Abstract
Background
The extent to which channels within scar are inter-connected is not known. The objective of the study was to evaluate the impact of local ablation of LPs on adjacent and remote areas of slow conduction with simultaneous multipolar mapping.
Methods and Results
Analysis was performed on consecutive patients referred for ablation of scar-mediated VT with double ventricular access. Ablation was performed targeting the earliest of LPs visualized on the multipolar catheter and the impact on later LPs was recorded. In 21 patients, a multipolar catheter placed within scar visualized spatially distinct LPs. Among 39 RF applications, ablation at earlier LPs had an effect on neighboring and remote LPs in 31 (80%), with delay in 8 (21%), partial elimination in 9 (23%), and complete elimination in 14 (36%). The mean distance where an ablation impact was detected was 17.6±14.7mm (range 2mm-50mm). Among all patients, 9.7±7.8 RF applications were delivered to homogenize the targeted scar region with a mean number of 23±12 LPs targeted.
Conclusions
Ablation can eliminate neighboring and remote areas of slow conduction, suggesting that channels within scar are frequently inter-connected. This is the first mechanistic demonstration to show that ablation can modify electrical activity in regions of scar outside of the known radius of an RF lesion. The targeting of relatively earlier LPs can expedite scar homogenization without the need for extensive ablation of all LPs.
Keywords: ablation, ventricular tachycardia, mapping, late potential
Introduction
Slow conduction via non-linear electrical impulse activation within complex scar architecture has been implicated in the pathogenesis of fractionated and delayed local electrical activity.1 Late potentials (LP) mapped in sinus rhythm within scar have been shown to have specificity for reentrant isthmuses.2-4 While the elimination of LPs has been demonstrated to be effective in preventing recurrent VT, “homogenization” of all abnormal local electrical activity within scar has been proposed as a more comprehensive endpoint for substrate-based VT ablation. When compared to inducibility, extensive ablation within scar has been more predictive of clinical success.5-7
The extent of ablation required to “homogenize” an entire scar can be variable and result in prolonged procedural times. The impact of radiofrequencey (RF) ablation of LPs on other spatially distinct LPs mapped within scar has not been previously quantified or reported. Double ventricular access using a multipolar mapping catheter8 and ablation catheter, which has been used for rapid identification of critical isthmuses during VT ablation, can be a useful method to monitor the effect of local ablation on neighboring and remote regions of slow conduction within scar.
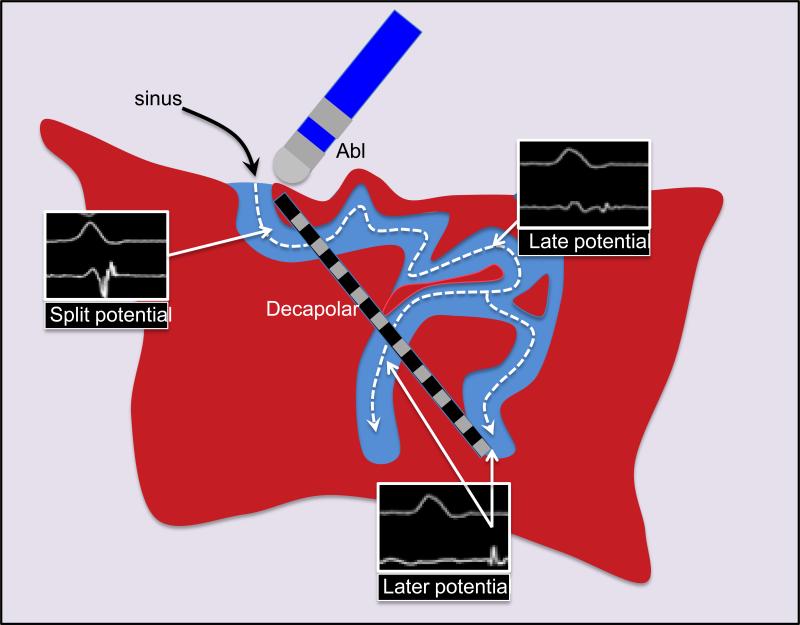
We hypothesized that 1) areas of LPs are necessarily activated through channels with earlier abnormal activation (FIGURE 1) 2) local ablation frequently impacts neighboring and even remote regions within scar and 3) ablation in the proximal part of a channel may be a more efficient method to “homogenize” scar in sinus rhythm.
Figure 1.
Schematic of activation within scar demonstrating progressively late activation after the QRS in sinus rhythm. A decapolar catheter oriented along this channel can detect multiple areas with LP and monitor the impact of ablation.
Methods
Patient Population
From 2009-2013, 128 patients at 2 centers underwent mapping of scar-mediated VT using a multipolar catheter. Among these patients, 21 underwent ablation with double ventricular access using a multipolar catheter to guide and monitor RF ablation. Patients with spatially distinct LPs (>2mm apart) represented on more than one electrode pair at a stable multipolar catheter position were included for analysis.
The diagnosis of ischemic cardiomyopathy (ICM) was established by prior history of infarction with Q waves, focal wall motion abnormality, or fixed perfusion defect correlated with coronary stenosis or prior coronary intervention. All ablations for scar-mediated VT were performed under general anesthesia. Written informed consent was obtained from all patients. The UCLA Medical Center and University of Texas Health Science institutional review board approved review of this data.
Electrophysiological Study and Electroanatomic Mapping
The approach and strategy for ablation of scar-mediated VT at our center has been previously reported.9 Entrainment mapping was performed when VT was hemodynamically tolerated. In cases of hemodynamically unstable VT, all LP sites were tagged and pacemapping was performed. Sites with multiple exit sites (MES) and pace-mapped induction (PMI) were considered isthmus surrogates.9 High-density electroanatomic maps were created in sinus rhythm (intrinsic= 13, RV paced=6, BiV paced=2) using CARTO (Biosense Webster, Diamond Bar, CA) or NAVX (St. Jude Medical, Minneapolis, MN) with greater density of sampling in regions of low voltage and border zone tissue (0.5-1.5mV). Multipolar mapping was performed using either a duodecapolar catheter (Livewire, 2-2-2 inter-electrode spacing, St. Jude Minneapolis MN), decapolar catheter (DecaNav, 2-8-2 interelectrode spacing, Biosense Webster, Diamond Bar, CA), or a 64-electrode basket catheter (Constellation 60 mm diameter, 5mm inter-electrode spacing, Boston Scientific, Natick, MA)
Multipolar monitoring of LP ablation
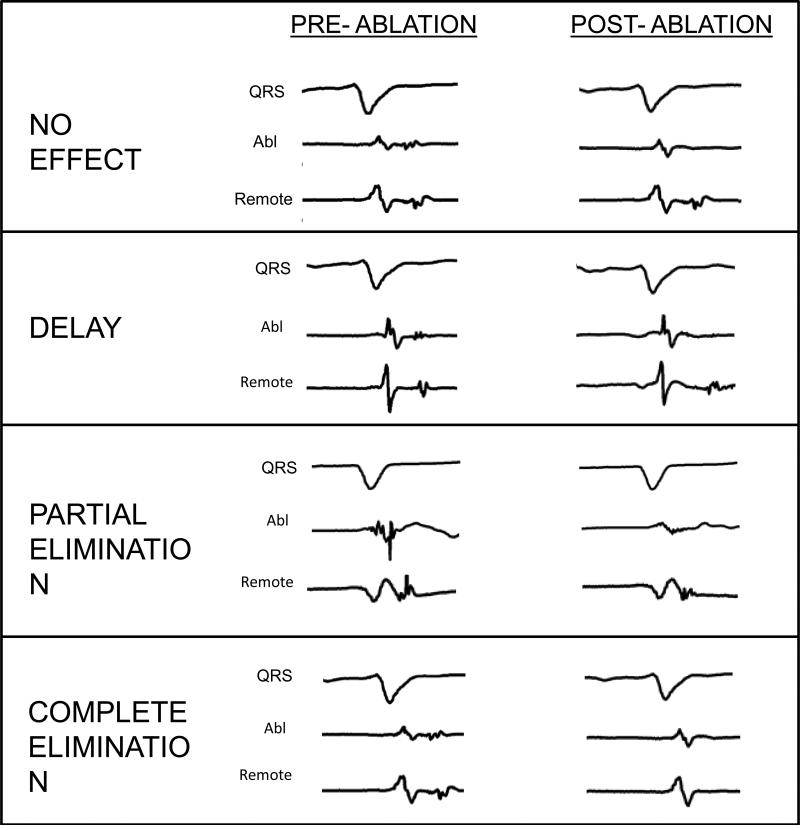
All LPs (<1.5mV, with onset after QRS offset or split potential separated by 20ms isoelectric segment) identified were tagged on electroanatomic mapping, whether detected with the multipolar or ablation catheter. The multipolar catheter was positioned within scar in an orientation that recorded the largest number of LPs with a gradient of early-to-late activation. Ablation was performed at the earlier electrograms with late activity and the impact of ablation on neighboring (within 8mm of RF site) and remote electrodes (>8mm away from RF site) was classified into four responses if the local distal ablation signal was from ablation: 1) no effect 2) delay in activation 3) partial elimination of LP (abolition of sharp, high-frequency component within a far-field ventricular electrogram)6 or 4) complete elimination of LP. (FIGURE 2)
Figure 2.
Categorization of the impact of local ablation (elimination of distal ablation electrogram) on neighboring and remote LPs. Local ablation can have no effect, delay, partially eliminate, and completely eliminate spatially distinct areas of late activity. Partial elimination is defined as elimination of the high frequency component of an electrogram within a far field signal.
If delay at the remote LP site was seen prior to elimination of the local LP, it was categorized as elimination. RF applications where instability of the multipolar catheter was suspected (change in far field electrogram of a given bipole pair or variability of electrograms in neighboring electrodes) were excluded. At least one multipolar catheter position to evaluate RF delivery was recorded in all patients and additional monitoring of ablation was left to the discretion of the operator.
In addition to analysis of individual RF applications, quantification of the total number of LPs mapped within the scar was performed. These were manually counted for each map and compared to the number of ablation lesions delivered to eliminate or modify the region of substrate targeted, which was verified by remapping after ablation.
RF ablation was performed using an open-irrigated catheter (ThermoCool or ThermCool SF, 3.5 mm, Biosense-Webster, Diamond Bar, CA) at 30-50W, temperature limit 45°C @ 30 ml flow rate or closed-loop irrigated catheter (Chilli, Boston Scientific, Natick MA) at 30-50W, temperature limit 45°C. The temperature limit for epicardial RF applications was 50°C. RF energy was applied for 60 seconds for each application. Ablation sites were tagged on the electroanatomic map and lesions were considered effective if they resulted in at least one of the following: a diminution or elimination of the local electrogram, failure to capture at 10mA at 2 ms pacing, or impedance drop >10Ω. Distances between LPs were calculated using the fluoroscopy, mapping system, and known interelectrode distances. When using a linear catheter, distances are reported as edge-to-edge to the more inner electrode of a pair. For example, the interelectrode distance between bipole 1,2 and bipole 5,6 using a 2-2-2 mm spacing with 1mm electrode size is 8mm.
Acute Outcomes and Clinical Follow-Up
After ablation was performed in the region of the targeted VT, programmed stimulation was repeated with ventricular extrastimulus testing at drive cycles of 600 and 400 ms, with decrement by 10 ms until refractoriness or 200 ms, up to four extrastimuli at a minimum of two sites. Acute procedural success was defined as noninducibility after ablation. Partial success was defined as inducibility of only faster non-clinical VTs. Follow-up for VT recurrence and survival data were assessed from outpatient clinic notes, device interrogation, patient charts, and contact with the patients or referring physician by phone.
Statistical analysis
All continuous data are reported as medians with 25% and 75% percentiles due to the small sample size, evidenced by the relatively large standard deviations observed in this cohort.
Results
21 patients (ICM=15, NICM=2, ARVD=1, sarcoid=1, Chagas=1, noncompaction=1) underwent double ventricular access for VT ablation using a multipolar catheter with the intention of monitoring RF application effects within scar. The median age was 63 (52-70) years and all patients were male. The median ejection fraction was 25% (25-30%). Approximately half (48%) of patients referred had prior ablation and 90% were on antiarrhythmic medications. Patient characteristics are shown in TABLE 1.
Table 1.
Patient characteristics
| Patient | Age | Sex | Etiology | EF | AAD | Prior ablation | ICD | VT storm |
|---|---|---|---|---|---|---|---|---|
| 1 | 74 | M | ICM | 10 | A | no | yes | yes |
| 2 | 49 | M | ICM | 20 | A/L | no | yes | yes |
| 3 | 59 | M | ICM | 35 | S | no | yes | no |
| 4 | 52 | M | Noncompaction | 25 | none | no | yes | yes |
| 5 | 70 | M | ICM | 35 | M/S | yes | yes | no |
| 6 | 63 | M | ICM | 25 | no | no | yes | no |
| 7 | 70 | M | ICM | 25 | A | yes | yes | yes |
| 8 | 48 | M | NICM | 30 | A/S | yes | yes | no |
| 9 | 80 | M | ICM | 20 | A | no | yes | yes |
| 10 | 57 | M | ICM | 35 | none | no | yes | no |
| 11 | 70 | M | ICM | 20 | A/M | no | yes | yes |
| 12 | 69 | M | ICM | 26 | A/M | yes | yes | no |
| 13 | 34 | M | Sarcoid | 35 | S | yes | yes | yes |
| 14 | 73 | M | ICM | 30 | A | yes | yes | no |
| 15 | 60 | M | Chagas | 25 | A/M | no | yes | no |
| 16 | 43 | M | ARVC | 55 | S | yes | yes | no |
| 17 | 56 | M | ICM | 25 | A/M/S | yes | yes | yes |
| 18 | 71 | M | ICM | 20 | A | yes | yes | no |
| 19 | 76 | M | ICM | 30 | A | no | yes | yes |
| 20 | 68 | M | NICM | 25 | A/M | yes | yes | no |
| 21 | 51 | M | ICM | 30 | A | no | yes | no |
A=amiodarone, S=sotalol, M=mexiletene
Electroanatomic mapping findings are summarized in TABLE 2. A median of 614 (417-833) points were obtained for each map. A multipolar catheter (duodecapolar=16, decapolar=3, basket=2) was placed within scar to visualize spatially distinct LPs at a single stable position. The basket catheter was chosen for patients with apical scar. 9 patients underwent epicardial mapping and ablation, where two sheaths were placed into the pericardium using a double-wire technique through a single puncture.10 Each patient had a median of 2 (1-2.5) RF applications targeted at eliminating multiple potentials visualized on the multipolar catheter. All other RF applications required to complete the ablation were performed without multipolar monitoring.
Table 2.
Electroanatomic mapping and electrophysiological findings
| Pt | EAM | Multi- polar |
Scar Area (cm2) |
Scar location | Mapping points |
MES/PMI | LP # |
Abl # |
Target VT (TCL) |
VTs induced |
VT Term |
Acute success |
VT recur |
Follow- up (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CARTO | DD | 96 | apical/septal | 861 | no/no | 11 | 4 | RBLS (380) | 2 | yes | non | no | 6 |
| 2 | CARTO | DD | 62 | inferior | 833 | no/no | 4 | 2 | RBLS (355) | 4 | yes | flutter | no | 11 |
| 3 | NAVX | DD | 57 | apical | 922 | no/no | 14 | 9 | RBLS (290) | 3 | no | non | no | 9 |
| 4 | NAVX | DD | 59 | epi lateral | 531 | no/no | 33 | 25 | RBRS (290) | 1 | no | non | no | 13 |
| 5 | NAVX | DD | 17 | anteroseptal | 745 | yes/no | 41 | 4 | RBRI (320) | 4 | no | non | no | 12 |
| 6 | CARTO | DD | 88 | anteroapical | 402 | yes/yes | 36 | 6 | RBLS (300) | 1 | yes | non | no | 18 |
| 7 | CARTO | Deca | 87 | inferior epi | 721 | no/no | 10 | 3 | RBRS (340) | 1 | no | partial | no | 21 |
| 8 | CARTO | Basket | 71 | anteroapical | 328 | yes/no | 4 | 3 | RBRI (360) | 4 | yes | non | yes | 8 |
| 9 | CARTO | Basket | 64 | inferoapical | 374 | yes/yes | 26 | 25 | LBS (360) | 4 | yes | non | no | 7 |
| 10 | CARTO | DD | 18 | apical | 399 | no/no | 11 | 1 | non | 0 | no | non | no | 17 |
| 11 | NAVX | DD | 58 | apex | 815 | no/no | 12 | 2 | LBI (340) | 2 | no | non | no | 7 |
| 12 | NAVX | DD | 83 | anterior | 817 | yes/yes | 23 | 12 | RBLS (440) | 4 | no | flutter | no | 44 |
| 13 | CARTO | DD | 68 | basal IL epi | 522 | no/no | 30 | 10 | non | 0 | no | non | no | 11 |
| 14 | NAVX | DD | 51 | epi basal IL | 1157 | yes/no | 24 | 14 | RBRI (530) | 2 | yes | non | no | 30 |
| 15 | NAVX | DD | 22 | epi inferoapex | 2890 | no/no | 22 | 6 | RBRI (400) | 3 | no | non | no | 1 |
| 16 | CARTO | Deca | 40 | epi RVOT | 430 | yes/no | 32 | 11 | LBI (320) | 1 | yes | non | no | 1 |
| 17 | NAVX | DD | 39 | epi IL | 185 | yes/yes | 39 | 26 | RBRI (540) | 1 | yes | non | yes | 2 |
| 18 | NAVX | DD | 70 | endo IL | 417 | yes/yes | 18 | 7 | RBRS (585) | 3 | yes | no | no | 46 |
| 19 | CARTO | DD | 55 | septal | 614 | yes/no | 37 | 16 | LBLS (360) | 3 | yes | non | no | 2 |
| 20 | NAVX | DD | 86 | epi-anteroapical | 583 | no/no | 15 | 5 | RBI (330) | 1 | no | non | no | 24 |
| 21 | CAROT | Deca | 127 | epi inferolat | 1309 | yes/yes | 42 | 14 | RBLI (420) | 3 | yes | failure | yes | 1 |
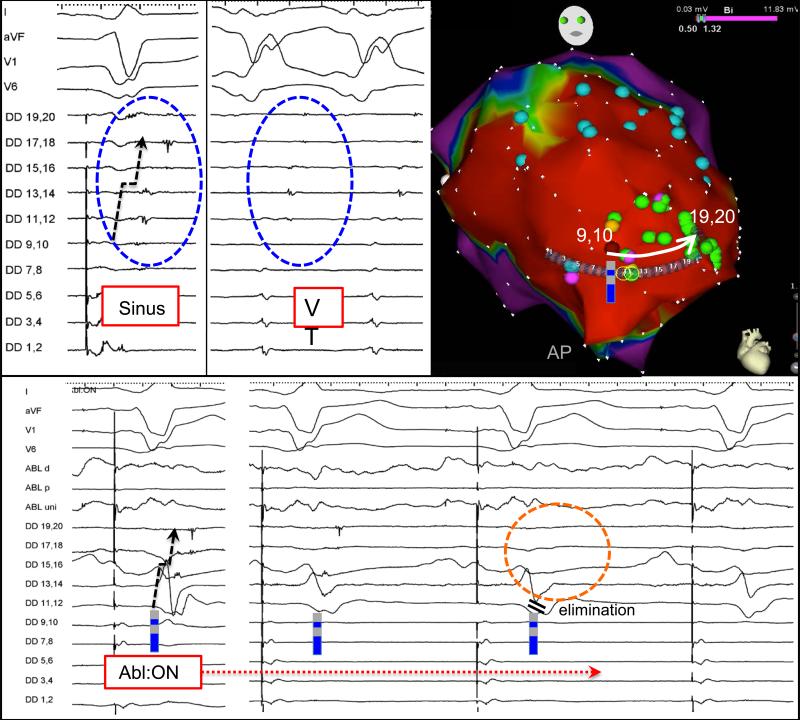
Among 39 RF applications, ablation at earlier abnormal potentials had an effect on neighboring LPs in 31 (80%), with delay in 8 (21%), partial elimination in 9 (23%), and complete elimination in 14 (36%). FIGURE 3 demonstrates ablation within a dense anteroapical infarct with ablation at DD 9,10 which results in elimination of all LP to 19,20 (26mm away). The median delay, when observed, was 26 ms (19-37 ms) on remote LPs. The median distance where an ablation effect was seen remotely was 15 mm (8-20 mm), with a range from 2mm up to 50mm from the RF application site. 61% (19/31) of the effects were remote (>8mm from RF site). FIGURE 4 demonstrates delay prior to elimination of a critical remote LP (mid-diastolic during VT) demonstrated with a basket catheter in a patient with history of a remote stab wound to the chest complicated cardiac perforation.
Figure 3.
Placement of a duodecapolar catheter across a region with multiple LPs in the apical region of anterior infarction. A single RF application targeted at the earliest later potential (9,10) eliminates a gradient of late activation down the channel to 19,20, which is 26 mm away.
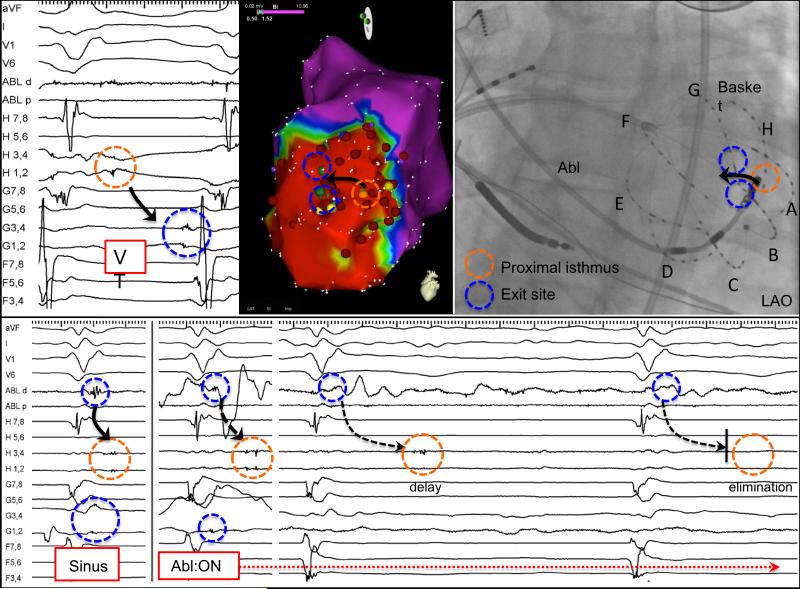
Figure 4.
Placement of basket catheter into trauma-induced (stab wound to chest with cardiac perforation) left ventricular apical aneurysm reveals diastolic activation from spline H1-4 (early diastolic) to G1-4 (presystolic). VT was terminated by ablation between these two splines. After termination, sinus rhythm LP activation proceeds from G1-4 to H1-4. A single RF application using retrograde approach magnetic navigation targeted at the earliest LP at G1-4 delays and eliminates H 1-4, which is 15 mm away.
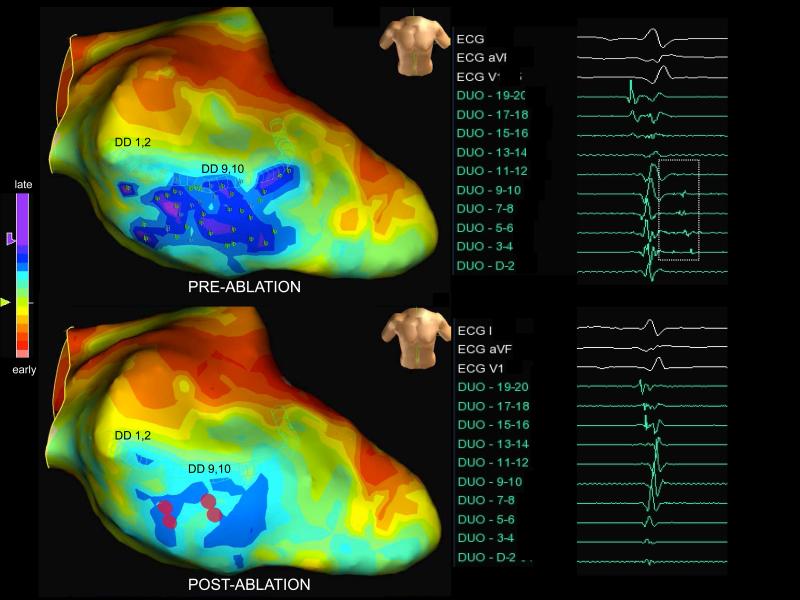
Among all patients, 7 (4-14) RF applications were delivered to homogenize the targeted scar region where a median number of 23 (12-33) LPs were mapped. FIGURE 5 is an example of ablation along the septal border zone of an anterior infarct, where the latest activity (dark blue and purple on propagation map setting) is seen in sinus rhythm. Only four ablations were required to eliminate 41 LPs mapped. Remapping in this region demonstrated elimination of late activity and LPs.
Figure 5.
Late activation map display in sinus rhythm within septal scar. A duodecapolar catheter was used to map LPs (lp tags) and the regions of latest activation are displayed as blue and purple. Four ablation lesions (red) applied within scar eliminates all 41 LPs identified, which is confirmed with remapping using the duodecapolar catheter where the latest activation is eliminated postablation
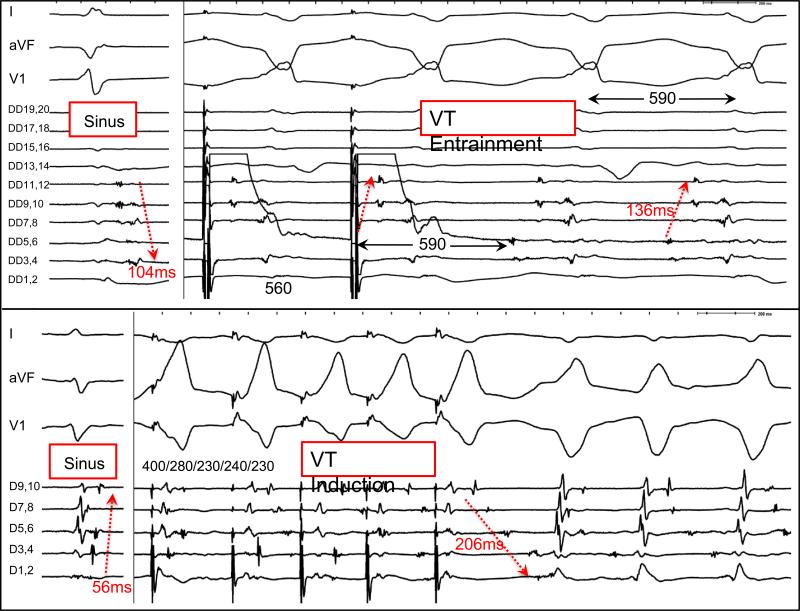
A median of 2 (1-3) VTs were induced with an average cycle length of 360 ms (325-410 ms) for the targeted VT. In five patients, diastolic activation during VT was recorded on the multipolar catheter at the exact same position that revealed a gradient of LPs in sinus rhythm. In all but one patient (FIGURE 3), the diastolic activation sequence during VT was reversed from LP activation in sinus rhythm. The interval between the earliest and latest LPs was longer during VT compared to sinus rhythm in all patients (136 ms (100-171 ms) vs. 57 ms (56-81 ms). The latest sinus rhythm LP in these patients (84 ms (90-128 ms) after QRS) was 33% (31-48%) of the TCL during diastole in VT. Concealed entrainment of an isthmus was demonstrated in two patients, and the activation of the LPs during entrainment was the same as during VT. (FIGURE 6)
Figure 6.
Evidence of interconnected LPs during sinus rhythm and VT in two patients. The first patient (top) had a history of inferolateral myocardial infarction and concealed entrainment is demonstrated from DD 3,4, which is latest in sinus and earliest in VT. The interval between the LPs (red arrows) is longer during VT (136ms) than sinus (104ms). Activation more distally (DD 9-12) is the same pattern during entrainment and VT. In the second patient (lower) with arrhythmogenic right ventricular cardiomyopathy, LPs are seen with a decapolar catheter placed across the epicardial RV outflow tract. During induction of VT with programmed stimulation, activation is reversed and the interval between the LPs (56ms) is significantly prolonged during VT (206ms) with pandiastolic activity recorded. Unidirectional block with emergence of the unopposed orthodromic wavefront is seen with progressive extrastimuli.
MES was seen in 52% of patients (11/21), PMI was observed in 29% (6/21) and termination of a targeted VT was achieved in 52% (11/31) of cases. Two patients were noninducible throughout the procedure and the acute endpoint of noninducibility was obtained in 84% of the remainder (16/19) and a partial success was seen in 16% (3/19). At a median of 11 (6-18) months, 86% of patients remained free of recurrent VT.
Discussion
The current study, to our knowledge, is the first to provide mechanistic evidence of the interconnected nature of channels within scar where 1) RF applications frequently modify regions of late activity within scar outside the known radius of an ablation lesion 2) the amount of ablation required to eliminate LPs is not as extensive as the number of LPs mapped and identified.
The intended elimination of all abnormal electrical activity within scar has been shown to be improve clinical freedom from recurrent VT compared to more limited strategies.5-7 These more extensive approaches, however, may prolong procedural time and the duration of RF application to “homogenize” scar may be longer. The nature of abnormally fractionated, split, or local electrical activity within scar has been attributed to the zig-zag propagation of heterogenous conducting channels interspersed and bounded by collagen.1, 11 In this construct, every LP deep within scar is necessarily activated by an “earlier” later potential. The ablation of a more proximal, or entrance site in sinus rhythm is likely to have an effect on more delayed activation “downstream” in a channel.
Jais et al demonstrated that epicardial LPs could be abolished during endocardial ablation in 5 patients who underwent combined epi-endo ablation of VT, with the goal of eliminating all local abnormal ventricular activitities (LAVA).6 However, the local effects of endocardial and epicardial ablation on the same surface have not been systematically studied and quantified. Such an analysis requires double ventricular access with a stable multipolar catheter to monitor the impact of ablation on spatially adjacent and remote regions of scar. As Jais et al classified direct ablation of LAVA into four categories, we similarly classified “indirect” ablation effects on neighboring and remote ablation into four categories only when the local electrogram was eliminated. In this analysis, we also demonstrate that fewer ablation lesions are required to eliminate or modify multiple LPs.
Irrigated ablation has been shown to created lesions 14±3 mm in diameter with a lesion depth 6±1 mm on infarcted epicardial tissue in vivo.12 Similar lesion diameters (14 mm) with high power irrigated technology have been shown in ex vivo thigh preparations. Therefore, any effects seen outside of the radius (>8mm) cannot be attributed to direct resistive or conductive heating. In this study, we observed ablation impact up to the entire length of a duodecapolar catheter which measures 50mm. The remote effects of ablation are likely to be underestimated with the current methodology when calculating the closest distance between the inner electrodes with edge-to-edge reporting. Theoretically the maximum distance between a bipole pair 1,2 and 3,4 with 2 mm spacing may be as high as 10mm.
An alternative to displaying scar as gradations of low voltage is displaying activation maps of progressively late electrical activity in sinus rhythm (FIGURE 5). This provides more of the “functional” characteristics of arrhythmogenic scar, which is complementary to “structural” low voltage areas. The current findings suggest that ablation targeted at the “border zone” of the latest activity region may be a more efficient approach to homogenize scar. This approach would be aimed at eliminating entrances into the latest activity in sinus rhythm, which would represent the exit during VT.
As we have previously demonstrated MES when pacing within scar, it is plausible to expect multiple entrances into a given LP. Delay of a LP with ablation of an earlier LP may be due to conduction slowing or alternatively, a shift to a different entrance into channel. Functional conduction slowing during reentry likely accounts for the prolongation in the interval between LPs seen during VT than in sinus rhythm. However, activation of a channel through multiple wavefronts during sinus rhythm, as opposed to a singular wavefront during VT, may also contribute to this phenomenon. Further studies are required to assess the impact of differential pacing from different scar border zones on the identification of LP as preferential entrance into scar may rely on directionality of the wavefront and anisotropic conduction.
Limitations
The intended purpose of the study is to provide mechanistic insight into the effects of ablation within scar via analysis of individual RF applications as well as the sum total extent of ablation required to eliminate the total number of LP mapped. The objective of the present study is not to propose monitoring of all ablation lesions with a multipolar mapping catheter, as this technique can be time-consuming. We recognize that only the minority of lesions delivered during the ablation procedure were monitored with the double access setup. This is largely due to the fact that these observations require a stable catheter position demonstrating multiple LPs and careful manipulation of the ablation catheter toward the targeted bipole without disturbing the multipolar catheter position or creating inter-catheter mechanical artifact.
The observed inter-connectedness of channels within scar may not be generalizable to all scar substrates as only patients in whom multiple potentials are recorded at a single catheter position were included. Multiple etiologies of scar were included in this cohort and the small sample size is inadequate to draw conclusions across all substrates.
Only electrograms with a gradient of late activation were chosen, which biases the ablation lesions towards orientation along a channel. However, the absence of remote impact detected during local ablation does not exclude effects on regions that may not contacted by the electrodes on the multipolar catheter. Different multipolar catheters were used within this cohort, and the use of smaller interelectrode spacing may increase the ability to detect ablation impact within scar. Further, remote impact on regions outside of 50 mm may be present but cannot be proven using a single duodecapolar catheter.
Distances of remote impact were estimated using “field scaling” from known interelectrode size and spacing as well as fluoroscopy. The linear catheter is frequently curved to improve mapping contact on the mapping surface contour and therefore, an overestimation of the distances may result as they are calculated based on a linear orientation. For this reason, we chose to report edge-to-edge distances between the most inner electrodes to avoid overestimation of ablation effects.
Conclusions
Local ablation frequently impacts and eliminates neighboring and spatially remote areas of slow conduction, suggesting that channels within scar are often inter-connected. This is the first mechanistic demonstration to show that ablation can modify electrical activity in regions of scar outside of the known radius of a RF application. The targeting of relatively earlier LPs may expedite a scar homogenization strategy without the need for extensive ablation. Simultaneous multipolar mapping during ablation may be of clinical value.
Supplementary Material
Acknowledgments
Funding Sources: K.S. is supported by the NHLBI (R01HL084261).
Footnotes
Conflict of Interest Disclosures: R.T. receives research grant from St. Jude Medical.
References
- 1.de Bakker JM, van Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, de Jonge N, Lahpor JR. Slow conduction in the infarcted human heart. ‘Zigzag’ course of activation. Circulation. 1993;88:915–926. doi: 10.1161/01.cir.88.3.915. [DOI] [PubMed] [Google Scholar]
- 2.Arenal A, Glez-Torrecilla E, Ortiz M, Villacastin J, Fdez-Portales J, Sousa E, del Castillo S, Perez de Isla L, Jimenez J, Almendral J. Ablation of electrograms with an isolated, delayed component as treatment of unmappable monomorphic ventricular tachycardias in patients with structural heart disease. J Am Coll Cardiol. 2003;41:81–92. doi: 10.1016/s0735-1097(02)02623-2. [DOI] [PubMed] [Google Scholar]
- 3.Bogun F, Bender B, Li YG, Groenefeld G, Hohnloser SH, Pelosi F, Knight B, Strickberger SA, Morady F. Analysis during sinus rhythm of critical sites in reentry circuits of postinfarction ventricular tachycardia. J Interv Card Electrophysiol. 2002;7:95–103. doi: 10.1023/a:1020832502838. [DOI] [PubMed] [Google Scholar]
- 4.Bogun F, Good E, Reich S, Elmouchi D, Igic P, Lemola K, Tschopp D, Jongnarangsin K, Oral H, Chugh A, Pelosi F, Morady F. Isolated potentials during sinus rhythm and pace-mapping within scars as guides for ablation of post-infarction ventricular tachycardia. J Am Coll Cardiol. 2006;47:2013–2019. doi: 10.1016/j.jacc.2005.12.062. [DOI] [PubMed] [Google Scholar]
- 5.Di Biase L, Santangeli P, Burkhardt DJ, Bai R, Mohanty P, Carbucicchio C, Dello Russo A, Casella M, Mohanty S, Pump A, Hongo R, Beheiry S, Pelargonio G, Santarelli P, Zucchetti M, Horton R, Sanchez JE, Elayi CS, Lakkireddy D, Tondo C, Natale A. Endo-epicardial homogenization of the scar versus limited substrate ablation for the treatment of electrical storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2012;60:132–141. doi: 10.1016/j.jacc.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 6.Jais P, Maury P, Khairy P, Sacher F, Nault I, Komatsu Y, Hocini M, Forclaz A, Jadidi AS, Weerasooryia R, Shah A, Derval N, Cochet H, Knecht S, Miyazaki S, Linton N, Rivard L, Wright M, Wilton SB, Scherr D, Pascale P, Roten L, Pederson M, Bordachar P, Laurent F, Kim SJ, Ritter P, Clementy J, Haissaguerre M. Elimination of local abnormal ventricular activities: a new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation. 2012;125:2184–2196. doi: 10.1161/CIRCULATIONAHA.111.043216. [DOI] [PubMed] [Google Scholar]
- 7.Vergara P, Trevisi N, Ricco A, Petracca F, Baratto F, Cireddu M, Bisceglia C, Maccabelli G, Della Bella P. Late potentials abolition as an additional technique for reduction of arrhythmia recurrence in scar related ventricular tachycardia ablation. J Cardiovasc Electrophysiol. 2012;23:621–627. doi: 10.1111/j.1540-8167.2011.02246.x. [DOI] [PubMed] [Google Scholar]
- 8.Tung R, Nakahara S, Maccabelli G, Buch E, Wiener I, Boyle NG, Carbucicchio C, Bella PD, Shivkumar K. Ultra high-density multipolar mapping with double ventricular access: a novel technique for ablation of ventricular tachycardia. J Cardiovasc Electrophysiol. 2011;22:49–56. doi: 10.1111/j.1540-8167.2010.01859.x. [DOI] [PubMed] [Google Scholar]
- 9.Tung R, Mathuria N, Michowitz Y, Yu R, Buch E, Bradfield J, Mandapati R, Wiener I, Boyle N, Shivkumar K. Functional pace-mapping responses for identification of targets for catheter ablation of scar-mediated ventricular tachycardia. Circ Arrhythm Electrophysiol. 2012;5:264–272. doi: 10.1161/CIRCEP.111.967976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horowitz BN, Vaseghi M, Mahajan A, Cesario DA, Buch E, Valderrabano M, Boyle NG, Ellenbogen KA, Shivkumar K. Percutaneous intrapericardial echocardiography during catheter ablation: a feasibility study. Heart Rhythm. 2006;3:1275–1282. doi: 10.1016/j.hrthm.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 11.de Bakker JM, van Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, de Jonge N, Lahpor JR. Fractionated electrograms in dilated cardiomyopathy: origin and relation to abnormal conduction. J Am Coll Cardiol. 1996;27:1071–1078. doi: 10.1016/0735-1097(95)00612-5. [DOI] [PubMed] [Google Scholar]
- 12.d'Avila A, Houghtaling C, Gutierrez P, Vragovic O, Ruskin JN, Josephson ME, Reddy VY. Catheter ablation of ventricular epicardial tissue: a comparison of standard and cooled-tip radiofrequency energy. Circulation. 2004;109:2363–2369. doi: 10.1161/01.CIR.0000128039.87485.0B. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.