Abstract
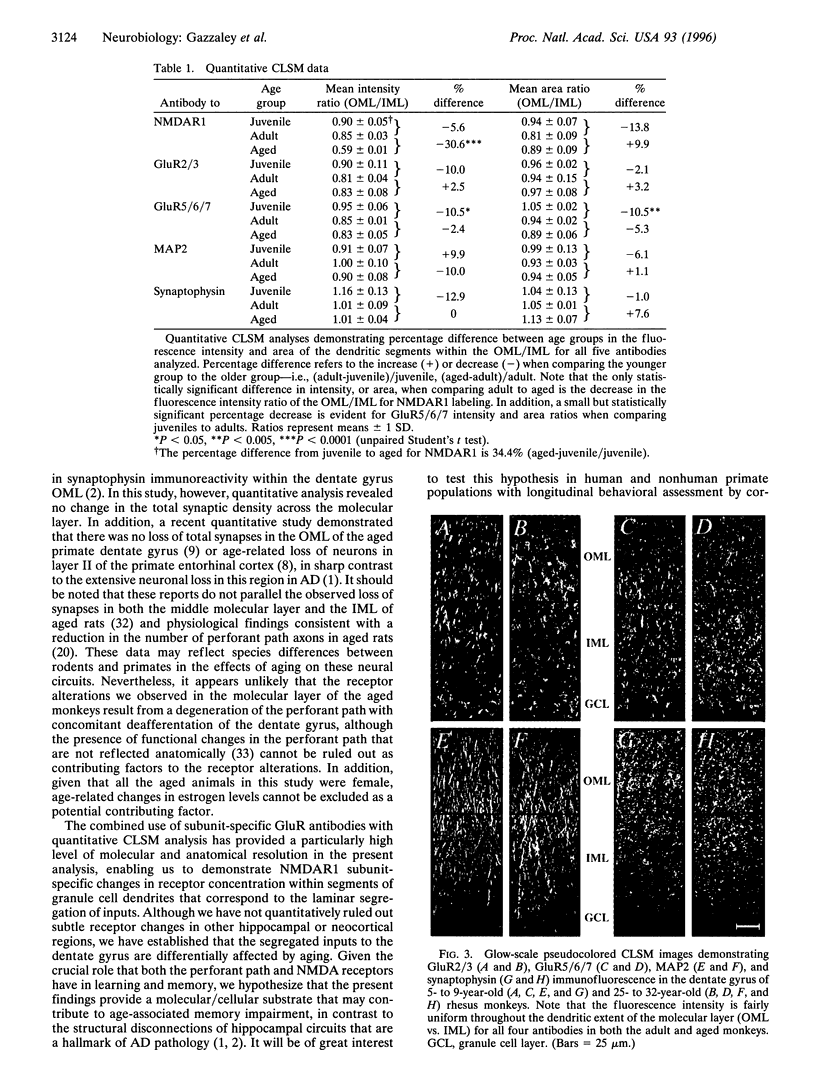
Age-associated memory impairment occurs frequently in primates. Based on the established importance of both the perforant path and N-methyl-D-aspartate (NMDA) receptors in memory formation, we investigated the glutamate receptor distribution and immunofluorescence intensity within the dentate gyrus of juvenile, adult, and aged macaque monkeys with the combined use of subunit-specific antibodies and quantitative confocal laser scanning microscopy. Here we demonstrate that aged monkeys, compared to adult monkeys, exhibit a 30.6% decrease in the ratio of NMDA receptor subunit 1 (NMDAR1) immunofluorescence intensity within the distal dendrites of the dentate gyrus granule cells, which receive the perforant path input from the entorhinal cortex, relative to the proximal dendrites, which receive an intrinsic excitatory input from the dentate hilus. The intradendritic alteration in NMDAR1 immunofluorescence occurs without a similar alteration of non-NMDA receptor subunits. Further analyses using synaptophysin as a reflection of total synaptic density and microtubule-associated protein 2 as a dendritic structural marker demonstrated no significant difference in staining intensity or area across the molecular layer in aged animals compared to the younger animals. These findings suggest that, in aged monkeys, a circuit-specific alteration in the intradendritic concentration of NMDAR1 occurs without concomitant gross structural changes in dendritic morphology or a significant change in the total synaptic density across the molecular layer. This alteration in the NMDA receptor-mediated input to the hippocampus from the entorhinal cortex may represent a molecular/cellular substrate for age-associated memory impairments.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes C. A., McNaughton B. L. Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J Physiol. 1980 Dec;309:473–485. doi: 10.1113/jphysiol.1980.sp013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C. A. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 1994 Jan;17(1):13–18. doi: 10.1016/0166-2236(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabalka L. M., Hyman B. T., Goodlett C. R., Ritchie T. C., Van Hoesen G. W. Alteration in the pattern of nerve terminal protein immunoreactivity in the perforant pathway in Alzheimer's disease and in rats after entorhinal lesions. Neurobiol Aging. 1992 Mar-Apr;13(2):283–291. doi: 10.1016/0197-4580(92)90041-u. [DOI] [PubMed] [Google Scholar]
- Duffy C. J., Rakic P. Differentiation of granule cell dendrites in the dentate gyrus of the rhesus monkey: a quantitative Golgi study. J Comp Neurol. 1983 Feb 20;214(2):224–237. doi: 10.1002/cne.902140210. [DOI] [PubMed] [Google Scholar]
- Flood D. G., Buell S. J., Horwitz G. J., Coleman P. D. Dendritic extent in human dentate gyrus granule cells in normal aging and senile dementia. Brain Res. 1987 Feb 3;402(2):205–216. doi: 10.1016/0006-8993(87)90027-8. [DOI] [PubMed] [Google Scholar]
- Geinisman Y., Morrell F., de Toledo-Morrell L. Axospinous synapses with segmented postsynaptic densities: a morphologically distinct synaptic subtype contributing to the number of profiles of 'perforated' synapses visualized in random sections. Brain Res. 1987 Oct 13;423(1-2):179–188. doi: 10.1016/0006-8993(87)90838-9. [DOI] [PubMed] [Google Scholar]
- Geinisman Y., deToledo-Morrell L., Morrell F., Persina I. S., Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992 Oct;2(4):437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Good M. J., Hage W. J., Mummery C. L., De Laat S. W., Boonstra J. Localization and quantification of epidermal growth factor receptors on single cells by confocal laser scanning microscopy. J Histochem Cytochem. 1992 Sep;40(9):1353–1361. doi: 10.1177/40.9.1506672. [DOI] [PubMed] [Google Scholar]
- Huber G., Matus A. Differences in the cellular distributions of two microtubule-associated proteins, MAP1 and MAP2, in rat brain. J Neurosci. 1984 Jan;4(1):151–160. doi: 10.1523/JNEUROSCI.04-01-00151.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley G. W., Rogers S. W., Moran T., Janssen W., Archin N., Vickers J. C., Cauley K., Heinemann S. F., Morrison J. H. Selective distribution of kainate receptor subunit immunoreactivity in monkey neocortex revealed by a monoclonal antibody that recognizes glutamate receptor subunits GluR5/6/7. J Neurosci. 1993 Jul;13(7):2965–2981. doi: 10.1523/JNEUROSCI.13-07-02965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman B. T., Penney J. B., Jr, Blackstone C. D., Young A. B. Localization of non-N-methyl-D-aspartate glutamate receptors in normal and Alzheimer hippocampal formation. Ann Neurol. 1994 Jan;35(1):31–37. doi: 10.1002/ana.410350106. [DOI] [PubMed] [Google Scholar]
- Hyman B. T., Van Hoesen G. W., Damasio A. R., Barnes C. L. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984 Sep 14;225(4667):1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Lambert J. D., Jones R. S. A reevaluation of excitatory amino acid-mediated synaptic transmission in rat dentate gyrus. J Neurophysiol. 1990 Jul;64(1):119–132. doi: 10.1152/jn.1990.64.1.119. [DOI] [PubMed] [Google Scholar]
- Magnusson K. R., Cotman C. W. Age-related changes in excitatory amino acid receptors in two mouse strains. Neurobiol Aging. 1993 May-Jun;14(3):197–206. doi: 10.1016/0197-4580(93)90001-r. [DOI] [PubMed] [Google Scholar]
- Maren S., Tocco G., Standley S., Baudry M., Thompson R. F. Postsynaptic factors in the expression of long-term potentiation (LTP): increased glutamate receptor binding following LTP induction in vivo. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9654–9658. doi: 10.1073/pnas.90.20.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E., Mallory M., Hansen L., DeTeresa R., Terry R. D. Quantitative synaptic alterations in the human neocortex during normal aging. Neurology. 1993 Jan;43(1):192–197. doi: 10.1212/wnl.43.1_part_1.192. [DOI] [PubMed] [Google Scholar]
- Meunier M., Bachevalier J., Mishkin M., Murray E. A. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci. 1993 Dec;13(12):5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H., Sprengel R., Schoepfer R., Herb A., Higuchi M., Lomeli H., Burnashev N., Sakmann B., Seeburg P. H. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992 May 22;256(5060):1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Anderson E., Lynch G. S., Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. 1986 Feb 27-Mar 5Nature. 319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Mufson E. J., Benzing W. C., Cole G. M., Wang H., Emerich D. F., Sladek J. R., Jr, Morrison J. H., Kordower J. H. Apolipoprotein E-immunoreactivity in aged rhesus monkey cortex: colocalization with amyloid plaques. Neurobiol Aging. 1994 Sep-Oct;15(5):621–627. doi: 10.1016/0197-4580(94)00064-6. [DOI] [PubMed] [Google Scholar]
- Nicoletti V. G., Condorelli D. F., Dell'Albani P., Ragusa N., Giuffrida Stella A. M. AMPA-selective glutamate receptor subunits in the rat hippocampus during aging. J Neurosci Res. 1995 Feb 1;40(2):220–224. doi: 10.1002/jnr.490400210. [DOI] [PubMed] [Google Scholar]
- Rapp P. R., Amaral D. G. Recognition memory deficits in a subpopulation of aged monkeys resemble the effects of medial temporal lobe damage. Neurobiol Aging. 1991 Sep-Oct;12(5):481–486. doi: 10.1016/0197-4580(91)90077-w. [DOI] [PubMed] [Google Scholar]
- Siegel S. J., Brose N., Janssen W. G., Gasic G. P., Jahn R., Heinemann S. F., Morrison J. H. Regional, cellular, and ultrastructural distribution of N-methyl-D-aspartate receptor subunit 1 in monkey hippocampus. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):564–568. doi: 10.1073/pnas.91.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel S. J., Janssen W. G., Tullai J. W., Rogers S. W., Moran T., Heinemann S. F., Morrison J. H. Distribution of the excitatory amino acid receptor subunits GluR2(4) in monkey hippocampus and colocalization with subunits GluR5-7 and NMDAR1. J Neurosci. 1995 Apr;15(4):2707–2719. doi: 10.1523/JNEUROSCI.15-04-02707.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges J., Herndon J. G., Rosene D. L. Mild age-related changes in the dentate gyrus of adult rhesus monkeys. Acta Anat (Basel) 1995;153(1):39–48. doi: 10.1159/000147713. [DOI] [PubMed] [Google Scholar]
- Wenthold R. J., Yokotani N., Doi K., Wada K. Immunochemical characterization of the non-NMDA glutamate receptor using subunit-specific antibodies. Evidence for a hetero-oligomeric structure in rat brain. J Biol Chem. 1992 Jan 5;267(1):501–507. [PubMed] [Google Scholar]
- Wiedenmann B., Franke W. W. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985 Jul;41(3):1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Witter M. P., Amaral D. G. Entorhinal cortex of the monkey: V. Projections to the dentate gyrus, hippocampus, and subicular complex. J Comp Neurol. 1991 May 15;307(3):437–459. doi: 10.1002/cne.903070308. [DOI] [PubMed] [Google Scholar]