tissues.5
A careful preoperative radiological examination is mandatory. CT scan and MRI or magnetic resonance cholangiopancreatography may not always reveal the exact nature of the choledochal dilatation. Laparoscopic intraoperative cholangiography is valuable for detection of biliary variants; even though, as in our case, may not be conclusive. Endoscopic retrograde cholangiopancreatography is not usually done as a routine approach in pediatric CC cases.13,14
Even without preoperative malignant diagnosis the surgical treatment won’t change: resection has to be the treatment of choice. Risk of malignant transformation, usually adenocarcinoma, is rare but increases with age in patients undergoing incomplete CC excision.15 This constrains that excision of the CC extends to the whole biliary tree. Surgery must be carried out as soon as possible to avoid further hepatic damage.16
While robotic surgery in adults have already been described for biliary tree malignancies, no evidence of safety of robotic approach for biliary tumors in pediatrics are reported yet in the literature.17 More recently only few authors advocate pediatric robotic surgery approach for complex hepatobiliary procedure. Furthermore, in pediatrics clear benefits with mini-invasive surgery at porta hepatis for congenital malformations are controversial.18-20
Several reports postulated that liver perfusion could be compromised during laparoscopic treatment of biliary atresia, for the temporary intraperitoneal high pressures inducing liver cells damage.19
Per contra recent encouraging reports advocate that robotic surgery facilitate the exposure of the porta hepatis in both biliary atresia and CC.19,20 In our case the dissection of the CC was smooth. High magnification and the 3D-visualization allowed a meticulous intrahepatic portal space dissection. To distinguish healthy tissue from pathologic one was effortless. Robotic approach seemed advantageous to obtain a radical excision of the tumor.
Two comments raised from our experience. First, could robotic surgery damage hepatic and biliary duct in cases of CC and portal tumor like ours? Risks of hepatic injury would be lower than laparoscopy procedures, having usually lower insufflations pressure, but further experiences are needed. Nevertheless could we exclude a real risk of cancer dissemination in performing a portal anastomosis by mini invasive surgery in biliary tree RMS?
Second, the feasibility of porta hepatis anastomosis has been proved in robotic surgery in the most recent literature but no contraindication are illustrated yet.19,20
Although several reports have already underlined the advantages of mini invasive or robotic surgery in biliary tree malformations, more experience is required to define the best attitude. Data from adult patients could be of benefit.17
References
- 1.Stocker JT. Hepatic tumors in children. Clin Liver Dis 2001;5:259-81 [DOI] [PubMed] [Google Scholar]
- 2.Meyers RL. Tumors of the liver in children. Surg Oncol 2007;16:195-203 [DOI] [PubMed] [Google Scholar]
- 3.Kebudi R, Görgun O, Ayan I, et al. Rhabdmyosarcoma of the biliary tree. Pediatr Int 2003;45:469-71 [DOI] [PubMed] [Google Scholar]
- 4.Duan F, Smith LM, Gustafson DM, et al. Genomic and clinical analysis of fusion gene amplification in rhabdomyosarcoma: a report from the Children’s Oncology Group. Genes Chromosomes Cancer 2012; 51:662-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tireli GA, Sander S, Dervisoglu S, et al. Embryonal rhabdomyosarcoma of the common bile duct mimicking choledochal cyst. J Hepatobiliary Pancreat Surg 2005;12:263-5 [DOI] [PubMed] [Google Scholar]
- 6.Nemade B, Talapatra K, Shet T, et al. Embryonal rhabdomyosarcoma of the biliary tree mimicking a choledochal cyst. JCRT 2007;3:40-2 [DOI] [PubMed] [Google Scholar]
- 7.Ali S, Russo AM, Margraf L. Biliary Rhabdomyosarcoma mimicking a choledochal cyst. J Gastrointest Liver Dis 2009; 18:95-7 [PubMed] [Google Scholar]
- 8.Kumar V, Chaudhary S, Kumar M, Gangopadhyay AN. Rhabdomyosarcoma of biliary tract: a diagnostic dilemma. Indian J Surg Oncol 2012;3:314-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zampieri N, Camoglio F, Corroppolo M, et al. Botryoid rhabdomyosarcoma of the biliary tract in children: a unique case report. Eur J Cancer Care (Engl) 2006;15:463-6 [DOI] [PubMed] [Google Scholar]
- 10.Margain-Deslandes L, Gelas T. A botryoid rhabdomyosarcoma diagnosed as a choledochal cyst. Pediatr Blood Cancer 201;60:2089-90 [DOI] [PubMed] [Google Scholar]
- 11.Flanigan DP. Biliary carcinoma associated with biliary cysts. Cancer 1977;40:880-3 [DOI] [PubMed] [Google Scholar]
- 12.Todani T, Tabuchi K, Watanabe Y, et al. Carcinoma arising in the wall of congenital bile duct cysts. Cancer 1979;44:1134-41 [DOI] [PubMed] [Google Scholar]
- 13.Roebuck DJ. Interventional radiology in children with hepatobiliary rhabdomyosarcoma. Med Pediatr Oncol 1998;31:187-8 [DOI] [PubMed] [Google Scholar]
- 14.De Angelis P, Foschia F, Romeo E, et al. Role of endoscopic retrograde cholangiopancreatography in diagnosis and management of congenital choledochal cysts: 28 pediatric cases. J Pediatr Surg 2012;47:885-8 [DOI] [PubMed] [Google Scholar]
- 15.Ohashi T, Wakai T, Kubota M, et al. Risk of subsequent biliary malignancy in patients undergoing cyst excision for congenital choledochal cyst. J Gastroenterol Hepatol 2013;28:243-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saluja SS, Nayeem M, Sharma BC, et al. Management of choledochal cysts and their complications. Am Surg 2012;78:284-90 [PubMed] [Google Scholar]
- 17.Liu QD, Chen JZ, Xu XY, et al. Incidence of port-site metastasis after undergoing robotic surgery for biliary malignancies. World J Gastroenterol 2012;18:5695-701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makin E, Davenport M. Understanding choledochal malformation. Arch Dis Child 2012;97:69-72 [DOI] [PubMed] [Google Scholar]
- 19.Yamataka A, Lane GJ, Cazares J. Laparoscopic surgery for biliary atresia and choledochal cyst. Semin Pediatr Surg 2012;21:201-10 [DOI] [PubMed] [Google Scholar]
- 20.Chang EY, Hong YJ, Chang HK, et al. Lessons and tips from the experience of pediatric robotic choledochal cyst resection. J Laparoendosc Adv Surg Tech A 2012;22:609-14 [DOI] [PubMed] [Google Scholar]


