Abstract
Estrogen receptor β (ERβ) is a member of the nuclear receptor family of homeostatic regulators that is frequently lost in breast cancer (BC), where its presence correlates with a better prognosis and a less aggressive clinical outcome of the disease. In contrast to ERα, its closest homolog, ERβ shows significant estrogen-independent activities, including the ability to inhibit cell cycle progression and regulate gene transcription in the absence of the ligand. Investigating the nature and extent of this constitutive activity of ERβ in BC MCF-7 and ZR-75.1 cells by means of microRNA (miRNA) sequencing, we identified 30 miRNAs differentially expressed in ERβ+ versus ERβ− cells in the absence of ligand, including up-regulated oncosuppressor miRs such miR-30a. In addition, a significant fraction of >1,600 unique proteins identified in MCF-7 cells by iTRAQ quantitative proteomics were either increased or decreased by ERβ, revealing regulation of multiple cell pathways by ligand-free receptors. Transcriptome analysis showed that for a large number of proteins regulated by ERβ, the corresponding mRNAs are unaffected, including a large number of putative targets of ERβ-regulated miRNAs, indicating a central role of miRNAs in mediating BC cell proteome regulation by ERβ. Expression of a mimic of miR-30a-5p, a direct target and downstream effector of ERβ in BC, led to the identification of several target transcripts of this miRNA, including 11 encoding proteins whose intracellular concentration was significantly affected by unliganded receptor. These results demonstrate a significant effect of ligand-free ERβ on BC cell functions via modulation of the cell proteome and suggest that miRNA regulation might represent a key event in the control of the biological and clinical phenotype of hormone-responsive BC by this nuclear receptor.
Estrogen receptors (ERs)1 α and β, ligand-inducible nuclear receptors, regulate the transcription of target genes such as those involved in cell cycle control, including proto-oncogenes and cyclin genes (1–3). ERs act through genomic and nongenomic signal transduction pathways (4–7) that integrate with one another to mediate the mitogenic actions of estrogens, hormones involved in the pathophysiology of the mammary gland through their ability to modulate cell growth and differentiation. Although the two receptor subtypes share a similar mechanism of action, they show substantial differences in their ability to control gene transcription, including specific properties defining subtype-specific functions and features (8–10). Indeed, upon hormonal activation they recruit different molecular partners in large multiprotein complexes exerting different effects in breast cancer (BC) cells (11–14). As a consequence, whereas ERα is mitogenic for hormone-responsive BC cells, ERβ inhibits cell proliferation by increasing the expression of growth-inhibitory genes and interfering with the activation of cell cycle and anti-apoptotic genes by ERα in response to 17β-estradiol (8, 15). Interestingly, ERβ is frequently lost in BC, and this is believed to represent a critical stage in estrogen-dependent tumor progression (16, 17). The presence of ERβ generally correlates with a better prognosis of the disease (18) and is a biomarker of a less aggressive clinical phenotype (19, 20). Unlike ERα, which is mainly inactive in the absence of estrogens, ERβ is able to regulate gene expression in the absence of steroid ligands, as unliganded receptor can translocate to the nucleus (21–23), where it recruits transcriptional co-regulators and modulates gene activity (24, 25).
The different regulatory roles of the two ERs in BC have recently been shown to relate also to the specific modulation of microRNA (miRNA) expression. Both ERs were found to bind in vivo to miRNA gene regulatory sites in BC cell chromatin upon activation by E2 (8, 26) and to control BC cell miRNome activity in vitro and in vivo (27, 28). This result is particularly intriguing in view of the known master regulatory role of these RNAs in normal and transformed cells. miRNAs are small (20 to 25 nucleotides) noncoding RNAs synthesized in the nucleus by RNA polymerase II or III as long primary transcripts (pri-miRNAs) that are then processed by a microprocessor complex comprising Drosha and DiGeorge syndrome critical region protein 8 proteins (29) to ∼70-nucleotide stem-loop RNAs (pre-miRNAs). Pre-miRNAs are exported from nucleus to cytoplasm by exportin 5 and Ran-GTP (30) and cleaved by Dicer/TRBP endoribonucleases to generate ∼22-nucleotide mature miRNAs (31, 32) that, in turn, are incorporated into RNA-induced silencing complexes and bind to the 3′ untranslated regions (UTRs) of target mRNAs determining gene silencing by either inhibition of translation or mRNA degradation (33). In this way miRNA regulates a wide variety of physiological and pathological cellular pathways at a post-transcriptional level, including cell proliferation, differentiation, and homeostasis, as well as neoplastic transformation (34). Interestingly, in solid tumors such as prostate, colon, stomach, pancreas, lung, and breast, the spectrum of expressed miRNAs (miRNome) is different from that of the corresponding normal tissues (35), suggesting the involvement of miRNAs in the transformed cell biology. Indeed, altered miRNA expression contributes to tumorigenesis, as some of them can function as either tumor suppressors or oncogenes (36).
We investigated here the ability of unliganded ERβ to influence hormone-responsive BC cell proteome via miRNAs. By combining massively parallel next-generation miRNA sequencing, gene expression profiling, and isobaric tags for relative and absolute quantitation (iTRAQ) quantitative proteomics for comparative analyses of MCF-7 cells expressing or not expressing ERβ, we observed a remarkable effect of this ER subtype on miRNome composition in the absence of steroidal ligands. This, in turn, results in significant quantitative and qualitative changes of the cell proteome that, in most cases, are not accompanied by comparable changes of the corresponding transcriptome. These results demonstrate that ERβ controls many BC cell functions via miRNA-mediated post-transcriptional regulation of the cell proteome also in the absence of estrogen ligands.
EXPERIMENTAL PROCEDURES
Cell Culture
Stable cell clones expressing either C-TAP-ERβ or Ν-TAP-ERβ (ERβ+) have been previously described (12). To generate ZRFlagβ cells expressing Flag-ERβ, the full-length coding region of human ERβ cDNA was PCR amplified, creating BamHI sites at both ends that were then used to excise the insert for cloning into the p3XFLAG-CMV expression vector (Sigma Aldrich) to obtain p3XFLAG-ERβ-CMV plasmid. p3XFLAG-ERβ-CMV was transfected into ZR75.1 cells by lipofection (Lipofectamine 2000, Invitrogen). Stable Flag-ERβ-expressing clones were isolated after antibiotic selection using 200 μg/ml G418. All cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM) (Sigma-Aldrich) supplemented with 10% FBS (HyClone) and antibiotics (100 U/ml penicillin, 100 mg/ml streptomycin, 250 ng/ml Amfotericin-B). Steroid deprivation (starvation) was performed by culturing in DMEM without phenol red and 5% dextran-coated charcoal stripped serum for 5 days, as described elsewhere (12).
Preparation of Nuclear and Cytosolic Extracts
ERβ+ or ERβ− cells were harvested by scraping in cold PBS, collected by centrifugation at 1,000 × g, and resuspended in three volumes with respect to the cell pellet of hypotonic buffer (20 mm HEPES pH 7.4, 5 mm NaF, 10 μm Na molybdate, 0.1 mm EDTA, 1 mm DTT, 1 mm PMSF, 1X protease inhibitor mixture). Cell lysis was induced by incubation on ice for 15 min; then 0.5% Triton X-100 was added and a cytosolic fraction was prepared by spinning the samples for 30 s at 4 °C at 15,000 × g. Nuclei were purified by stratification on 25% sucrose cushion in hypotonic buffer and centrifuged for 15 min at 4 °C at 9,000 × g to remove cytosolic contaminants. The obtained nuclear pellets were then dissolved in one volume of nuclear lysis buffer (20 mm HEPES, pH 7.4, 25% glycerol, 420 mm NaCl, 1.5 mm MgCl2, 0.2 mm EDTA, 1 mm DTT, 1X Sigma-Aldrich protease inhibitor mixture, and 1 mm PMSF), incubated for 30 min at 4 °C with gentle shaking, and centrifuged for 30 min at 4 °C at 15,000 × g. Finally, nuclear extracts were diluted with 2X volumes of nuclear lysis buffer without NaCl for a final salt concentration of 140 mm (11).
Western Blotting
SDS-PAGE and Western blot analyses were performed using standard protocols as previously described (11). Protein samples from cytosolic and nuclear extracts were denatured, separated on 10% polyacrylamide and 0.1% SDS (SDS-PAGE), and electro-transferred onto a nitrocellulose membrane (Whatman GmbH-Schleicher & Schuell, Dassel, Germany). The membrane was blocked using 5% (w/v) fat-free milk powder in 1× TBS supplemented with 0.1% (v/v) Tween-20 (TBS-T). The following primary antibodies were used: rabbit anti-TAP (CAB1001, Thermo Scientific-Pierce), rabbit anti-ERα (sc-543, Santa Cruz Biotechnology, Dallas, TX), mouse anti-α-tubulin (T6199, Sigma Aldrich), mouse anti-β-actin (A1978, Sigma Aldrich), and mouse M2 anti-Flag (F1804, Sigma Aldrich). After extensive washing with the TBS–Tween-20 mixture, the immunoblotted proteins were incubated with the appropriate horseradish-peroxidase-conjugated secondary antibodies (GE Healthcare) and detected via enhanced chemiluminescence (Pierce ECL Western blotting Substrate, Thermo Scientific) and exposure to a medical x-ray film (FujiFilm, Dusseldorf, Germany).
Cell Proliferation Assay
Hormone-starved MCF-7 cells expressing or not expressing ERβ (3,000 per well) were seeded in 96-well plates and the proliferation rate was monitored for 10 days. At the established time (every 2 days) cells were washed in phosphate-buffered saline (PBS) and fixed with 12.5% glutaraldehyde for 20 min at room temperature. This was followed by two washes with distilled water, incubation with 0.05% methylene blue for 30 min, accurate rinsing, and incubation with 0.33 m HCl for 18 h. Absorption was measured at 620 nm. Hormone-starved ZR75.1 cells expressing or not expressing ERβ (3,000/well) were seeded in 96-well plates and the proliferation rate was assayed at 3, 6, and 12 days. At the established time a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide cell proliferation assay (Invitrogen) was performed. At each time point 200 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (final concentration of 1 mg/ml) reagent was added to each well, incubated for 4 h in a humidified 5% CO2 incubator at 37 °C, and resuspended in 100 μl of 0.4 m HCl in 2-propanol. Absorbance was then measured at 570 and 620 nm (background) wavelengths.
Cell Cycle Analysis
ERβ+ and ERβ− cells (1.5 × 105 cells per dish) were starved in 60-mm culture dishes for 5 days and collected in PBS containing 50 μg/ml propidiumiodide, 0.1% (v/v) sodium citrate, 0.1% (v/v) Nonidet P-40. Cell samples were incubated in the dark for at least 15 min at room temperature, or overnight at 4 °C, and analyzed by a FACScalibur flow cytometer using the CellQuest software package (BD Biosciences) according to standard protocols suggested by the manufacturer. Data analysis was performed with Modfit software (Verity Software, Topsham, ME). Results shown were obtained from several independent experiments.
Transient Transfections and Luciferase Assays
Wild-type (wt) MCF-7, C-TAP-ERβ (Ct-ERβ), and Ν-TAP-ERβ (Nt-ERβ) clones were starved as described above. Then 5 × 105 cells per dish were seeded in 60-mm culture dishes and transfected by using 25 μg of polyethylenimine per dish (Polysciences, Inc., Eppelheim, Germany) with 2.5 μg of DNA per dish, including 300 ng of ERE-tk-Luc, pSG-Δ2-NLS-LacZ vector (β-galactosidase) co-transfected as an internal control for transfection efficiency, and carrier DNA (Bluescribe M13+). At 48 h after transfection, cells were washed with cold PBS and lysed in 100 μl of lysis buffer (Promega, Madison, WI). Luciferase activity was measured in extracts using the luciferase assay reagent (Promega), according to the manufacturer's instructions, and values were expressed as relative light units normalized to the β-galactosidase activity. Average luciferase activity was calculated from the data obtained from three independent replicates.
miRNA Sequencing and Data Analysis
The identification of miRNA expression profiles was performed by means of next-generation sequencing with sequencing-by-synthesis technology; this allowed a dynamic range of detection (from very low to highly abundant RNAs) and measurement of relatively limited differences in expression between samples. For MCF7 miRNA profiling, 7.5 μg of total RNA was used in a library preparation according to the Illumina TruSeq small RNA sample preparation protocol (Rev. B, Illumina, San Diego, CA). Libraries were multiplexed by using Illumina indices set A with indices 10 (RPI10) and 11 (RPI11) for ERβ− and ERβ+ cells, respectively. Sized miRNA libraries were gel purified and sequenced on GAIIx (Illumina) at a concentration of 10 pm for 36 plus 7 additional cycles for index sequencing. For ZR75.1 small RNA sequencing, an updated version of the protocol, recommending library preparation from 1 μg of RNA input, was applied (Rev. E). In this case samples were indexed by using indices 2 (RPI2) and 3 (RPI3) for ERβ+ and ERβ− cells, respectively, and sequenced on a HiSeq1500 sequencer (Illumina) at a concentration of 10 pm for 50 plus 7 additional cycles for index sequencing. Raw sequencing data were filtered following several criteria. Because the sequence of the adapter was known, a perl script was used to trim the adaptors from the raw data. Sequence reads were then filtered for quality and clustered to unique sequences to remove redundancy, retaining their individual read count information. Unique sequences 18 nucleotides or more in length were mapped, without any mismatch, on miRNA annotation according to miRBase version 18 using iMir, a modular pipeline for comprehensive analysis of small RNA sequencing data, comprising specific tools for adapter trimming, quality filtering, identification of known small noncoding RNAs, novel miRNA prediction, differential expression analysis, and target prediction (37). The pipeline created has proven to be efficient and flexible enough to allow a user to select the preferred combination of analytical steps. It detects the reads corresponding to known miRNAs, giving a precise estimation of their expression level. The miRBase (38–40) repository is used because it offers information about mature (the mature sequence of known miRNAs), mature-star (the sequence that pairs with the mature miRNA in the miRNA secondary structure), and precursor miRNA sequences (sequence of the hairpin). miRNAs have been considered as expressed when they were detected by at least five reads per miRNA. The identification of differentially expressed miRNAs was performed with the Bioconductor DESeq package (41). Starting from the expression values, the first step was to minimize the effect of the systematic technical variations, and then a negative binomial distribution model was used to test differential expression in deep sequencing datasets. Data were filtered according to read count value (threshold: 10 reads), and those showing fold-changes of ≤−1.3 or ≥1.3 with a p value ≤ 0.05 were considered as differentially expressed.
Raw miRNA sequencing data have been deposited in the EBI ArrayExpress database (www.ebi.ac.uk/arrayexpress) according to the Minimum INformation about a high-throughput SEQuencing Experiment (MINSEQE) guidelines (accession numbers E-MTAB-1495, E-MTAB-2085, and E-MTAB-2069).
iTRAQ Labeling and Mass Spectrometry
Cytosolic and nuclear extracts obtained as described above from two independent cell cultures, in two experiments performed on different days, were quantified using an EZQ protein quantitation kit (Invitrogen). ∼75 μg of each sample was used for iTRAQ analyses. Samples were precipitated using a 2-D Clean Up Kit (Amersham Biosciences) and the precipitated proteins were dissolved in 20 μl of iTRAQ dissolution buffer. 1 μl of each sample was subjected to SDS-PAGE followed by silver staining to verify that protein precipitation was comparable in all samples. For each of the two cellular fractions, equal protein amounts from each sample were used for the following steps of the iTRAQ procedure. Protein alkylation, trypsin digestion, and labeling of the resulting peptides (114 and 115 for ERβ−, 116 and 117 for ERβ+) were performed according to the manufacturer's instructions (AB Sciex, Foster City, CA). After labeling, the samples were pooled, dried, and dissolved into 20 mm KH2PO4 (pH 3). Labeled peptides were fractionated by strong cation exchange chromatography (SCX). SCX separations were performed with an Ettan HPLC system (Amersham Biosciences) using a PolySULFOETHYL A column (200 × 2.1 mm, PolyLC, Columbia, MD). The LC was performed at 0.2 ml/min, and KH2PO4 buffer (pH 3) was used with a gradient of 0–0.4 M KCl over 35 min. The eluting peptides were collected in 1-min fractions. Each SCX fraction containing labeled peptides was analyzed twice (technical replicates) with nano-LC electrospray ionization MS/MS using an Ultimate 3000 nano-LC (Dionex) and QStar Elite mass spectrometer (AB Sciex). Samples were first injected into a ProteCol C18 trapping column (0.15 × 10 mm) (SGE, Griesheim, Germany) and then subjected to peptide separation on PepMap100 C18 analytical column (0.075 × 150 mm) (LC Packings/Dionex, Sunnyvale, CA) at 200 nl/min with a linear 120-min gradient of 0% to 40% acetonitrile in 0.1% formic acid. MS data were acquired automatically using Analyst QS 2.0 software. The information-dependent acquisition method consisted of a 0.5-s TOF-MS survey scan of m/z 400–1400. From every survey scan, the two most abundant ions with charge states of +2 to +4 were selected for product ion scans. Once an ion was selected for MS/MS fragmentation, it was put on an exclusion list for 60 s.
Quantitative Proteomics Data Analysis
Protein identification and relative quantitation were performed with the ParagonTM search algorithm (42) using the ProteinPilot 3.0 interface (AB Sciex). Database searches were performed against human protein sequences in the UniProt database (version 15.12.2009, 20,328 human entries). The search criteria were cysteine alkylation with methyl methanethiosulfonate, trypsin digestion, biological modifications allowed, thorough search, and detected protein threshold of 95% confidence (Paragon Unused ProtScore > 1.3). Additionally, automatic bias correction was used to correct for uneven protein loading. The false discovery rates for protein identifications were calculated using a target-decoy strategy against a concatenated database of forward and reversed human protein sequences (43) and were 2% for the cytosolic fraction and 1% for the nuclear fraction. The global distribution of iTRAQ fold-change was assessed, and only proteins showing significant (p value ≤ 0.05 for at least one of the biological replicates) and reproducible differences in concentration (showing the some modulation in both biological replicates, fold-change 1.5) between ERβ+ and ERβ− replicate samples were considered. The iTRAQ data have been deposited in the ProteomeXchange Consortium (proteomecentral.proteomexchange.org) via the PRIDE partner repository (44) with the dataset identifier PXD000499.
Gene Ontology Analysis
To identify statistically overrepresented “biological process” Gene Ontology terms among the differentially modulated proteins, we used the Database for Annotation, Visualization and Integrated Discovery (DAVID) functional annotation tool (45, 46). All down- or up-regulated proteins (fold-change 1.5) in the two cellular compartments investigated were included in this analysis, and the list of all protein-coding mRNAs detected in steroid-free Ct-ERβ cells by gene expression profiling (see below) was used as background.
Transfections and Luciferase Reporter Assay
To confirm miR-30a-5p overexpression in ERβ+ versus ERβ− cells, a plasmid carrying the 3′ UTR of SNAI1 mRNA (47), which harbors a binding site for miR-30a-5p, was transiently transfected in the above-mentioned cells using a nonliposomal reagent (attractene transfection reagent, Qiagen, Hilden, Germany). To assess the effects of miR-30a-5p overexpression in ERβ+ cells, 5 and 10 nm miSript miRNA mimic (syn-hsa-miR-30a-5p, Qiagen) were used in transient transfection. As a control, a scrambled oligonucleotide (all star negative control siRNA, Qiagen) was transfected in parallel samples at the same concentration (48, 49). Starved ERβ+ cells were plated in 24-well plates and transfected using Attractene transfection reagent according to the manufacturer's protocol. Luciferase reporter assays were performed 24 and 48 h post-transfection using the luciferase assay system (Promega). Transfection efficiency was evaluated by co-transfecting a β-galactosidase expression vector. Once the optimal condition had been chosen, starved ERβ+ cells were sited in 60-mm cell plates and transfected using HiPerFect Transfection Reagent (Qiagen) with either mimic or control oligonucleotides. RNA was extracted 24 h after transfection for subsequent microarray hybridization.
RNA Purification and Microarray Analysis
Total RNA was extracted from ERβ+ cells and transiently transfected as described above, using the standard RNA extraction method with TRIzol (Invitrogen), as described previously (27, 28). Before use, the RNA concentration in each sample was assayed with an ND-1000 spectrophotometer (NanoDrop, Thermo Scientific, Rockford, IL) and its quality was assessed with an Agilent 2100 Bioanalyzer with the Agilent RNA 6000 nano kit (Agilent Technologies, Santa Clara, CA). mRNA microarrays were performed using 500 ng of total RNA as starting material for the synthesis of cDNA and biotinylated cRNA, according to the Illumina TotalPrep RNA Amplification Kit (Ambion, Austin, TX, catalog number IL1791) protocol. For each sample, 750 ng of cRNA were hybridized for 18 h at 55 °C on Illumina HumanHT-12 v4.0 BeadChips (Illumina Inc.) according to the manufacturer's instructions and subsequently scanned with the Illumina iSCAN. Data analyses were performed with GenomeStudio software version 2011.1 (Illumina Inc.) by comparing all values obtained in the miR-30a overexpressed condition against the scramble values. Data were normalized with the quantile algorithm, and genes were considered detected if the detection p value was less than 0.01. Statistical significance was calculated with Illumina DiffScore, a proprietary algorithm that uses the bead standard deviation to build an error model. Only genes with a DiffScore of ≤−30 or ≥30, corresponding to a p value of 0.001, were considered as statistically significant. Raw microarray data have been deposited, in a format complying with the Minimum Information about a Microarray Gene Experiment guidelines of the Microarray Gene Expression Data Society, in the EBI ArrayExpress database (www.ebi.ac.uk/arrayexpress) with accession number E-MTAB-1493.
miRNA Target Predictions
Focusing on miR-30a-5p, we predicted its putative mRNA targets considering results obtained from four different resources, miRanda (50), miRDB (51, 52), TarBase (53), and TargetScan (54–57). miRDB is based on a computational algorithm that uses the features linked to miRNA targets binding to train a bioinformatics target prediction model based on a machine learning approach. TarBase is a collection of manually edited and experimentally supported miRNA targets. TargetScan provides computationally predicted miRNA gene targets by searching for the presence of 8-mer and 7-mer sites matching the seed region of each miRNA, and miRanda target prediction incorporates current knowledge on target rules and on the use of a compendium of mammalian miRNAs. The lists of predicted targets obtained with each program were independently compared with the list of miR-30a-5p down-regulated genes and to the list of ERβ down-regulated proteins.
RESULTS
Functional Characterization of Ligand-independent ERβ Activity in MCF-7 Cells
ERβ controls gene expression at transcriptional and post-transcriptional levels via its ability to recruit different molecular partners that assemble in large multiprotein complexes (12, 13) to modulate mRNA (8) and miRNA (28) gene transcription. This has been studied so far mainly in the presence of E2, a natural ligand of this nuclear receptor. However, recent evidence suggests that ERβ is able to elicit genomic responses in human osteosarcoma U2OS cells in the absence of a steroid ligand (22), suggesting that this receptor is likely to exert a biological role in physiological and pathological conditions when serum concentrations of estrogen are low. To explore this possibility in the case of hormone-responsive BC, we investigated here the ability of unliganded ERβ to control BC cell functions in a model represented by MCF-7 cell clones stably expressing full-length human ERβ carrying a TAP tag at the N terminus (Nt-ERβ) or C terminus (Ct-ERβ), which has been shown to be a good model for studying the activity of this ER subtype in hormone-responsive BC cells (8, 12, 28). Expression and nuclear localization of ERβ in the absence of estrogen were first measured in Ct-ERβ and Nt-ERβ cells via SDS-PAGE and immunoblotting. As shown in Fig. 1A, 40% to 50% of cellular ERβ is localized in the nucleus in the absence of estrogen, whereas ERα resides primarily in the cytosol. The absence of α-tubulin in the nuclear fractions analyzed indicated a lack of cytosolic contamination. Liganded ERβ is able to inhibit BC cell proliferation by interfering with ERα-mediated activation of cell cycle and anti-apoptotic genes (8, 28). This is the case also for the unliganded form of the receptor, as ERβ-expressing cells grow considerably slower than ERβ− ones (Fig. 1B). This is likely to be due to a reduction of cell cycle kinetics causing an accumulation of ERβ+ cells in the G1 phase, resulting in a reduction in the replication rate, demonstrated by a decrease in the cultures of cells in S and G2/M cells (Fig. 1C). This result suggests a constitutive activity of ERβ toward the inhibition of BC cell proliferation, independent of the well-documented estrogen-dependent activity that relies on direct interference with the mitogenic actions of ERα (8, 28). In order to evaluate whether this estrogen-independent activity of ERβ also involves the transcriptional pathway, Ct-ERβ and Nt-ERβ cells were transiently transfected with a synthetic ER reporter gene comprising an estrogen response element cloned upstream of the viral tk promoter linked and the luciferase coding sequence (ERE-tk-luc; 1), and reporter activity was measured and compared with that in wt (ERβ−) cells. The results reported in Fig. 1D show that unliganded ERβ was able to induce significant repression of promoter activity (50% to 60% reduction) relative to ERβ− cells. Because it is known that ERβ binds DNA even in the absence of ligand, this result indicates that the unliganded receptor can exert marked transcriptional activity in BC cells. In the case of the artificial reporter tested here, that activity resulted in trans-repression, but it could also enhance transcription, depending upon the nature of the co-regulator complexes recruited on target promoters.2 When combined, these results demonstrate that this cellular model is suitable for investigating the effects of the ligand-free, constitutive activity of ERβ in BC cells.
Fig. 1.
Functional characterization of the effects of unliganded ERβ in MCF-7 cells. A, nuclear translocation of ERα and ERβ shown by Western blot analysis on cytosolic (C) and nuclear (N) protein extracts obtained from wt MCF-7, Ct-ERβ, and Nt-ERβ cells. The presence of α-tubulin was also assessed to control for the absence of cytosolic contaminants in the nuclear fractions. B, wt MCF-7, Ct-ERβ, and Nt-ERβ cell proliferation rate in the absence of estrogen, assessed with a colorimetric assay as indicated in the text. C, cell cycle analysis in wt and TAP-ERβ-expressing MCF-7 cells. Percentages of G1, S, and G2/M phase cells were determined via flow cytometry in estrogen-free cultures. D, effects of ERβ on basal activity of the ER-responsive ERE-tk-luc reporter gene. Results were normalized by co-transfection of a β-galactosidase-expressing vector. In B, C, and D, results shown are the mean ± S.D. of triplicate determinations from a representative experiment.
Effects of Unliganded ERβ on MCF-7 Cell miRNome
Because miRNAs have been shown to play a major role in the cellular response to ERs in BC (see Refs. 27 and 28 and references therein), we investigated the possibility that unliganded ERβ might influence miRNA expression in BC cells. To this end, RNA was extracted from ERβ− and ERβ+ (Ct-ERβ and Nt-ERβ) cells maintained in steroid-free medium, and massively parallel miRNA sequencing was applied to detect global changes in miRNome expression induced by this receptor. The results revealed that unliganded ERβ indeed had a significant effect on the composition of the cell miRNome, comparable to what has been reported for E2-bound receptor (28). Among >450 miRNAs detected (e.g. expressed), 101 were differentially expressed in both ERβ+ clones (p value < 0.05, fold-change 1.3), including 83 regulated in the same way (up- or down-regulation) in both clones (Fig. 2A and supplemental Table S1). To confirm this result, ZR75.1 cells with (ZRFlagβ) or without (wt) stable expression of Flag-ERβ were also analyzed (Fig. 2B). The expression of ERβ in this cell line significantly reduced cell proliferation (Fig. 2C), although to a lesser extent than observed in MCF-7 cells (Fig. 1B), most likely due to the fact that ZRFlagβ cells express less ERβ than Ct- or Nt-ERβ cells as detected via mRNA quantitation and radioligand estrogen binding (data not shown). Interestingly, of 120 miRNAs differently expressed in ERβ+ versus ERβ− ZR75.1 cells, 30 showed a response to ERβ comparable to that observed in MCF-7 cells (Fig. 2D and supplemental Table S1). These results confirm that this ER subtype exerts a significant effect on miRNA expression in BC cells. The unliganded ERβ-responsive miRNAs discovered include several that have been shown to play a crucial role in BC initiation or progression, epithelial-mesenchymal transition and metastasis, and/or cell death and apoptosis. Interestingly, among the miRNAs identified here, several also were differentially expressed in ERβ+ breast tumors (28), indicating that the in vitro model adopted reflects, at least partially, the behavior of BC cells in vivo. These results demonstrate that the control of the BC cell miRNome by ERβ can occur independently of hormonal intervention, an observation that bears significance when considering that ERβ+ breast tumors are less aggressive and that BC often strikes post-menopausal patients with very low circulating levels of estrogens.
Fig. 2.
Effects of unliganded ERβ on BC cell miRNome detected via miRNA-Seq. A, heatmap summarizing the fold-changes relative to 83 miRNAs differentially expressed in both Nt- and Ct-ERβ MFC-7 cell clones in estrogen-free culture conditions. B, analysis of ERβ expression in ZRFlagβ cells by Western blot. C, wt and Flag-ERβ ZR75.1 cell proliferation rate in the absence of estrogen, assessed with MTT assay as indicated in the text. D, miRNAs responsive to ERβ in both MFC-7 and ZR75.1 cells in estrogen-free culture conditions.
Quantitative Analysis of the Effects of Unliganded ERβ on MCF-7 Cell Proteome
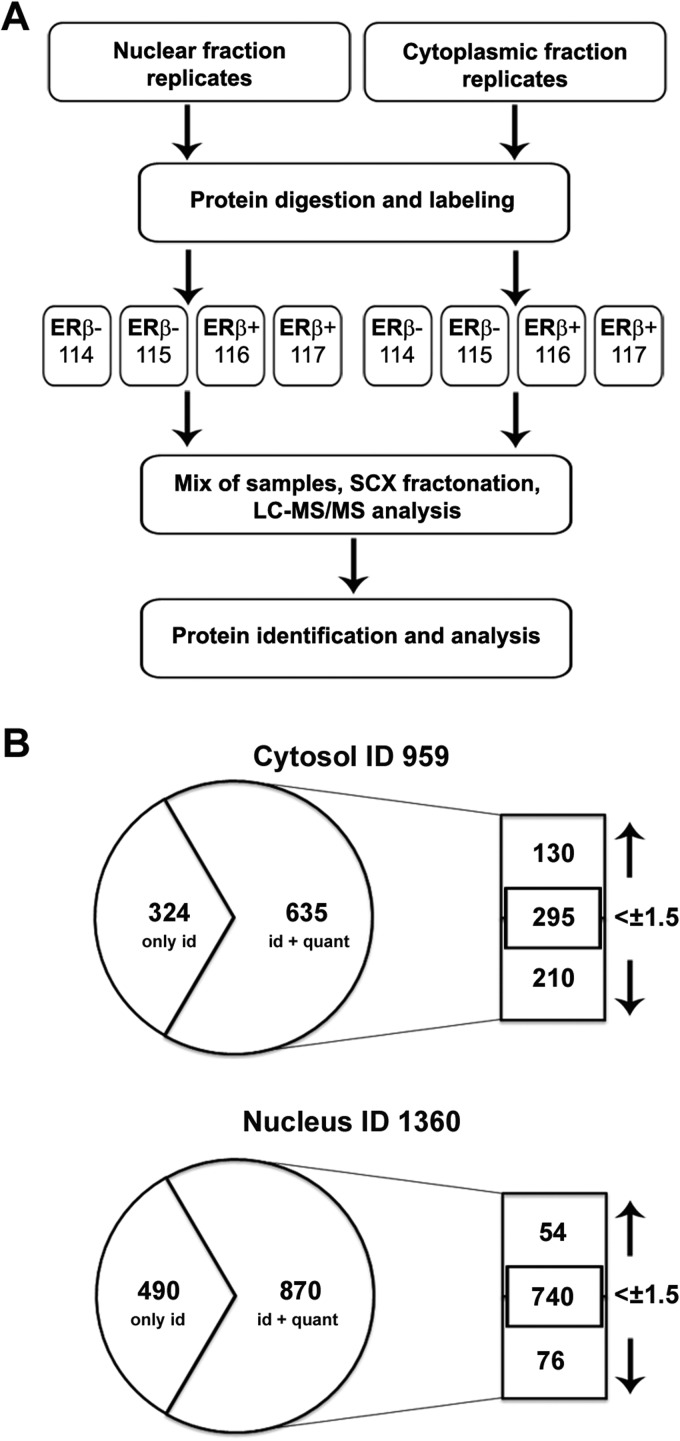
The best-known role of miRNAs within the cell is to modulate gene expression at the post-transcriptional level by binding to target mRNAs and thereby promoting their degradation or, more often, preventing their translation (33, 34). Reprogramming of the cell miRNome by ERβ is thus likely to induce significant changes in the BC cell proteome, resulting in multiple structural and functional changes that underlie the biological and clinical phenotypes of ERβ+ tumors. To test this possibility, Ct-ERβ and wt MCF-7 cells were subjected to iTRAQ quantitative proteomics analysis. To gather additional information, nuclear and cytosolic extracts from the same cultures were analyzed via quantitative proteomics. Two biological replicates from nuclear and cytosolic preparations were analyzed twice with iTRAQ. The iTRAQ labeling scheme of the samples is shown in Fig. 3A: the two nuclear extracts from ERβ− cells were labeled with iTRAQ labels 114 and 115, respectively, and the two from ERβ+ were labeled with iTRAQ labels 116 and 117, respectively. The same labeling scheme was used for the two cytosolic biological replicates. After cation exchange fractionation of the iTRAQ-labeled peptides, each SCX fraction was analyzed twice with LC-MS/MS, and data from these technical replicates were processed together to increase the reliability of the quantitative data. In all, 1,690 unique proteins were identified, including 959 proteins from the cytosolic extracts and 1,360 from the nuclear extracts (Fig. 3B). Among them, several were differentially expressed in one or both cellular compartments analyzed (130 up and 210 down in the cytosol, and 54 up and 76 down in the nucleus) considering a fold-change between confronted samples of at least 1.5, with a p value ≤ 0.05 in at least one of the biological replicates and/or a comparable change in both biological replicates (Fig. 3B; supplemental Tables S2–S5 and S8). When the quantitative proteomics data obtained were analyzed in further detail, it was possible to distinguish several subsets of proteins, each showing a different response to unliganded ERβ in one or both cellular compartments (Fig. 4, supplemental Fig. S1, supplemental Table S3). Fig. 4A reports proteins identified in both cellular compartments, grouped in four distinct classes, including proteins (a) down-regulated in the cytosol (fold-change ≥ 1.5) and, to a lesser extent, in the nucleus; (b) up-regulated in the cytosol (fold-change ≥ 1.5) and, again less markedly, the nucleus; (c) down-regulated in the cytosol (fold-change ≥ 1.5) but showing a minor, although still significant, up-regulation in the nucleus; and (d) up-regulated in the cytosol (fold-change ≥ 1.5) but slightly down-regulated in the nucleus (supplemental Table S3). Within each of the four classes described above, only a few proteins showed a fold-change ≥ 1.5 in both compartments (supplemental Fig. S1), suggesting in this case the possibility that ERβ promotes a significant redistribution of the proteins within these two cell compartments. Fig. 4B displays instead the proteins detected in only one cell compartment (most likely due to the technical limitations of the method) where they are significantly down- or up-regulated (fold-change ≥ 1.5) by ERβ. Then, to gather a general view of the biological processes involving the proteins significantly modulated (>1.5 fold-change) by unliganded ERβ, Gene Ontology analysis was performed. Several Gene Ontology terms were overrepresented in cytosol, nuclei, or both compartments. Among these, RNA splicing, translation, signal transduction, and cell cycle were the processes most represented (supplemental Fig. S2). This result, which confirms many of the known effects of ERβ in BC cells (8, 12, 13), supports the notion that the observed changes in proteome composition induced by the receptor reflect its known activities in this cell type. The identification of hundreds of differentially regulated proteins in ERβ+ versus ERβ− BC cells that well reflect the ability of this receptor to modulate gene expression in BC cells led us to focus our attention on levels of those mRNAs whose corresponding proteins were modulated by ERβ. To this end we compared the data obtained with iTRAQ with gene expression profiles measured in the same cell lines under comparable experimental conditions (steroid-free cultures, control array data from Ref. 8). Interestingly, the correlation between protein and mRNA levels was moderate, as summarized in Figs. 5A and 5B. A comparison between protein (fold-change ≥ 1.5) and corresponding mRNA levels showed that changes in the expression of most proteins occurred in the absence of or at a much higher level than those of the corresponding mRNAs (Fig. 5C). This result, although not fully surprising, is striking, as we are investigating here the response of the cell proteome to a transcription factor whose main localization is the nucleus and whose mechanism of action is the regulation of gene transcription. For this reason, among the many possible explanations for the results obtained, we considered the possibility that ERβ might control the cell proteome via secondary mechanisms involving the products of primary response genes, including those encoding miRNAs (8, 28).
Fig. 3.
Identification of ERβ-regulated proteins by quantitative proteomics profiling with iTRAQ tagging. A, schematic representation of the workflow for quantitative proteomics analysis applied in this study. Two independent nuclear and cytosolic protein extracts from ERβ+ and ERβ− MCF-7 cells maintained in estrogen-free medium were subjected to enzymatic digestion, labeling (ERβ−, 114 and 115; ERβ+, 116 and 117), SCX fractionation, and LC-MS/MS analysis. Two technical replicates of each sample were analyzed with LC-MS/MS, and raw data from the technical replicates were processed together to increase the robustness of identification and quantitation. B, overview of quantitative proteomics results obtained from cytosolic and nuclear fractions.
Fig. 4.
Quantitative changes of nuclear and cytosolic MCF-7 cell proteomes induced by unliganded ERβ. Heatmaps showing proteins differentially expressed in the nuclear and/or cytosolic fractions of ERβ+ versus ERβ− cells in absence of estrogen. Differentially expressed proteins are grouped as follows: displaying the same (left) or opposite trend (right) in both cellular compartments (A); or quantified either in the cytosolic (left) or nuclear (right) fraction only (B). Only proteins showing ±1.5-fold changes were considered and are shown; the gray color marks a lack of identification of the protein in a cell compartment.
Fig. 5.
Correlations between unliganded ERβ-dependent protein and mRNA changes. Scatter plots correlating ERβ-dependent changes in expression of proteins, detected in the cytosolic (A) and nuclear (B) fractions (blue-yellow heatmaps), and the corresponding mRNAs (green-red heatmaps). The same data are represented as heatmaps in C, where only those proteins showing ±1.5-fold changes are shown.
Identification of mRNA Targets of ERβ-responsive miR-30a-5p in MCF-7 Cells
miRNAs modulate the half-life and/or function of their target mRNAs by binding directly to their 3′-UTR and thereby promoting mRNA degradation or translation inhibition. As a consequence, an increase in the intracellular concentration of a given miRNA often results in reduced levels of the proteins encoded by its target mRNAs. Interestingly, many of the proteins identified here whose concentration was affected by the presence of ERβ are encoded by mRNAs that represent putative targets of the ERβ-responsive miRNAs described above (Fig. 2 and supplemental Table S1). Considering the ERβ-responsive miRNAs expressed at higher levels in MCF-7 cells, and thus most likely to exert a significant effect on the cell via this mechanism (58), we focused our attention on miR-30a-5p. This small RNA, responsive to ERβ in all cell lines (Fig. 2D), has been shown to have oncosuppressive functions in breast and other cancer cells (59, 60), where its reduction maintains self-renewal and inhibits apoptosis in tumor-initiating cells (61). More important, miR-30a-5p is expressed at higher levels in ERβ+ breast tumor biopsies (28). In order to identify experimentally the mRNA targets of this miRNA in MCF-7 cells, we transiently transfected a synthetic miR-30a-5p mimic and performed a gene expression profiling analysis of transfected cells with oligonucleotide microarrays to detect significant changes in mRNA concentration induced by the transfected oligonucleotide. As a control, cells were transfected in parallel with a “scrambled” oligonucleotide and used as reference. The main advantage of this experimental approach is that transcripts destabilized by a high intracellular concentration of miR-30a-5p mimic are likely to comprise a large proportion of the natural targets of this miRNA expressed in this cell type. The biological activity of the mimics was tested first with the SNAI1 3′UTR luciferase reporter plasmid, which comprises the miR-30a-5p binding site from SNAI1 mRNA cloned downstream of the luciferase coding sequence (47). The reporter was transfected into MCF-7 cells expressing or not expressing ERβ, together with a β-galactosidase plasmid used as a control for transfection efficiency. Analyses were performed at 24 and 48 h after transfection, and the results are shown in supplemental Fig. S3. Interestingly, activity of the reporter was significantly lower in ERβ+ cells, where the concentration of miR-30a-5p was higher than in wt cells (supplemental Fig. S3A). Upon co-transfection of the miRNA mimic, reporter activity was further reduced in these cells in a concentration-dependent manner (supplemental Fig. S3B). As this was more pronounced after 24 h, this timing was selected for the gene profiling assay with two concentrations of miRNA mimic or scramble control oligonucleotide. Results summarized in supplemental Fig. S4 and supplemental Tables S6A and S6B show a marked effect of the mimic on the cell transcriptome involving 1,344 mRNAs. This is not surprising, as affected mRNAs are likely to include not only those directly targeted by miR-30a-5p but also transcripts responding to the intracellular changes consequent to effects on the primary targets. To pursue the primary aim of this experiment (e.g. to identify mRNA targets of miR-30a-5p in BC cells), we focused our attention on the 1,107 mRNAs significantly down-regulated by the mimic and screened them for the presence of one or more miR-30a-5p target sequences. In this way we identified 249 mRNAs that can be considered putative targets of this miRNA in MCF-7 cells (Fig. 6A and supplemental Table S7). Gene Ontology analysis of the cellular processes involving the proteins encoded by these mRNAs suggested a putative role of miR-30a-5p in the control of RNA processing and splicing and in cell cycle progression, in particular in cell phase transition (Fig. 6B), a result consistent with the known effects of ERβ in this cell type (see above). When we compared the list of primary miR-30a-5p targets with that of the proteins found significantly down-regulated in ERβ+ via quantitative proteomics, we found the 11 entries listed in Fig. 6C. Interestingly, five among them also showed lower levels of the corresponding mRNAs in ERβ+ versus ERβ− cells, whereas the remaining (including the strongly underexpressed SERBP1, TPD52L1, and CSDE1) appeared to be affected by ERβ only at the protein level. It is worth mentioning that CAT, MTA2A, NAP1L1, and TMED3 have been reported as validated direct targets of miR-30a-5p by independent means, a result that can be considered a partial confirmation of the data reported here.
Fig. 6.
Effects of the expression of a miR-30a-5p mimic on MCF-7 cell transcriptome. A, experimental strategy for the identification of miR-30a-5p target mRNAs in MCF-7 cells. Following transient transfection of MCF7 cells with 10 nm synthetic miR-30a-5p mimic or scramble mimic (control), RNA was extracted and profiled on microarrays for gene expression analysis; down-regulated transcripts identified in this way were filtered for the presence of the miR30a-5p seed sequence, leading to the identification of 249 putative targets of this miRNA. B, Gene Ontology analysis of the biological processes involving miR-30a-5p target transcripts identified. C, heatmap summarizing the effects of unliganded ERβ− on 11 proteins and the corresponding mRNAs present among miR-30a-5p targets identified in this study. All proteins showed significantly lower expression in ERβ+ cells, where miR-30a-5p is up-regulated with respect to ERβ− cells.
DISCUSSION
Nuclear receptors (NRs) are classified in four classes, based on subcellular distribution, binding of the cognate ligand, dimerization, and DNA binding properties of the protein (62). ERs are Class I NRs, and in the absence of ligand they are sequestered in an inactive multiprotein complex in the cytoplasm. ERβ, however, escapes this rule, as in its ligand-free state it is not confined in the cytoplasm and freely diffuses into the nucleus, where it binds with high affinity to chromatin and modulates gene transcription activation (21, 63, 64). This property of ERβ has important implications in the case of hormone-responsive BC, as this receptor subtype has been shown to exert growth-inhibitory and oncosuppressive functions (65, 66). When considering the decline in estrogen levels during menopause or locally induced by endocrine therapies based on the use of aromatase inhibitors (67), a better characterization of the cellular pathways influenced by unliganded ERβ in hormone-responsive BC cells becomes important. In previous studies by our laboratory, we observed that the expression of ERβ in MCF-7 cells induces significant changes in the ER-responsive transcriptome (8) and promotes reprogramming of the cellular miRNome (28). Focusing on this last observation, and considering the central role of miRNAs in controlling the cellular proteome, we set forth to investigate the global effects of ERβ on miRNA expression and the cell proteome in the absence of hormonal ligands. To this end, in order to identify small noncoding RNAs constitutively regulated by ERβ, we performed a global analysis of the cell miRNome by means of RNA sequencing. This led to the identification of several miRNAs differentially expressed in ERβ+ cells in the absence of estrogen, including many known to be involved in the control of the malignant phenotype of BC cells. Among these, focusing on those down-regulated by ERβ (Fig. 2 and supplemental Table S1), we observed miRNAs previously reported to act as oncogenes. The first example comprises miRNAs belonging to the miR-200 family, which has been linked to cancer metastasis (68). Their down-regulation by ERβ, together with that of miR-10b (69, 70), suggests a potential mechanism for the low metastatic potential of BC cells expressing this ER subtype. Among the miRNAs down-regulated by ERβ, miR-221 and miR-222 were identified as basal-like cell-specific miRNAs and were shown to function as regulators of epithelial-to-mesenchymal transition, with high expression in luminal-like BC cells resulting in increased cell migration and invasion due to the regulation of mesenchymal-specific genes (71), whereas low miR-9 expression has been associated with a less aggressive BC phenotype (72). The up-regulated miR-125b, in contrast, has been found down-regulated in HER2-overexpressing BCs (73) and is able to suppress HER2 and HER3 mRNA and protein levels, leading to a reduction in anchorage-dependent cell growth, motility, and invasiveness (74). The effect of ERβ on these miRNAs correlates with the potential role of this nuclear receptor as a tumor suppressor, a conclusion further supported by the up-regulation of miR-30a-5p, known to be involved in epithelial-to-mesenchymal transition, leading to inhibited cell migration and invasion and tumor-initiating cell self-renewal (60, 61). Given the remarkable effects of unliganded ERβ on the cell miRNome and the potential significance of this finding with regard to the biological and clinical phenotypes of ERβ+ breast tumors, we set forth to investigate in the same model system global changes in protein expression via quantitative proteomics. Using iTRAQ, we identified several hundred proteins whose cellular levels were affected by ligand-free ERβ (Fig. 3 and supplemental Tables S2–S5). When we compared protein and mRNA expression profiles, we observed that in most cases changes in protein level occurred in the absence of similar changes of the corresponding mRNA (Fig. 4) and, more important, that a large number of proteins whose expression changed in response to unliganded ERβ are encoded by mRNAs targeted by ERβ-responsive miRNAs. This last finding, although quite exciting, as it would suggest that miRNA regulation is a key upstream event in the control of BC cell behavior by the unliganded receptor, should be taken with caution because of the controversy in defining the true targets of miRNAs. Indeed, the computational and experimental approaches available to date for identifying miRNA targets are still not fully reliable because of the complexity of miRNA-mediated mRNA regulation and the fact that a given mRNA often targets multiple miRNAs, with each miRNA able to affect, directly and indirectly, hundreds of mRNAs and proteins (75, 76). This complexity, along with the uncertainties it raises, emphasizes the need for experimental validation of hypotheses derived from quantitative analysis of miRNA expression changes. In this respect, quantitative proteomics represents a suitable approach for miRNA target identification under controlled experimental conditions (74). Based on this assumption, and to search for an experimental validation of the hypothesis stated above, we focused our attention on miR-30a-5p, whose expression is affected by the presence of ERβ both in BC MCF-7 and ZR75.1 cells in vitro and in tumor biopsies (Fig. 2 and Ref. 28). Expression of this miRNA is low in hormone-responsive BC cells and increases in the presence of unliganded ERβ (Fig. 2 and supplemental Table S1), as a result of direct regulation of the corresponding gene mediated by two receptor binding sites within the chromosomal locus (8). Focusing on miR-30a-5p, we performed gene expression profiling in MCF-7 cells before and after overexpression of a mimic oligonucleotide and screened the differentially expressed mRNAs for the presence of one or more miR-30a-5p target sequences in the 3′-UTR. This led to the identification of 249 transcripts whose expression was significantly down-regulated by overexpression of this miRNA (Fig. 6 and supplemental Tables S6 and S7). Among these, 11 were found to encode proteins identified by iTRAQ as down-regulated in ERβ+ cells (Fig. 6C), including the 4 validated miR-30a targets catalase, methionine adenosyltransferase II α, nucleosome assembly protein 1-like 1, and transmembrane emp24 domain-containing protein 3 (53) and 7 newly identified downstream effectors, among which it is worth mentioning the Band 4.1-like protein 5, known to promote epithelial-to-mesenchymal transition (77), and Tumor protein D52-like 1, a member of the tumor protein D52 family first identified as overexpressed in human breast carcinomas, where their deregulation adversely affects the completion of mitosis (78). Considering the partial view of the cell proteome obtained in our study as representative of the whole cell proteome, these results confirm the key role of miRNome regulation in the control of BC cell functions by unliganded ERβ. Furthermore, they suggest that the experimental strategy adopted here is a valid approach to defining the role of ERβ in BC cell biology, identifying at the same time new tumor markers exploitable for diagnostic and prognostic evaluation of these tumors.
Supplementary Material
Acknowledgments
We are grateful to Dr. Heike Allgayer for kindly providing the Snai1 mRNA 3′-UTR luciferase reporter plasmid. C.S. is a Ph.D. student in Molecular Oncology, Experimental Immunology and Innovative Therapy Development at the University of Catanzaro Magna Graecia, and M.R.D.F. is a Ph.D. student in Computational Biology and Bioinformatics at the University of Napoli Federico II.
Footnotes
Author contributions: G.N., C.A., M.B., T.A.N., and A.W. designed research; G.N., R.T., G.G., M.R., F.R., C.S., N.L., and T.A.N. performed research; G.N., R.T., G.G., M.D., M.R., F.R., M.B., N.L., T.A.N., and A.W. analyzed data; G.N., R.T., G.G., M.D., M.R., F.R., C.S., C.A., M.B., T.A.N., and A.W. wrote the paper.
* This work was supported by the Italian Association for Cancer Research (Grant IG-13176), the Italian Ministry for Education, University and Research (Grant PRIN 2010LC747T 002 to A.W. and FIRB RBFR12W5V5 003 to R.T.), the University of Salerno (Fondi FARB 2011–2012), the Federation of European Biochemical Societies (FEBS Short-term Fellowship to G.N.), and EU COST Action SEQAHEAD: Next Generation Sequencing Data Analysis Network (BM1006). G.N. is supported by a Mario e Valeria Rindi fellowship of the Italian Foundation for Cancer Research. M.R. is supported by a Vladimir Ashkenazy fellowship of the Italian Association for Cancer Research. F.R. is supported by a Young Investigator Programme fellowship of the Fondazione Umberto Veronesi.
 This article contains supplemental material.
This article contains supplemental material.
2 R. Tarallo, G. Nassa, A. Weisz, manuscript in preparation.
1 The abbreviations used are:
- ER
- estrogen receptor
- BC
- breast cancer
- iTRAQ
- isobaric tags for relative and absolute quantitation
- miRNA
- microRNA
- miRNA-Seq
- next-generation microRNA sequencing
- UTR
- untranslated region
- wt
- wild type
- SCX
- strong cation exchange chromatography.
REFERENCES
- 1. Cicatiello L., Addeo R., Sasso A. R., Altucci L., Belsito Petrizzi V., Borgo R., Cancemi M., Caporali S., Caristi S., Scafoglio C., Teti D., Bresciani F., Perillo B., Weisz A. (2004) Estrogens promote persistent G1 activation of the CCND1 gene by inducing transcriptional de-repression via c-Jun/c-Fos/ER complex assembly to a distal regulatory element and recruitment of cyclin D1 to its own gene promoter. Mol. Cell. Biol. 24, 7260–7274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heldring N., Pike A., Andersson S., Matthews J., Cheng G., Hartman J., Tujague M., Strom A., Treuter E., Warner M., Gustafsson J. A. (2007) Estrogen receptors: how do they signal and what are their targets. Physiol. Rev. 87, 905–931 [DOI] [PubMed] [Google Scholar]
- 3. Weisz A., Rosales R. (1990) Identification of an estrogen response element upstream of the human c-fos proto-oncogene that binds the estrogen receptor and the AP-1 transcription factor. Nucleic Acids Res. 18, 5097–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klinge C. M., Jernigan S. C., Mattingly K. A., Risinger K. E., Zhang J. (2004) Estrogen response element-dependent regulation of transcriptional activation of estrogen receptors alpha and beta by coactivators and corepressors. J. Mol. Endocrinol. 33, 387–410 [DOI] [PubMed] [Google Scholar]
- 5. Leclercq G. (2002) Molecular forms of the estrogen receptor in breast cancer. J. Steroid Biochem. Mol. Biol. 80, 259–272 [DOI] [PubMed] [Google Scholar]
- 6. Manavathi B., Kumar R. (2006) Steering estrogen signals from the plasma membrane to the nucleus: two sides of the coin. J. Cell. Physiol. 207, 594–604 [DOI] [PubMed] [Google Scholar]
- 7. Pettersson K., Gustafsson J. A. (2001) Role of estrogen receptor beta in estrogen action. Annu. Rev. Physiol. 63, 165–192 [DOI] [PubMed] [Google Scholar]
- 8. Grober O. M., Mutarelli M., Giurato G., Ravo M., Cicatiello L., De Filippo M. R., Ferraro L., Nassa G., Papa M. F., Paris O., Tarallo R., Luo S., Schroth G. P., Benes V., Weisz A. (2011) Global analysis of estrogen receptor beta binding to breast cancer cell genome reveals an extensive interplay with estrogen receptor alpha for target gene regulation. BMC Genomics 12, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McInerney E. M., Tsai M. J., O'Malley B. W., Katzenellenbogen B. S. (1996) Analysis of estrogen receptor transcriptional enhancement by a nuclear hormone receptor coactivator. Proc. Natl. Acad. Sci. U.S.A. 93, 10069–10073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Watanabe T., Inoue S., Ogawa S., Ishii Y., Hiroi H., Ikeda K., Orimo A., Muramatsu M. (1997) Agonistic effect of tamoxifen is dependent on cell type, ERE-promoter context, and estrogen receptor subtype: functional difference between estrogen receptors alpha and beta. Biochem. Biophys. Res. Commun. 236, 140–145 [DOI] [PubMed] [Google Scholar]
- 11. Ambrosino C., Tarallo R., Bamundo A., Cuomo D., Franci G., Nassa G., Paris O., Ravo M., Giovane A., Zambrano N., Lepikhova T., Janne O. A., Baumann M., Nyman T. A., Cicatiello L., Weisz A. (2010) Identification of a hormone-regulated dynamic nuclear actin network associated with estrogen receptor alpha in human breast cancer cell nuclei. Mol. Cell. Proteomics 9, 1352–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nassa G., Tarallo R., Ambrosino C., Bamundo A., Ferraro L., Paris O., Ravo M., Guzzi P. H., Cannataro M., Baumann M., Nyman T. A., Nola E., Weisz A. (2011) A large set of estrogen receptor beta-interacting proteins identified by tandem affinity purification in hormone-responsive human breast cancer cell nuclei. Proteomics 11, 159–165 [DOI] [PubMed] [Google Scholar]
- 13. Nassa G., Tarallo R., Guzzi P. H., Ferraro L., Cirillo F., Ravo M., Nola E., Baumann M., Nyman T. A., Cannataro M., Ambrosino C., Weisz A. (2011) Comparative analysis of nuclear estrogen receptor alpha and beta interactomes in breast cancer cells. Mol. Biosyst. 7, 667–676 [DOI] [PubMed] [Google Scholar]
- 14. Tarallo R., Bamundo A., Nassa G., Nola E., Paris O., Ambrosino C., Facchiano A., Baumann M., Nyman T. A., Weisz A. (2011) Identification of proteins associated with ligand-activated estrogen receptor alpha in human breast cancer cell nuclei by tandem affinity purification and nano LC-MS/MS. Proteomics 11, 172–179 [DOI] [PubMed] [Google Scholar]
- 15. Chang E. C., Frasor J., Komm B., Katzenellenbogen B. S. (2006) Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology 147, 4831–4842 [DOI] [PubMed] [Google Scholar]
- 16. Bardin A., Boulle N., Lazennec G., Vignon F., Pujol P. (2004) Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr. Relat. Cancer 11, 537–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roger P., Sahla M. E., Makela S., Gustafsson J. A., Baldet P., Rochefort H. (2001) Decreased expression of estrogen receptor beta protein in proliferative preinvasive mammary tumors. Cancer Res. 61, 2537–2541 [PubMed] [Google Scholar]
- 18. Sugiura H., Toyama T., Hara Y., Zhang Z., Kobayashi S., Fujii Y., Iwase H., Yamashita H. (2007) Expression of estrogen receptor beta wild-type and its variant ERbetacx/beta2 is correlated with better prognosis in breast cancer. Jpn. J. Clin. Oncol. 37, 820–828 [DOI] [PubMed] [Google Scholar]
- 19. Novelli F., Milella M., Melucci E., Di Benedetto A., Sperduti I., Perrone-Donnorso R., Perracchio L., Venturo I., Nistico C., Fabi A., Buglioni S., Natali P. G., Mottolese M. (2008) A divergent role for estrogen receptor-beta in node-positive and node-negative breast cancer classified according to molecular subtypes: an observational prospective study. Breast Cancer Res. 10, R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaaban A. M., Green A. R., Karthik S., Alizadeh Y., Hughes T. A., Harkins L., Ellis I. O., Robertson J. F., Paish E. C., Saunders P. T., Groome N. P., Speirs V. (2008) Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clin. Cancer Res. 14, 5228–5235 [DOI] [PubMed] [Google Scholar]
- 21. Leung Y. K., Ho S. M. (2011) Estrogen receptor beta: switching to a new partner and escaping from estrogen. Sci. Signal. 4, pe19. [DOI] [PubMed] [Google Scholar]
- 22. Vivar O. I., Zhao X., Saunier E. F., Griffin C., Mayba O. S., Tagliaferri M., Cohen I., Speed T. P., Leitman D. C. (2010) Estrogen receptor beta binds to and regulates three distinct classes of target genes. J. Biol. Chem. 285, 22059–22066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ivanova M. M., Mazhawidza W., Dougherty S. M., Klinge C. M. (2010) Sex differences in estrogen receptor subcellular location and activity in lung adenocarcinoma cells. Am. J. Respir. Cell Mol. Biol. 42, 320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tremblay A., Tremblay G. B., Labrie F., Giguere V. (1999) Ligand-independent recruitment of SRC-1 to estrogen receptor beta through phosphorylation of activation function AF-1. Mol. Cell 3, 513–519 [DOI] [PubMed] [Google Scholar]
- 25. Tremblay A., Giguere V. (2011) Contribution of steroid receptor coactivator-1 and CREB binding protein in ligand-independent activity of estrogen receptor beta. J. Steroid Biochem. Mol. Biol. 77, 19–27 [DOI] [PubMed] [Google Scholar]
- 26. Cicatiello L., Mutarelli M., Grober O. M., Paris O., Ferraro L., Ravo M., Tarallo R., Luo S., Schroth G. P., Seifert M., Zinser C., Chiusano M. L., Traini A., De Bortoli M., Weisz A. (2010) Estrogen receptor alpha controls a gene network in luminal-like breast cancer cells comprising multiple transcription factors and microRNAs. Am. J. Pathol. 176, 2113–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferraro L., Ravo M., Nassa G., Tarallo R., De Filippo M. R., Giurato G., Cirillo F., Stellato C., Silvestro S., Cantarella C., Rizzo F., Cimino D., Friard O., Biglia N., De Bortoli M., Cicatiello L., Nola E., Weisz A. (2012) Effects of oestrogen on microRNA expression in hormone-responsive breast cancer cells. Horm. Cancer 3, 65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paris O., Ferraro L., Grober O. M., Ravo M., De Filippo M. R., Giurato G., Nassa G., Tarallo R., Cantarella C., Rizzo F., Di Benedetto A., Mottolese M., Benes V., Ambrosino C., Nola E., Weisz A. (2012) Direct regulation of microRNA biogenesis and expression by estrogen receptor beta in hormone-responsive breast cancer. Oncogene 31, 4196–4206 [DOI] [PubMed] [Google Scholar]
- 29. Han J., Lee Y., Yeom K. H., Kim Y. K., Jin H., Kim V. N. (2004) The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 18, 3016–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim V. N., Han J., Siomi M. C. (2009) Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 10, 126–139 [DOI] [PubMed] [Google Scholar]
- 31. Chendrimada T. P., Gregory R. I., Kumaraswamy E., Norman J., Cooch N., Nishikura K., Shiekhattar R. (2005) TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 436, 740–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okamura K., Phillips M. D., Tyler D. M., Duan H., Chou Y. T., Lai E. C. (2008) The regulatory activity of microRNA* species has substantial influence on microRNA and 3′ UTR evolution. Nat. Struct. Mol. Biol. 15, 354–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Newman M. A., Hammond S. M. (2010) Emerging paradigms of regulated microRNA processing. Genes Dev. 24, 1086–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garzon R., Calin G. A., Croce C. M. (2009) MicroRNAs in cancer. Ann. Rev. Med. 60, 167–179 [DOI] [PubMed] [Google Scholar]
- 35. Volinia S., Calin G. A., Liu C. G., Ambs S., Cimmino A., Petrocca F., Visone R., Iorio M., Roldo C., Ferracin M., Prueitt R. L., Yanaihara N., Lanza G., Scarpa A., Vecchione A., Negrini M., Harris C. C., Croce C. M. (2006) A microRNA expression signature of human solid tumors defines cancer gene targets. Proc. Natl. Acad. Sci. U.S.A. 103, 2257–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Croce C. M. (2009) Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 10, 704–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Giurato G., De Filippo M. R., Rinaldi A., Hashim A., Nassa G., Ravo M., Rizzo F., Tarallo R., Weisz A. (2013) iMir: an integrated pipeline for high-throughput analysis of small non-coding RNA data obtained by smallRNA-Seq. BMC Bioinformatics 14, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kozomara A., Griffiths-Jones S. (2011) miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 39, D152–D157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Griffiths-Jones S., Saini H. K., van Dongen S., Enright A. J. (2008) miRBase: tools for microRNA genomics. Nucleic Acids Res. 36, D154–D158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Griffiths-Jones S., Grocock R. J., van Dongen S., Bateman A., Enright A. J. (2006) miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 34, D140–D144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Anders S., Huber W. (2010) Differential expression analysis for sequence count data. Genome Biol. 11, R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shilov I. V., Seymour S. L., Patel A. A., Loboda A., Tang W. H., Keating S. P., Hunter C. L., Nuwaysir L. M., Schaeffer D. A. (2007) The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol. Cell. Proteomics 6, 1638–1655 [DOI] [PubMed] [Google Scholar]
- 43. Elias J. E., Gygi S. P. (2007) Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods 4, 207–214 [DOI] [PubMed] [Google Scholar]
- 44. Vizcaino J. A., Côté R. G., Csordas A., Dianes J. A., Fabregat A., Foster J. M., Griss J., Alpi E., Birim M., Contell J., O'Kelly G., Schoenegger A., Ovelleiro D., Pérez-Riverol Y., Reisinger F., Ríos D., Wang R., Hermjakob H. (2013) The Proteomics Identifications (PRIDE) database and associated tools: status in. Nucleic Acids Res. 41(Database issue), 1063–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang da W., Sherman B. T., Lempicki R. A. (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang da W., Sherman B. T., Lempicki R. A. (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 [DOI] [PubMed] [Google Scholar]
- 47. Kumarswamy R., Mudduluru G., Ceppi P., Muppala S., Kozlowski M., Niklinski J., Papotti M., Allgayer H. (2012) MicroRNA-30a inhibits epithelial-to-mesenchymal transition by targeting Snai1 and is downregulated in non-small cell lung cancer. Int. J. Cancer 130, 2044–2053 [DOI] [PubMed] [Google Scholar]
- 48. Miyazaki Y., Adachi H., Katsuno M., Minamiyama M., Jiang Y. M., Huang Z., Doi H., Matsumoto S., Kondo N., Iida M., Tohnai G., Tanaka F., Muramatsu S., Sobue G. (2012) Viral delivery of miR-196a ameliorates the SBMA phenotype via the silencing of CELF2. Nat. Med. 18, 1136–1141 [DOI] [PubMed] [Google Scholar]
- 49. Merkel O., Hamacher F., Laimer D., Sifft E., Trajanoski Z., Scheideler M., Egger G., Hassler M. R., Thallinger C., Schmatz A., Turner S. D., Greil R., Kenner L. (2010) Identification of differential and functionally active miRNAs in both anaplastic lymphoma kinase (ALK)+ and ALK- anaplastic large-cell lymphoma. Proc. Natl. Acad. Sci. U.S.A. 107, 16228–16233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Betel D., Wilson M., Gabow A., Marks D. S., Sander C. (2008) The microRNA.org resource: targets and expression. Nucleic Acids Res. 36, D149–D153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang X. (2008) miRDB: a microRNA target prediction and functional annotation database with a wiki interface. RNA 14, 1012–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang X., El Naqa I. M. (2008) Prediction of both conserved and nonconserved microRNA targets in animals. Bioinformatics 24, 325–332 [DOI] [PubMed] [Google Scholar]
- 53. Papadopoulos G. L., Reczko M., Simossis V. A., Sethupathy P., Hatzigeorgiou A. G. (2009) The database of experimentally supported targets: a functional update of TarBase. Nucleic Acids Res. 37, D155–D158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lewis B. P., Burge C. B., Bartel D. P. (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20 [DOI] [PubMed] [Google Scholar]
- 55. Friedman R. C., Farh K. K., Burge C. B., Bartel D. P. (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19, 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Grimson A., Farh K. K., Johnston W. K., Garrett-Engele P., Lim L. P., Bartel D. P. (2007) MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol. Cell 27, 91–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Garcia D. M., Baek D., Shin C., Bell G. W., Grimson A., Bartel D. P. (2011) Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nat. Struct. Mol. Biol. 18, 1139–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wee L. M., Flores-Jasso C. F., Salomon W. E., Zamore P. D. (2012) Argonaute divides its RNA guide into domains with distinct functions and RNA-binding properties. Cell 151, 1055–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Baraniskin A., Birkenkamp-Demtroder K., Maghnouj A., Zollner H., Munding J., Klein-Scory S., Reinacher-Schick A., Schwarte-Waldhoff I., Schmiegel W., Hahn S. A. (2012) MiR-30a-5p suppresses tumor growth in colon carcinoma by targeting DTL. Carcinogenesis 33, 732–739 [DOI] [PubMed] [Google Scholar]
- 60. Cheng C. W., Wang H. W., Chang C. W., Chu H. W., Chen C. Y., Yu J. C., Chao J. I., Liu H. F., Ding S. L., Shen C. Y. (2012) MicroRNA-30a inhibits cell migration and invasion by downregulating vimentin expression and is a potential prognostic marker in breast cancer. Breast Cancer Res. Treat. 134, 1081–1093 [DOI] [PubMed] [Google Scholar]
- 61. Yu F., Deng H., Yao H., Liu Q., Su F., Song E. (2010) Mir-30 reduction maintains self-renewal and inhibits apoptosis in breast tumor-initiating cells. Oncogene 29, 4194–4204 [DOI] [PubMed] [Google Scholar]
- 62. Mangelsdorf D. J., Thummel C., Beato M., Herrlich P., Schütz G., Umesono K., Blumberg B., Kastner P., Mark M., Chambon P., Evans R. M. (1995) The nuclear receptor superfamily: the second decade. Cell 83, 835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Charn T. H., Liu E. T., Chang E. C., Lee Y. K., Katzenellenbogen J. A., Katzenellenbogen B. S. (2010) Genome-wide dynamics of chromatin binding of estrogen receptors alpha and beta: mutual restriction and competitive site selection. Mol. Endocrinol. 24, 47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Levy N., Paruthiyil S., Zhao X., Vivar O. I., Saunier E. F., Griffin C., Tagliaferri M., Cohen I., Speed T. P., Leitman D. C. (2010) Unliganded estrogen receptor-beta regulation of genes is inhibited by tamoxifen. Mol. Cell. Endocrinol. 315, 201–217 [DOI] [PubMed] [Google Scholar]
- 65. Paruthiyil S., Parmar H., Kerekatte V., Cunha G. R., Firestone G. L., Leitman D. C. (2004) Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 64, 423–428 [DOI] [PubMed] [Google Scholar]
- 66. Ström A., Hartman J., Foster J. S., Kietz S., Wimalasena J., Gustafsson J. A. (2004) Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc. Natl. Acad. Sci. U.S.A. 101, 1566–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chumsri S., Howes T., Bao T., Sabnis G., Brodie A. (2011) Aromatase, aromatase inhibitors, and breast cancer. J. Steroid Biochem. Mol. Biol. 125, 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Iliopoulos D., Polytarchou C., Hatziapostolou M., Kottakis F., Maroulakou I. G., Struhl K., Tsichlis P. N. (2009) MicroRNAs differentially regulated by Akt isoforms control EMT and stem cell renewal in cancer cells. Sci. Signal. 2, ra62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Edmonds M. D., Hurst D. R., Vaidya K. S., Stafford L. J., Chen D., Welch D. R. (2009) Breast cancer metastasis suppressor 1 coordinately regulates metastasis-associated microRNA expression. Int. J. Cancer 125, 1778–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ma L., Reinhardt F., Pan E., Soutschek J., Bhat B., Marcusson E. G., Teruya-Feldstein J., Bell G. W., Weinberg R. A. (2010) Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat. Biotechnol. 28, 341–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stinson S., Lackner M. R., Adai A. T., Yu N., Kim H. J., O'Brien C., Spoerke J., Jhunjhunwala S., Boyd Z., Januario T., Newman R. J., Yue P., Bourgon R., Modrusan Z., Stern H. M., Warming S., de Sauvage F. J., Amler L., Yeh R. F., Dornan D. (2011) TRPS1 targeting by miR-221/222 promotes the epithelial-to-mesenchymal transition in breast cancer. Sci. Signal. 4, ra41. [DOI] [PubMed] [Google Scholar]
- 72. Zhou X., Marian C., Makambi K. H., Kosti O., Kallakury B. V., Loffredo C. A., Zheng Y. L. (2012) MicroRNA-9 as potential biomarker for breast cancer local recurrence and tumor estrogen receptor status. PLoS One 7, e39011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Scott G. K., Goga A., Bhaumik D., Berger C. E., Sullivan C. S., Benz C. C. (2007) Coordinate suppression of ERBB2 and ERBB3 by enforced expression of micro-RNA miR-125a or miR-125b. J. Biol. Chem. 282, 1479–1486 [DOI] [PubMed] [Google Scholar]
- 74. Li C., Xiong Q., Zhang J., Ge F., Bi L. J. (2012) Quantitative proteomic strategies for the identification of microRNA targets. Expert Rev. Proteomics 9, 549–559 [DOI] [PubMed] [Google Scholar]
- 75. Baek D., Villen J., Shin C., Camargo F. D., Gygi S. P., Bartel D. P. (2008) The impact of microRNAs on protein output. Nature 455, 64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Selbach M., Schwanhausser B., Thierfelder N., Fang Z., Khanin R., Rajewsky N. (2008) Widespread changes in protein synthesis induced by microRNAs. Nature 455, 58–63 [DOI] [PubMed] [Google Scholar]
- 77. Hirano M., Hashimoto S., Yonemura S., Sabe H., Aizawa S. (2008) EPB41L5 functions to post-transcriptionally regulate cadherin and integrin during epithelial-mesenchymal transition. J. Cell Biol. 182, 1217–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Boutros R., Byrne J. A. (2005) D53 (TPD52L1) is a cell cycle-regulated protein maximally expressed at the G2-M transition in breast cancer cells. Exp. Cell Res. 310, 152–165 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.