Abstract
This study in participants with type 2 diabetes and obstructive sleep apnea evaluated changes in activity, sleep quality and daytime sleepiness after 4 weeks of continuous positive airway pressure (CPAP). This pilot study was a double-blind, randomized, placebo-controlled trial. Sleep apnea was quantified with an overnight sleep study. Sleep quality was measured by the Pittsburgh Sleep Quality Index, daytime sleepiness by the Epworth Sleepiness Scale, vigor and fatigue with the Profiles of Mood States, subjective activity with the Functional Outcomes of Sleep Questionnaire, and objective activity with the Bodymedia SenseWear Armband™. Subjects were randomized to either continuous positive airway pressure (n=12) or a sham-devices (n=11). The intervention group had reduced apneas and hypopneas, daytime sleepiness and fatigue; they also had improved sleep quality, increased objective activity, and vigor. The study suggested that treatment of obstructive sleep apnea results in a modest improvement of activity in persons with type 2 diabetes.
Keywords: sleep, type 2 diabetes mellitus, randomized clinical trial
An increasing body of evidence suggests that untreated obstructive sleep apnea (OSA) is additive or synergistic to determinants of poor glycemic control in persons with type 2 diabetes (T2DM) (Aronsohn, Whitmore, Van Cauter, & Tasali, 2010; Punjabi, 2009). However, it remains unclear whether treatment of OSA is accompanied by improved behavioral outcomes such as increased physical activity, an important component in management of T2DM. A joint statement by the American College of Sports Medicine and the American Diabetes Association suggests regular physical activity to be beneficial for improved diabetes management; unfortunately many individuals with T2DM have a sedentary lifestyle (Colberg et al., 2010). Therefore, understanding the possible effect of OSA on factors associated with glycemic control, such as physical activity, is salient to achieving improved diabetes management.
In the U.S., approximately 25.8 million people were estimated in 2010 to have diabetes, with 90 to 95% having T2DM (National Diabetes Statistics, 2011). Diabetes is a costly chronic illness both to the individual and to the national economy. Poorly controlled diabetes is associated with significant health problems including kidney failure, lower limb amputation, heart disease and stroke. Recent research estimated that the total cost of diabetes in 2007 was $218 billion, including $153 billion in excess medical expenditures and $65 billion in reduced national productivity (Dall et al., 2010). Results of multiple studies have found that untreated OSA has a negative impact 24 hours a day, not only disrupting nighttime sleep but also resulting in excessive daytime sleepiness that interferes with daytime functioning (Engleman et al., 1999; Faccenda, Mackay, Boon, & Douglas, 2001; Jenkinson, Davies, Mullins, & Stradling, 1999; Weaver et al., 2012). Previous studies found that excessive daytime sleepiness negatively affects cognition (Ferini-Strambi et al., 2003), mood (Bardwell, Berry, Ancoli-Israel, & Dimsdale, 1999), and physical activity (Chasens, Sereika, Houze, & Strollo, 2011). Continuous positive airway pressure (CPAP) treats the apneas (cessation of breathing for at least 10 seconds) and hypopneas (reduction in breathing by at least 30% accompanied by a 4% or greater decrease in oxygen saturation) that characterize OSA by pneumatically splinting open the airway. Previous research found that individuals who are adherent to CPAP at least 4-hours a night have a dose-related effect; there is reduced daytime sleepiness and improved sleep quality (Weaver et al., 2007). Baseline data from the current study suggests that poor sleep quality was associated with decreased diabetes management including decreased attitude, adherence to diabetes self-care activities, and worse dietary adherence (Chasens, Korytkowski, Sereika, & Burke, 2013). However, among adults with T2DM, it remains unclear whether the effect of treatment of OSA improves factors associated with improved glycemic control such as physical activity level.
The aim of this study in participants with T2DM and OSA was to 1) determine the feasibility of conducting a double blind, randomized, placebo-controlled study in subjects treated with CPAP compared to subjects on sham-CPAP, 2) to examine physical activity in subjects treated with CPAP compared to subjects on sham-CPAP, and 3) to explore pre- to post-therapy changes in sleep quality, daytime sleepiness, activity, vigor and fatigue.
Methods
Research Design and Sample
The study had a 2 group, double-blind, randomized, placebo-controlled design. Participants who were originally randomized to the sham-CPAP group were offered a trial of active CPAP treatment for 4 weeks after they were debriefed on group assignment. Data from the baseline evaluation of participants (n = 107) on the effect of poor sleep quality and daytime sleepiness on factors associated with diabetes self-management activity has already been published (Chasens et al., 2013). The Institutional Review Board at the University of Pittsburgh approved the study protocol and the clinical trial was registered with http://www.clinicaltrials.gov/.
Participants were recruited from the community over 15 months. Potential subjects were provided with an explanation of the purpose of the study and what it entailed if they chose to participate. After verbal consent was obtained, a set script was used for telephone screening to determine if they met the initial inclusion and exclusion criteria. See Table 1 for list of inclusion and exclusion criteria.
Table 1.
Study Inclusion and Exclusion Criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
| |
| • T2DM (verified by primary care provider or medication typically ordered for diabetes) | • Diagnosis of another sleep disorder, in addition to OSA, based on PSG (e.g., periodic limb movement disorder ≥ 10 limb movements/hr, or insomnia) |
| • AHI (from PSG) greater than ≥ 10 | • Oxygen or Bi-level positive airway pressure required for treatment of OSA |
| • A1C< 9.0% | • Oxygen saturation <75% for >10% of the diagnostic PSG or if subject has oxygen saturation <75% for >25% of the first 4 h of the diagnostic PSG |
| • ESS ≥ 10 (Screening) | • Any individual in the household currently or with history of CPAP treatment |
| • Able to ambulate independently or with a cane | • Type 1 or gestational diabetes |
| • Age 30 years or older | • Prescribed insulin for treatment of T2DM |
| • No acute medical and psychiatric illness in past 3 months | • Changes in diabetic medications in prior 3 months |
| • Self-reported sleep duration of at least 6 hours by baseline Sleep/Activity Diary | • Regular use (> 3 times/week) of hypnotic or alerting medications (i.e. modafinil) |
| • Telephone access | • Prior treatment for OSA with CPAP or surgery |
| • Able to perform study tests (e.g., speak, read and write in English). | • History of a near-miss or automobile accident due to sleepiness |
| • Willing to be randomized to CPAP or sham-CPAP | • Employed in transportation-related safety sensitive (i.e. airline pilot, truck driver, or train engineer) |
| • Currently working night or rotating shifts | |
| • Routine consumption of alcohol as determined by >2 drinks day | |
| • Claustrophobia that prevents wearing the CPAP mask | |
| • Pregnancy | |
T2DM= type 2 diabetes mellitus; AHI= apnea + hypopnea index; ESS= Epworth Sleepiness Scale; CPAP= continuous positive airway pressure, OSA= obstructive sleep apnea; PSG= polysomnography
Measures
Activity Measure
The Bodymedia SenseWare Pro Armband® is an objective measurement of physical activity that calculates the duration and intensity of activity for each wearer by monitoring physiological and movement variables; age, gender, height, and weight are manually entered for each participant. The activity endpoint was summarized as the participant's number of steps walked on days when the monitor was worn ≥ 95% of the time.
Sleep Measures
Pittsburgh Sleep Quality Index (PSQI) (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989) is a validated questionnaire that differentiates “poor” from “good” sleep. The PSQI evaluates seven components: sleep duration, sleep latency, usual sleep efficiency, perceived sleep quality, sleep disturbances, use of medications for sleep, and difficulty in being active during the daytime because of sleepiness. Yielding a global score that can range from 0 “excellent sleep quality” to 21 “extremely poor sleep quality”, each component is graded between 0 “better” to 3 “worse”. A global score greater than 5 is associated with poor sleep quality. The PSQI has an internal consistency of 0.83 and test-retest reliability of 0.85 over approximately 4-weeks.
Epworth Sleepiness Scale (ESS) (Johns, 1991, 1992) is a single factor, 8-item questionnaire where respondents rate their probability from 0 “no chance” to 3 “high chance” of falling asleep in different situations. The ESS is a reliable and valid instrument with test-retest reliability (r=.82) and internal consistency (Cronbach's alpha =.88). The ESS has sensitivity (93.5%) and specificity (100%) for distinguishing pathological from normal sleepiness at the cut-point of ≥ 10.
Functional Outcomes of Sleep Questionnaire (FOSQ) (Weaver et al., 1997) is designed to evaluate difficulty in carrying out activities that are sensitive to sleepiness. The Activity Level subscale (Cronbach's alpha =.91) queries aspects of subjective difficulty because of sleepiness in being active.
Mood Measure
Profile of Mood States (POMS) (McNair, Lorr, & Druppleman, 1971) is a reliable and valid measure of mood states that has been shown to be sensitive to sleep impairment with OSA and to treatment of OSA with CPAP (Derderian, Bridenbaugh, & Rajagopal, 1988; Tomfohr, Ancoli-Israel, Loredo, & Dimsdale, 2011). The POMS asks participants to rate 65 adjectives on a 5-point scale as “not at all” to “extremely” that represent their feelings to during the past week (e.g. “friendly”, “helpful”, “blue”). For this study, we are reporting the Vigor-Activity and the Fatigue-Inertia subscales. Worse Fatigue-Inertia is indicated by high scores; worse Vigor-Activity by low scores.
Demographic and Clinical Measures
Demographic and Co-Morbidities. Information including age, sex, race, and education were obtained at baseline using a self-administered questionnaire developed at the University of Pittsburgh School of Nursing Center for Research in Chronic Disorders. Participants were questioned about having a physician diagnosis of 16 comorbidities in addition to T2DM.
A1C. An A1C level was obtained at the baseline visit to screen for persons with impaired glucose control (>9%) that required medical intervention. The A1C measures hemoglobin glycation to indicate participant's glucose control over the previous 2 to 3 months.
Body mass index (BMI/kg/m2). Height and weight were obtained to calculate the BMI. Height was measured at baseline with a wall-mounted stadiometer. A Tanita-300GS scale was used to weigh subjects at each assessment while they wore light clothing.
Protocol Fidelity
Feasibility of the study was determined by evaluation of achieving recruitment goals which included recruitment of women and minorities, retention rate, intervention fidelity, adherence to CPAP, success in maintaining blinding to CPAP or sham-CPAP, and maintenance of confidentiality and participant safety. Staff and principal investigator had weekly meetings to discuss issues related to protocol fidelity; there were meetings with the team of co-investigators and safety officers semi-annually and consultations with individual co-investigators as needed.
Procedure
Persons meeting the initial eligibility criteria were invited to a baseline clinical evaluation (Assessment 1) where written informed consent was obtained and a clinical evaluation was completed. Participants were given a single channel apnea screening device to wear one night; participants with a low risk for apnea,(less than 15 episodes of decreased airflow per hour while asleep, were excluded from the study. In addition, participants were given the Bodymedia SenseWare Pro Armband® to wear 24 hours a day for 7 days and questionnaires to be completed at home with pre-paid mailers to return the materials. There was no exercise intervention to the protocol and participants were not instructed to change their current habits.
Description of the protocol including the CPAP and sham-CPAP devices, diagnostic polysomnogram (PSG), titration PSG to therapeutic or sham-CPAP has been previously described in detail (Chasens, Drumheller, & Strollo, 2012). A nurse practitioner performed a history and physical on all participants scheduled for overnight PSG to further screen for medical or psychological conditions that may make the participant ineligible.
Full-night PSG were conducted at the Neuroscience Clinical Translational Research Center (N-CTRC) with a montage that recorded electroencephalogram (EEG), electrooculogram (EOG), submentalis and anterior tibialis electromyograms (EMG), airflow, air pressure, thoracic and abdominal excursion, and pulse oximetry. Participants were requested to refrain from caffeinated beverages and not to nap on the day of the study. The PSGs were scored by a single PSG technician, with a random sample of PSGs rescored each month by a second PSG technician to check for inter-rater reliability, there was approximately a 95% agreement on the apnea + hypopnea index (AHI). The PSG results were interpreted by the co-investigator physician who is board certified in Sleep Medicine. Sleep and wake episodes were scored with 30 second epochs using established criteria. Apneas were defined as a cessation of breathing for ≥ 10 seconds; hypopneas were defined as a decrease in airflow by ≥ 30% associated with an arousal or 4% oxygen desaturation (American Academy of Sleep Medicine, 2005).
Randomization
Participants were randomly assigned by a computer program using a minimization algorithm with equal allocation to one of two treatment groups: 1) CPAP set at a level of pressure that will abolish/decrease to less than 5 events/hour nocturnal respiratory disturbances, or 2) sham-CPAP (placebo) where the level of pressure was set at 0.5 to 1.0 cm, a pressure that will not abolish nocturnal respiratory disturbances. The use of a minimization algorithm ensures treatment balance on the participant's baseline physical activity levels in terms of mean number of steps per day based on the SenseWear Pro Armband® (< 5,000, ≥ 5000). Minimization is a form of adaptive treatment allocation in which the probability of assignment to the treatment groups is determined by the current composition of the groups.(Friedman, Furberg, & DeMets, 2010) Following baseline assessment, information on the participant's baseline physical activity level were entered in the randomization program by an unblinded research staff member and the treatment group assignment was generated and stored in a closed envelope. The participant and all other research staff were blinded as to which group the subjects were assigned except for the night PSG technician who performed the overnight titration CPAP/sham-CPAP study and the study's sleep physician co-investigator.
The CPAP and sham-CPAP titration studies consisted of a second overnight visit to the N-CTRC. The sham-CPAP apparatus (RemStar Pro, Respironics, Inc.) was similar to the one used in previous studies (Quan et al., 2011; Rodway et al., 2010) and consisted of an enlarged air leak incorporated into the exhalation valve between the mask and the CPAP tubing and an orifice restrictor in the CPAP circuit with the pressure at the mask interface less than 1 cm H2O. During the 4-wk protocol (8-wk for the sham-CPAP group), CPAP machines were provided free of charge to the subjects from devices that were loaned by the manufacturer (Respironics, Inc.).
All randomized participants had a second assessment (Assessment 2) after 4 weeks of treatment with CPAP or sham-CPAP. They were mailed the Bodymedia SenseWare Pro Armband® to wear for 7 days to determine activity and the questionnaires to fill out at home. After 7 days, participants returned for the second clinical evaluation and were then debriefed and told to which group they had been randomized. Participants who were originally in the sham-CPAP group were encouraged to continue in the study with a second CPAP titration study and crossover to active treatment for 4 weeks.
Adherence to CPAP/sham-CPAP
The CPAP/sham-CPAP devices contain a software system (Respironics Encore® SmartCard®) that recorded adherence to nightly CPAP treatment on a microchip contained on the SmartCard® that slides into a port on the side of the CPAP machine. Subjects sent their Smart Cards® to the Project Manager for weekly follow-up evaluations and for the data to be uploaded to the project server. Adherence was encouraged in both treatment arms with weekly telephone calls.
Data Management
Data were recorded with only participant identification codes. Oracle (version 11g, Oracle Corp., Redwood Shores, CA) was used for data management and the database was password protected and only trained staff had access. Teleform Elite (Version 10, Verity, Inc., Sunnyvale, CA), a Windows-based software package for automated data entry and verification, was used for paper-and-pencil instruments. Data collected using electronic data capture methods (SenseWear Pro Armband® and SmartCard® adherence data) were uploaded from the devices to the project computer, interpreted using the software, exported as an ASCII file, and merged into the Oracle database. The interpreted PSG diagnostic and titration studies were directly downloaded into an Excel data base by research staff at the N-CTRC. The biological data were manually entered in a Teleform and then merged with the project's database.
Sample Size Justification
No formal power analysis was done for this feasibility study. Our goal was to recruit a final sample of 20 subjects to randomized to CPAP or sham-CPAP who met all of the inclusion and exclusion criteria and were adherent to CPAP (≥ 4 hours/night). By design, while all male and female subjects with T2DM who met the eligibility criteria were included, we made special efforts to recruit women and a sample with a minority racial distribution consistent with or greater than, that of the greater Pittsburgh area (27% African American).
Statistical Analysis
The statistical methods used in analysis were primarily descriptive and exploratory in nature. An intent-to-treat approach was used with all participants included in the data analyses as randomized. Descriptive statistics were computed with all continuous type variables reported as mean ± SD and categorical variables reported as frequency and percent. While the majority of the sample (65%) averaged more than 5,000 steps a day, several participants differed greatly in the number of steps walked between work days (high) and days when they did not work (low). Because of this, we utilized the median number of steps walked from the Bodymedia SenseWare Pro Armband®.
Baseline characteristics of participants in the CPAP group were compared to those in the sham-CPAP group using two-sample t-tests for continuous variables and Fisher's Exact tests for categorical variables. Standardized mean differences, Cohen's standardized mean difference, d, was used to estimate the relative magnitude of change in using Cohen's definitions of a small (0.2), medium (0.5) and larger (0.8 and larger) effect size (Cohen, 1992). Analyses were conducted using IBM SPSS Statistics Version 19.
Results
Description of Sample
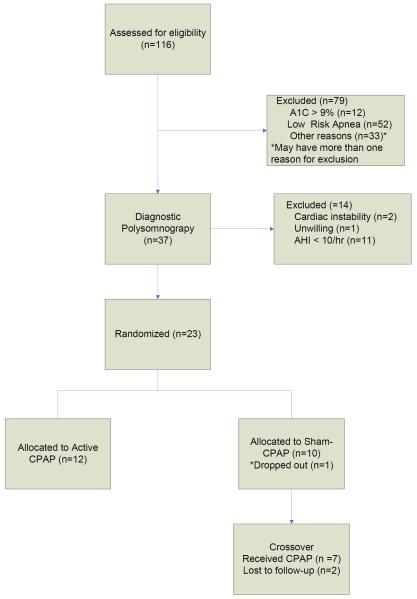
A total of 116 adults were assessed for eligibility (see Figure 1 for CONSORT diagram for participant progression through phases of study). A total of 79 subjects were excluded after Assessment 1, primarily because of low apnea events (<15/hour) sensed by a single channel (airflow pressure) apnea screening device (n=52) or because of high A1C values (n= 12, A1C 9.0% to 12.8%). A total of 37 participants underwent overnight diagnostic PSG; 9 of the 11 persons excluded because of having an AHI < 10 were premenopausal females. In addition, two subjects were found to have significant cardiac arrhythmias during their overnight PSG and were excluded from the study, referred for evaluation, and eventually started directly on CPAP therapy.
Figure 1.
CONSORT Diagram
The demographic characteristics of the randomized sample are depicted in Table 2. Overall, our randomized sample (N= 23) was well distributed by race, primarily male, middle-aged (mean age = 55.6 years, SD 10.6, range 34–80 years), and overweight or obese (mean BMI = 35.5, SD 6.2, 80% with BMI ≥ 30). Slightly more than half the sample (mean A1C = 6.9, SD = 1.15) had relatively good glucose control at baseline (A1C levels < 7.0%). A large number of participants (44%) reported a physician diagnosis of 3 or more comorbid conditions in addition to T2DM. A comparison of participants in the CPAP and sham-CPAP groups, showed no significant statistical difference in age, sex, BMI, A1C, racial composition, education level or number of comorbidities between groups.
Table 2.
Description of the Randomized Participants at Baseline
| Characteristics Mean±SD or n (%) | Total (N= 23) | Treatment Group | ||
|---|---|---|---|---|
|
| ||||
| Active CPAP n=12 |
Sham-CPAP n=11 |
p-value | ||
|
| ||||
| Sex | ||||
| Male | 61% (14) | 58% (7) | 64% (7) | .795 |
| Female | 39% (9) | 42% (5) | 36% (4) | |
|
| ||||
| Race | 2 | |||
| White | 48% (11) | 58% (7) | 37% (4) | .292 |
| Black/Biracial * | 52% (12) | 42% (5) | 63% (7) | |
|
| ||||
| Education | ||||
| ≤ High School | 48% (11) | 50% (6) | 46% (5) | .510 |
| > High School | 52% (12) | 50% (6) | 54% (6) | |
|
| ||||
| Comorbidities † | ||||
| 0–3 | 56% (13) | 50% (6) | 64% (7) | .762 |
| 4 or more | 44% (10) | 50% (6) | 36% (4) | |
One participant identified biracial Black and Native American
Comorbidities diagnosed by health care provider, possible range from 0–17.
Diagnostic and Titration Sleep Studies
A description of baseline PSG data for the total sample and a comparison by treatment group is displayed in Table 3. There was no significant difference in sleep latency, sleep maintenance, time awake after sleep onset, or sleep architecture (percentage of time spent in each of the sleep stages) between CPAP and sham-CPAP participants. At baseline, participants in the sham-CPAP group had about 30 minutes longer sleep duration (p=.026). The groups were not equal in apnea severity, partially because in the small sample there were 3 participants in the CPAP group with a high AHI (≥ 60). While there was no significant difference in the baseline apnea index between groups, the AHI and the oxygen desaturation indexes were significantly higher (p<.05) in the active CPAP group.
Table 3.
Baseline polysomnographic data for randomized subjects and comparison by treatment group
| Sleep Variables Mean±SD | Total Randomized (N= 23) | Randomized Sample | p-value | |
|---|---|---|---|---|
| Active CPAP n=12 |
Sham-CPAP n=11 |
|||
| Total Sleep (minutes). Time from sleep onset to good morning time. | 464±64 | 436±58 | 494±59 | .026 |
| Sleep latency (minutes). Time from good night time to sleep onset | 17±23 | 17±30 | 16±14 | .903 |
| % Sleep Efficiency after good night time | 78.7±12.6 | 76.9±15.8 | 80.8±8.3 | .475 |
| % Sleep maintenance after Sleep onset | 81.2±11.2 | 79.4±13.8 | 83.3±7.7 | .419 |
| REM latency (minutes) from sleep onset to onset of first REM period | 110±67 | 132± 83 | 88±40 | .129 |
| Awake after sleep onset (minutes) – WASO | 86±50 | 88±61 | 83±39 | .794 |
| Stage 1 (% total sleep time) | 11.4±7.2 | 13.7±8.3 | 9.0±4.9 | .117 |
| Stage 2 (% total sleep time) | 65.1±8.3 | 65.9±8.2 | 64.3±8.6 | .635 |
| Delta Sleep (% total sleep time) | 3.3±4.5 | 2.6±4.2 | 4.0±4.8 | .439 |
| Stage REM (% total sleep time) | 20.2±8.4 | 17.8±8.2 | 22.7±8.3 | .166 |
| Apnea + hypopnea index | 38.9±26.1 | 50.2±30.9 | 26.5±11.2 | .026 |
| Apnea index | 21.4±25.1 | 27.0±32.8 | 15.2±11.2 | .269 |
| Oxygen desaturation (4%) index | 31.1±24.7 | 41.9±29.6 | 19.2±9.1 | .023 |
| Periodic leg movement with arousal index | 1.7±2.9 | 1.2±2.2 | 2.3±3.6 | .371 |
Good morning time = the time when a participant was woken according to their self-reported normal wake time. Good night time = time participant was requested to be according to their self-reported normal bedtime. REM = rapid eye movement; WASO= wake after sleep onset; Index = mean score per hour for total time asleep
The AHI and oxygen desaturation index were, as expected, significantly improved with active CPAP compared with the sham-CPAP group (respectively, CPAP AHI =7, sham-CPAP AHI = 21; active CPAP oxygen desaturation index = 5, sham-CPAP oxygen desaturation index = 15, p-values <.05). The majority (74%) of participants (n=17) were adherent to CPAP ≥ 240 minutes/night (average usage, on days used, ranged from 96 to 489 minutes) and 40% (n= 9) used their devices 100% of the nights. One participant in the active CPAP group did not use his device after his first night at home and one participant in the sham-CPAP group dropped out because “his machine wasn't helping him”, although he stated he understood he may have been randomized to the sham-CPAP group. Seven of the nine participants who were originally in the sham-CPAP group continued on in the study to active CPAP treatment. After completion of the study, 50% of the sample (n =12) reported seeking follow-up medical care and continuing on their CPAP treatment.
Study Feasibility
The study achieved the goal of demonstrating feasibility by achieving recruitment and retention goals, a randomized sample that was almost 40% female and approximately 50% non-white, and a 95% retention rate of participants randomized to either CPAP or sham-CPAP. The protocol was maintained, although original aspects of the study, such as excluding persons with a BMI greater than 40 kg/m2, were changed because of the goal of protocol enhancement. Data management resulted in little missing data, most important, measures to ensure participant safety and confidentiality were strictly followed and maintained.
Physical Activity Before and After CPAP Treatment
Table 4 summarizes objective and subjective sleep data and activity for participants at baseline and after 4-weeks of CPAP or sham-CPAP. At baseline, there was no difference in the mean or median number of steps walked between participants in the active CPAP and the sham-CPAP group, indicating that the minimization plan used for randomization was successful. Participants in both groups increased their activity while in the study; however, participants in the active CPAP group walked more steps than those in the sham-CPAP group (d=0.24). This indicated there was a “small effect” in increasing physical activity in participants with OSA treated with CPAP compared to participants with OSA randomized to sham-CPAP.
Table 4.
Descriptive statistics and Effect Sizes for Outcome Variables by Treatment Group (Active and Sham-CPAP) and Time Point
| Variables Mean ± SD | CPAP Baseline n=12 |
Sham-CPAP baseline n=11 |
CPAP After 4-wks n=12 |
Sham-CPAP After 4-wks n=11 |
Effect Size (Cohen's d) |
|---|---|---|---|---|---|
| Objective and Subjective Sleep | |||||
| Apnea + hypopnea index* | 50.15±30.9 | 26.52±11.2 | 6.98±6.2 | 21.20±14.6 | −1.67 |
| Epworth Sleepiness Scale | 11.42±4.62 | 10.55±3.72 | 9.08±5.70 | 10.50±3.62 | −0.76 |
| Pittsburgh Sleep Quality Index: Global Score | 9.58±3.06 | 9.27±5.42 | 7.33±3.92 | 9.60±3.53 | −0.62 |
| Activity | |||||
| Steps Walked | 5892±3841 | 6071±2714 | 6284±3480 | 6684±2509 | 0.24 |
| POMS Vigor-Activity Subscale | 15.08±5.16 | 17.45±7.02 | 16.75±6.63 | 16.80±6.29 | 0.57 |
| POMS Fatigue-Inertia Subscale | 11.75±4.33 | 9.36±6.04 | 9.08±6.19 | 10.70±5.23 | −0.72 |
| FOSQ Activity Level | 3.15±.55 | 3.21±.73 | 3.32±.51 | 2.99±.91 | 0.86 |
Effect Size = standardized mean differences (e.g., Cohen's d) in change scores between CPAP baseline and after 4-weeks compared to sham-CPAP baseline and after 4-weeks. .
Sleep Quality, Daytime Sleepiness, Activity, Vigor and Fatigue
As expected from study inclusion requirements, participants were sleepy at baseline and had impaired sleep quality. While all participants reported subjective sleepiness (ESS>10) during the telephone screening, ESS scores at Assessment 1 ranged from 4 to 19 (mean ESS 11±4.15); almost 80% of participants had poor sleep quality (PSQI > 5) at the baseline assessment. Before treatment, there were no statistically significant differences in subjective sleepiness or sleep quality between participants randomized to CPAP compared to those participants on sham-CPAP. However, the CPAP group had, on average, moderate-to-large reductions in subjective sleepiness (d =−0.76) and improved sleep quality (d=−0.62) after 4 week of treatment when compared to the sham-CPAP group.
Subjective indicators of activity also showed improvement in participants treated with therapeutic CPAP. At baseline there was no statistically significant difference between participants in the active CPAP and the sham-CPAP groups in scores on the POMS Vigor-Activity subscale or the POMS Fatigue-Inertia subscales. After 4 weeks of treatment there was a moderate increase in POMS Vigor-Activity (d=0.57) and a large decrease in POMS Fatigue-Inertia (d=−0.72). In addition, while there was no significant difference in FOSQ Activity Level at baseline, participants in the CPAP group had a large improvement in the FOSQ Activity Level (d= 0.86) compared to participants in the sham-CPAP group.
Discussion
We were able to demonstrate feasibility in recruiting and retaining participants and in implementing the protocol that used sham-CPAP. The results of this study suggest that treatment of OSA in adults with T2DM may result in increased activity. These findings are consistent with the results of a randomized clinical trial (n =59) conducted by Tomfohr et al. (Tomfohr et al., 2011) who found that 3 weeks of active CPAP resulted in significantly reduced fatigue and increased vigor (POMS) in adults. However, Tomfohr's study excluded persons with T2DM and the current study extends the findings to this population. In addition, our study suggests that treating OSA objectively impacts measured physical activity.
The hypothesis that treatment of OSA in adults with coexisting T2DM is associated with improved clinical outcomes requires further study. Although results of a recent meta-analysis of five randomized clinical trials found a modest overall effect of treatment of OSA with CPAP to reduce insulin resistance in participants without diabetes (Iftikhar, Khan, Das, & Magalang, 2013), significant improved glycemic control in individuals with T2DM requires a multi-faceted approach. This study provided preliminary data on the feasibility of conducting a full-scale randomized clinical trial in persons with T2DM and OSA. The complaints of feeling fatigued and having decreased vigor may be favorably impacted by therapeutic CPAP. The modest results on physical activity suggest that treatment of OSA with CPAP alone may not be sufficient to change entrenched behavior such as sedentary lifestyle.
We would like to acknowledge that this pilot study had limitations. Because the sample size was small, formal hypothesis testing was precluded and individual cases may have unduly influenced the findings. While the study met or exceeded the rate of adherence to CPAP reported in previous studies (Weaver & Sawyer, 2010), it did not reach the goal of all-night, every-night adherence to CPAP. This suggests that future studies evaluating the effects of CPAP treatment need to incorporate recent knowledge about early intervention to facilitate better adaptation to this therapy.
Conclusions
In summary, the study utilized a sham-CPAP device in a 4-week double blind, randomized study to examine physical activity in participants with T2DM with moderate to severe sleep apnea manifesting daytime sleepiness to enhance. This study increased the understanding of mechanisms by which sleep disturbances affect chronic disease and ways that improving sleep may improve treatment outcomes. Despite previous strong evidence that T2DM and OSA frequently co-exist and may exacerbate each other, few previous studies examined whether OSA-induced sleepiness hinders health related behavior that is important in the self-management of T2DM. The study was innovative in testing the hypothesis that OSA severity and excessive daytime sleepiness are associated with decreased physical activity, and that CPAP treatment of OSA reduces excessive daytime sleepiness and thus has the potential to increase physical activity, a leading health indicator.
The findings of this study provide feasibility data to support doing a full-scale randomized controlled clinical trial testing the effect of CPAP on improving behavior needed for optimal diabetes control. Because behavior is difficult to change, we suggest adding a standard diabetes educational intervention and evaluating the difference in physical activity and glycemic control after 3 months of active CPAP or sham-CPAP. If this larger study has positive results, it would have the potential to significantly impact diabetes management to include the routine evaluation and treatment of OSA.
Acknowledgments
This research was supported by a grant from the National Institutes of Health, National Heart Lung and Blood Institute HL 089522 (E. Chasens) and through grants UL1 RR024153 and UL1TR000005. CPAP and sham-CPAP devices obtained via loan agreement from Philips-Respironics, Inc.
Registered with ClinicalTrials.gov # NCT00801892
References
- American Academy of Sleep Medicine . International Classification of Sleep Disorders, revised: Diagnostic and Coding Manual. 2nd ed. American Academy of Sleep Medicine; Rochester, Minnesota: 2005. [Google Scholar]
- Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181(5):507–513. doi: 10.1164/rccm.200909-1423OC. doi: 200909-1423OC [pii] 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell WA, Berry CC, Ancoli-Israel S, Dimsdale JE. Psychological correlates of sleep apnea. Journal of Psychosomatic Research. 1999;47(6):583–596. doi: 10.1016/s0022-3999(99)00062-8. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Chasens ER, Drumheller OJ, Strollo PJ., Jr. Success in blinding to group assignment with sham-CPAP. Biol Res Nurs. 2012 doi: 10.1177/1099800412461711. doi: 10.1177/1099800412461711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasens ER, Korytkowski M, Sereika SM, Burke LE. Effect of poor sleep quality and excessive daytime sleepiness on factors associated with diabetes self-management. Diabetes Educ. 2013;39(1):74–82. doi: 10.1177/0145721712467683. doi: 10.1177/0145721712467683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasens ER, Sereika SM, Houze MP, Strollo PJ. Subjective and objective appraisal of activity in adults with obstructive sleep apnea. J Aging Res. 2011;2011:751819. doi: 10.4061/2011/751819. doi: 10.4061/2011/751819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Braun B. Exercise and type 2 diabetes: The American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care. 2010;33(12):2692–2696. doi: 10.2337/dc10-1548. doi: 33/12/2692 [pii] 10.2337/dc10-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall TM, Zhang Y, Chen YJ, Quick WW, Yang WG, Fogli J. The economic burden of diabetes. Health Aff (Millwood) 2010;29(2):297–303. doi: 10.1377/hlthaff.2009.0155. [Research Support, Non-U.S. Gov't] doi: 10.1377/hlthaff.2009.0155. [DOI] [PubMed] [Google Scholar]
- Derderian SS, Bridenbaugh RH, Rajagopal KR. Neuropsychologic symptoms in obstructive sleep apnea improve after treatment with nasal continuous positive airway pressure. Chest. 1988;94(5):1023–1027. doi: 10.1378/chest.94.5.1023. [DOI] [PubMed] [Google Scholar]
- Engleman HM, Kingshott RN, Wraith PK, Mackay TW, Deary IJ, Douglas NJ. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep Apnea/Hypopnea syndrome. Am J Respir Crit Care Med. 1999;159(2):461–467. doi: 10.1164/ajrccm.159.2.9803121. [Clinical Trial Comparative Study Randomized Controlled Trial Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163(2):344–348. doi: 10.1164/ajrccm.163.2.2005037. [Clinical Trial Randomized Controlled Trial Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- Ferini-Strambi L, Baietto C, Di Gioia MR, Castaldi P, Castronovo C, Zucconi M, Cappa SF. Cognitive dysfunction in patients with obstructive sleep apnea: Partial reversibility after continuous positive airway pressure. Brain Research Bulletin. 2003;61:87–92. doi: 10.1016/s0361-9230(03)00068-6. [DOI] [PubMed] [Google Scholar]
- Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials. 4th ed. Springer; New York: 2010. [Google Scholar]
- Iftikhar IH, Khan MF, Das A, Magalang UJ. Meta-analysis: Continuous Positive Airway Pressure Improves Insulin Resistance in Patients with Sleep Apnea without Diabetes. Ann Am Thorac Soc. 2013;10(2):115–120. doi: 10.1513/AnnalsATS.201209-081OC. doi: 10.1513/AnnalsATS.201209-081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson C, Davies RJO, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. The Lancet. 1999;353:2100–21005. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Druppleman L. EITS Manual for the Profile of Mood States. Educational and Industrial Test Services; San Diego: 1971. [Google Scholar]
- National Diabetes Statistics [Accessed July 15, 2011];2011 Retrieved. , from http://www.diabetes.niddk.nih.gov/dm/pubs/statistics/
- Punjabi NM. Do sleep disorders and associated treatments impact glucose metabolism? Drugs. 2009;69(Suppl 2):13–27. doi: 10.2165/11531150-000000000-00000. doi: 3 [pii] 10.2165/11531150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Quan SF, Chan CS, Dement WC, Gevins A, Goodwin JL, Gottlieb DJ, Kushida CA. The association between obstructive sleep apnea and neurocognitive performance--the Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2011;34(3):303–314B. doi: 10.1093/sleep/34.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodway GW, Weaver TE, Mancini C, Cater J, Maislin G, Staley B, Kuna ST. Evaluation of sham-CPAP as a placebo in CPAP intervention studies. Sleep. 2010;33(2):260–266. doi: 10.1093/sleep/33.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomfohr LM, Ancoli-Israel S, Loredo JS, Dimsdale JE. Effects of continuous positive airway pressure on fatigue and sleepiness in patients with obstructive sleep apnea: data from a randomized controlled trial. Sleep. 2011;34(1):121–126. doi: 10.1093/sleep/34.1.121. [Randomized Controlled Trial Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver TE, Laizner AM, Evans LK, Maislin G, Chugh DK, Lyon K, Dinges DF. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20(10):835–843. [PubMed] [Google Scholar]
- Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, Pack AI. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver TE, Mancini C, Maislin G, Cater J, Staley B, Landis JR, Kuna ST. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. Am J Respir Crit Care Med. 2012;186(7):677–683. doi: 10.1164/rccm.201202-0200OC. [Randomized Controlled Trial Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] doi: 10.1164/rccm.201202-0200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver TE, Sawyer AM. Adherence to continuous positive airway pressure treatment for obstructive sleep apnoea: Implications for future interventions. Indian J Med Res. 2010;131:245–258. [PMC free article] [PubMed] [Google Scholar]