Abstract
The human epidermal receptor (HER) family of receptor tyrosine kinases, including EGFR, HER2, HER3 and HER4, transduce growth promoting signals in response to ligand-binding to their extra cellular domains. This family is deregulated in numerous cancers, with mutations in EGFR and HER2 often serving as ‘driver’ events to activate key growth factor signaling pathways such as the RAS-ERK and PI3K-AKT pathways. Less attention has been paid to the oncogenic functions of HER3 due to its lack of intrinsic kinase activity. Recent work, however, has placed HER3 in the spotlight as a key signaling hub in several clinical contexts. First, HER3 has been shown to play a major role in mediating resistance to HER2 and PI3K pathway directed therapies due to its feedback regulation via AKT signaling. Second, activating mutations in HER3 have recently been identified in multiple cancer types, including gastric, colon, bladder, and non-small cell lung cancers. As a result, HER3 is now being examined as a direct therapeutic target. Absent a strong enzymatic activity to target, the focus has been on strategies to prevent HER3 activation including blocking its most relevant dimerization partner’s kinase activity (erlotinib, gefitinib, lapatinib), blocking its most relevant dimerization partner’s ability to dimerize with HER3 (trastuzumab, pertuzumab), and directly targeting the HER3 extracellular domain (MM-121, U3-1287, and LJM716).
Whereas drugs targeting EGFR and HER2 have proven effective even as single agents, the preclinical and clinical data on the antibodies directly targeting HER3 suggest more limited potential for single agent activity. Possible reasons for this include the lack of a suitable biomarker for activated HER3, the lack of potency of the antibodies, and the lack of relevance of HER3 for growth of some of the cancer types analyzed. Nevertheless, clear improvements in activity are being observed for many of these compounds when they are given in combination. In this snapshot, we will highlight the basis for HER3 activation in cancer, the different pharmacologic strategies being utilized, and opportunities for further development.
BACKGROUND
The v-erb-b2 erythroblastic leukemia viral oncogene (ErbB)/human epidermal receptor (HER) family of receptor tyrosine kinases (RTKs), consisting of HER1 (EGFR), HER2, HER3 and HER4, are key regulators of cell growth and differentiation. These receptors share a common domain structure consisting of an extra cellular ErbB ligand-binding domain, an intracellular tyrosine kinase domain, and an intracellular C-terminal tail with multiple tyrosine residues which, when phosphorylated, activate downstream signaling cascades. Members of this family interact with a variety of ligands. As its name suggests, EGFR has been shown to interact with epidermal growth factor (EGF) as well as other ligands including betacellulin (BTC), epigen (EPG), epiregulin (EPR), amphiregulin (AR), heparin binding EGF-like growth facor (HB-EGF) and transforming growth factor α (TGFα). HER3 has only been shown to interact with neuregulin (NRG)-1 and -2. HER4 can interact with all four neuregulins (NRG-1,-2,-3,-4), EPR, HB-EGF, and BTC. HER2 is distinct in having no known ligand and is thought to not require ligand for its activation. Deregulation of ErbB kinase activity has been strongly implicated in tumorigenesis with mutational activation of EGFR and HER2 frequently observed in a variety of cancer histologies. Members of the family, particularlyHER2 and EGFR, have made excellent therapeutic targets selectively in those tumors showing evidence of receptor activation (1). More recent attention has been placed on HER3 as a potential therapeutic target as there is mounting evidence for its frequent activation in RTK driven tumors.
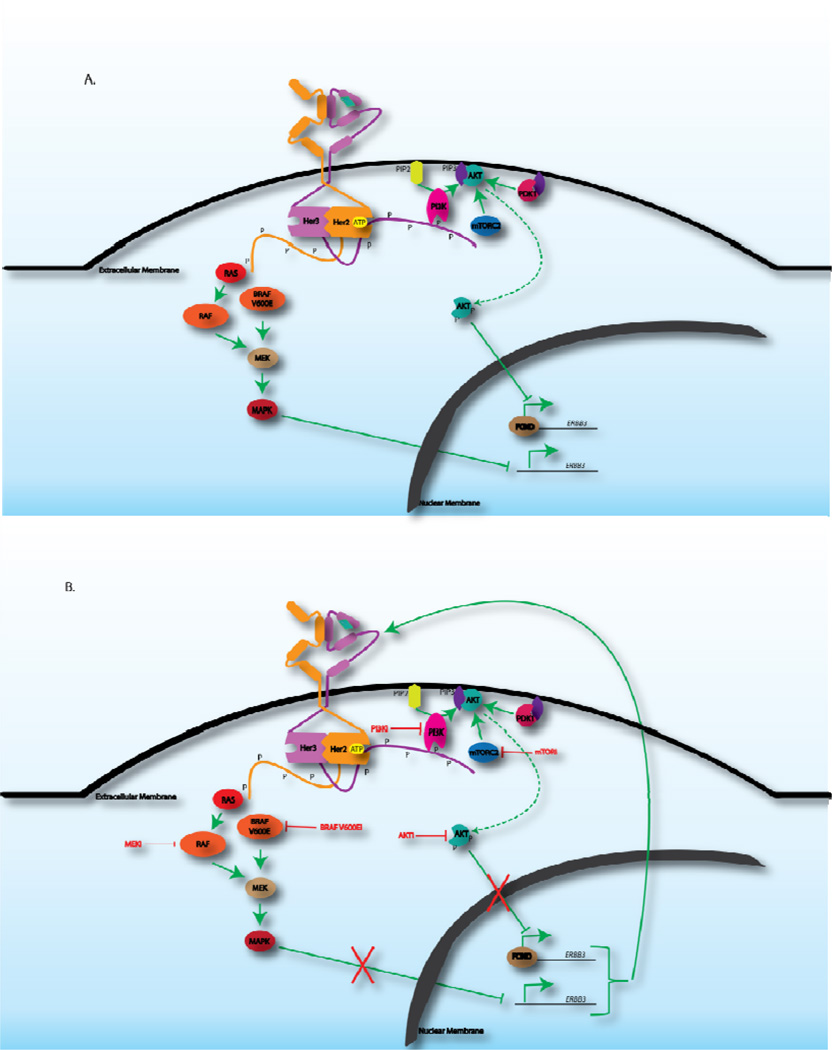
HER3 is distinguished from other ErbB family members by two attributes. First, HER3 lacks a functioning kinase domain (2). While Her3 is able to bind ATP (3), multiple lines of evidence demonstrate that it is catalytically impaired for the phospho-transfer reaction (2). This likely contributed to HER3 being somewhat ignored as a therapeutic target while multiple drugs against EGFR and HER2 have moved forward. Second, HER3 is a potent inducer of the phosphatidylinositol 3-kinase(PI3K)-protein kinase B (AKT) pathway through six consensus phosphotyrosine sites on its C-terminal tail which bind the PI3K p85 subunit (Figure 1 4–6). Binding of p85 to tyrosine phosphorylated HER3 induces PI3K activity which then potentiates multiple signals essential for the transformed phenotype, including activation of AKT (7). In many tumors it appears that HER3 functions as the major link between RTK and PI3K activation and thus, it has more recently gained attention as a selective means of inhibiting PI3K signaling in RTK-driven tumors.
Figure 1.
Negative feedback inhibition of HER3. A. The HER2-HER3 dimer potently activates both the PI3K-AKT and MAPK pathways, both of which result in inhibition of HER3 transcription. Activation of PI3K by phosphorylated (P) HER3 results in the PI3K-mediated conversion of PIP2 to PIP3. The production of PIP3 recruits AKT to the extracellular membrane where it can become phosphorylated by mTORC2 as well as PDK1. Phosphorylated AKT can then inhibit the FOXO family of transcription factors that activate RTK gene expression. Signaling through the MAPK pathway begins with RTK-mediated activation of RAS which signals downstream to RAF, MEK and eventually, MAPK. Specifically in the context of mutant V600E BRAF expression in thyroid cancer, it has been shown that MAPK signaling can negatively regulate transcription of HER3. B. Inhibition of the PI3K-AKT pathways, with either PI3K, AKT or mTOR inhibitors, as well as inhibition of the MAPK pathway with vemurafinib (BRAF V600E inhibitor) has been shown to result in transcriptional upregulation of HER3, eventually leading to reactivation of its downstream targets.
With the exception of HER2, ErbB family members are activated by ligand binding to the extracellular domain which promotes conformational changes that enable the receptors to homo- or heterodimerize. Dimerization is followed by allosteric activation of one dimer partner by the other, after which the activated receptor can phosphorylate the c-terminal tyrosine residues of its binding partner (8). The phosphotyrosine residues then bind and recruit proteins with SH2 domains as well as PTB binding proteins, eventually resulting in activation of downstream pathways. Due to its lack of kinase activity, HER3 cannot activate signaling within homodimers; however, in the presence of HER3 ligands, HER3 can promote the kinase activity of EGFR or HER2 and thereby induce phosphorylation of the HER3 C-terminal tail. In the absence of ligand(s), it is thought that the HER3 C-terminal tail acts in trans to block its activation domain, preventing HER3 from inappropriate activation by partner kinases (2). It should be noted that studies by Junttila and colleagues suggest a mechanism of ligand-independent HER2-HER3 dimerization in HER2 amplified cells. In this setting the abundance of HER2 forces a HER2-HER3 dimer, without the need for ligand, in a conformation distinct from that which is taken during ligand-dependent dimerization (9). While the HER2-HER3 dimer is the most potent HER family dimer, HER3 has been shown to dimerize with EGFR, as well as non-HER family members such as c-MET (10, 11). This complex mechanism of activation of ErbB family members presents multiple aspects that can be pharmacologically targeted including inhibition of ligand binding to the extracellular domain (ECD), inhibition of receptor dimerization, and inhibition of the partner tyrosine kinase activity, all of which have been exploited and proven to be successful in a variety of oncologic contexts.
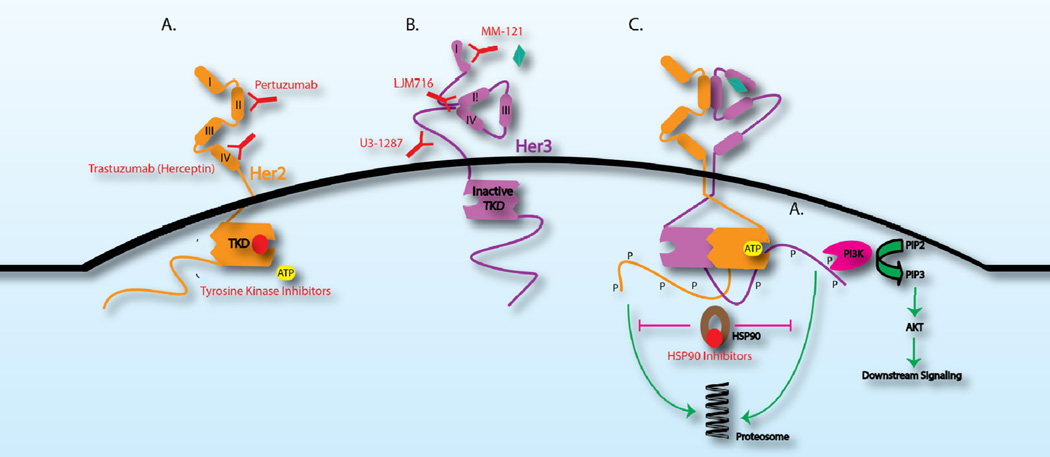
The essential function of HER3 in linking RTK and PI3K activation has been most readily demonstrated in the context of HER2 amplified breast cancer. Within such tumors, the HER2-HER3 dimer has been shown to be essential for tumor formation and tumor maintenance (12–15). The significance of this finding has been most powerfully illustrated by the benefit of targeting the HER2-HER3 dimer using the HER2 targeting antibodies, trastuzumab and pertuzumab. While trastuzumab promotes several antitumor actions including ADCC, a major portion of its action is to block dimers between HER2 and HER3 that occur in the absence of ligand by binding to domain IV of the HER2 extracellular domain (9, 16) (Figure 2). Meanwhile, pertuzumab acts almost exclusively through its blockade of ligand dependent HER2-HER3 dimers by binding to domain II of the HER2 extracellular domain (9, 17) (Figure 2). Preclincally, the combination of these two antibodies has been shown to be more potent at downregulating HER3-PI3K signaling than either alone (18), which has translated to an increased response rate and overall survival for administration of the combination (19). The studies on this combination have largely focused on HER2+ breast cancers; however, the potential exists for effective blockade of HER3 activity using this doublet in other tumor types driven by HER2 (20).
Figure 2.
Multiple modes of inhibiting HER3 pathway activation. Inhibitors are depicted in red. A. Targeted therapy against HER2 prevents HER2-dependent activation of HER3. Pertuzumab, a HER2 monoclonal antibody, binds domain II of the HER2 ECD and prevents ligand-dependent dimerization between HER2 and HER3. Trastuzumab binds domain IV of the HER2 ECD and prevents ligand-independent dimerization between HER2 and HER3 in the context of HER2-amplified breast cancer. HER2 tyrosine kinase inhibitors, including lapatinib, afatinib and neratinib, bind the tyrosine kinase domain (TKD), compete with ATP-binding and prevent phosphorylation (P) of the HER3 C-terminal tail. B. The monoclonal HER3 antibody, MM-121 prevents ligand binding while LJM716 specifically binds an epitope created by ECD domains II and IV in the HER3 closed conformation. U3-1287 is another HER3 monoclonal antibody that binds the extracellular domain. C. HSP90, a chaperone protein, inhibits the proteosome-mediated degradation of phosphorylated HER2 and HER3. Inhibitors of HSP90, therefore, promote RTK degradation.
The significance of HER3 in HER2 driven tumors has been further underscored by multiple studies implicating upregulation of HER3 in resistance to HER2 targeted therapy. Moreover, these studies have shown that upregulation of HER3 may promote resistance to a number of signaling inhibitors that are designed to directly or indirectly antagonize activated PI3K signaling (21–25). As growth factor signaling is physiologically regulated by negative feedback, mutationally activated growth factor signaling seen in tumors is associated with elevated levels of negative feedback. The consequence is that, in the steady state, the tumors feature suppression of upstream signals such as RTKs. We and others have observed that inhibitors of oncoprotein-driven signaling pathways cause‘relief’ of this elevated feedback. This ‘relief of feedback’ is manifested as increased expression and activity of RTKs that themselves have oncogenic functions. The induced RTK activity can function as a mechanism of resistance to the drug. Among RTKs, HER3 is very frequently observed as an RTK induced in this setting (25–27) as it seems to be a key node for feedback regulation of PI3K/AKT signaling in most cells as well as MEK/ERK signaling in more select cellular contexts.
The role of HER3 in feedback regulation of PI3K signaling emerged from work done by the Moasser group showing that inhibition of HER2-HER3 signaling by EGFR/HER2 tyrosine kinase inhibitors (TKIs) caused only transient downregulation of HER3 phosphorylation. A rebound in Her3 activity was demonstrated to occur and mediate resistance to EGFR/HER2 TKIs (26). We examined feedback regulation of PI3K/AKT signaling by studying the effects of AKT inhibition upon RTK activation and found multiple RTKs (HER2, IGF-1R, and insulin receptor) to be transcriptionally regulated by AKT signaling (21). Notably, these RTKs are known to be powerful activators of PI3K/AKT signaling, pointing to a true feedback response. These effects on HER3 were also predictably noted in examining the effects of an mTOR kinase inhibitor (22)and PI3K inhibitor (23) both of which result in AKT inhibition. Thus, various mechanisms that result in AKT inhibition subsequently result in a FOXO dependent induction of transcription of HER3 (21–23, 25–27) (Figure 1). Beyond the induction of HER3 expression, it has become clear that other mechanisms contribute to HER3 activation in response to PI3K/AKT inhibition including effects on HER3 localization (26) and on expression of HER3 ligands. Thus, inhibition of PI3K/AKT signaling serves as a powerful stimulus to drive HER3 activation and this may subsequently promote reactivation of PI3K/AKT signaling or other growth factor signaling cascades, such as the Ras pathway. In several of these studies, it was shown that blocking the induced HER3 via RNAi, antibodies, EGFR/HER2 kinase inhibitors, or even HSP90 inhibitors could significantly improve the antitumor effects (21–24, 26, 27).
While the aforementioned studies focused on feedback regulation of PI3K/AKT signaling in tumors featuring PI3K pathway activation, HER3 was also implicated in feedback regulation of MEK/ERK signaling in the specific context of BRAF V600E driven thyroid cancer. Montero-Conde and colleagues noted that treatment of such tumors with the RAF inhibitor vemurafinib only led to very transient inhibition of downstream targets such as pMEK and pERK with concomitant induction of several RTKs including HER2 and HER3 (28) (Figure 1). This study along with others in BRAF driven tumors suggested that combined inhibition of RAF/MEK along with ErbB kinases (that can activate the feedback induced HER3) led to superior antitumor effects (29, 30). What is less clear from these studies is the specific role of HER3 activation in mediating resistance as blockade of EGFR or HER2 may reasonably be enough to augment inhibition of RAS/RAF/MEK signaling irrespective of blocking HER3 activation. Further studies on selective HER3 ablation in this context will be needed to tease out the function of HER3 in these tumors.
A function for HER3 in tumor initiation and maintenance has been somewhat elusive until recently. Several studies examined steady state tumoral expression of HER3 (reviewed in31) and found little meaningful correlation with prognosis. Indeed, it does not seem that there is a clear correlation between levels of HER3 expression in tumor cell lines and its function in proliferation. Sheng and colleagues analyzed the effects of RNAi against Her3 in multiple ovarian cancer cell lines and found that HER3 knockdown only curtailed the proliferation of cell lines in which HER3 was activated despite relatively equal levels of HER3 protein expression between cell lines (32). More recently, evidence has begun to emerge on possible activation of HER3 through mutations mainly in the extracellular domain (33–39). Jaiswal and colleagues surveyed multiple cancers and found recurrent mutations in the ERBB3 coding region that had transforming capabilities in vivo and in vitro (40). The authors found the ERBB3 mutations in 12% and 11% of gastric and colon cancers, respectively, and also found the mutations in 1% NSCLC. Most of the recurrent mutations are within in the ECD of HER3(with a few located in the kinase domain), however the mechanism(s) by which the ECD mutations activate HER3isunknown. The authors speculate that the ECD mutations may promote an untethered, open conformation for HER3 that is primed for dimerization and activation, but further structural studies are needed to support such a hypothesis. It is important to note that none of the HER3 mutations remove the need for an active kinase to phosphorylate HER3, and therefore, it remains relevant to consider drugs that inhibit dimerization or drugs that inhibit the partner kinase as means to therapeutically target the HER3 mutants. In fact, the authors show that cells expressing both the HER3 mutants and HER2 can be effectively arrested by antibodies targeting the ECD of HER2orHER3, as well as with anEGFR/HER2 TKI or a PI3K inhibitor. The functional significance of the kinase domain mutants is less clear. The authors speculate that these mutations may enhance phosphotransferase activity to the typically inactive HER3 kinase domain or may promote a new conformation that promotes HER3 dimerization with partner kinases; however there has yet to be data confirming either of these hypotheses. In fact, under the assays conducted by the authors, they failed to see an increase in kinase activity by the kinase domain mutants.
CLINICAL-TRANSLATIONAL ADVANCES
Given the key roles for HER3 in both serving as a conduit between RTK activation and the PI3K pathway and in functioning as a feedback regulator that can contribute to resistance to PI3K/AKT directed therapy across a wide variety of tumors, interest in drugging this receptor has been very high. However, targeting this protein has proven to be a challenge as HER3 lacks enzymatic activity. Therefore, attention has been placed on targeting the HER3 extracellular domain through antibodies. The various antibodies being developed have unique ways of inhibiting HER3 pathway activation reflecting the various mechanisms by which HER3 can become activated. For example, MM-121, a human anti-HER3 monoclonal antibody, is thought to compete with ligand binding and specifically prevent ligand-dependent HER3-heterodimerization (41). LJM716, another monoclonal antibody, is selective for an epitope created by domains II and IV of the Her3 ECD (42) (Figure 2). These two domains are specifically responsible for mediating the closed and inactive conformation of HER3 (3). In theory, LJM716 can lock HER3 in a closed conformation and thereby prevent both ligand-dependent and ligand-independent activation (42). The mechanism of action of U3-1287, another monoclonal antibody targeting the HER3 ECD, is less clear; however it does seem to downregulate HER3 expression, possibly through increased endocytosis of the antibody-bound HER3 protein (43).
Another challenge in the attempt to therapeutically target HER3 is that there is no effective biomarker to define HER3 activation in patients. This issue has proven to be a difficult hurdle for effective patient selection. Consideration has been given to markers for activation/overexpression of partner kinases, such as HER2, as assayed by immunohistochemistry of tumor biopsies, markers for the expression levels of HER3, and even markers for the expression levels of ligands that activate HER3. For example, the researchers who developed MM-121quantified expression levels of NRG-1 and BTC to distinguish cell lines that would respond to MM-121 between those that would not using computational modeling. Their data suggests that the use of ligand expression as a biomarker has the potential to identify a select group of patients where MM-121 might prove to be effective over the use of receptor expression as biomarkers (44). However, while their computational modeling data implicates BTC and NRG-1 as the most potent inducers of phosphorylated HER3, it does not exclude the potential for other HER family ligands to continue to be relevant in the clinical setting. Additionally, ligand expression is likely quite limited as an effective biomarker as multiple other routes to HER3 activation exist, such as mutation and ligand-independent dimers. Therefore, the presence of activated HER3 may be an even more powerful biomarker for efficacy than the presence of ligand alone. As such, a caveat for all of the compounds targeting HER3 in the clinic is that it is unknown how well patients with bona fide HER3 activated/driven disease may fare on a HER3 selective therapy.
While multiple Phase I and Phase II trials have been opened for various HER3 targeted antibodies, clinical benefits of the antibodies as single agents have not been reported. The lack of early signals of single agent activity suggests a revisiting of the preclinical data to ascertain if this is a case of a limited target, limited set of drugs, or limited biomarkers to match the right patients and drugs. Models of HER2+ breast cancer are an obvious genotype to assess the efficacy of HER3 targeted therapy as the importance of HER3 has been very well defined in this context. Activated HER2 can drive HER3 signaling in both ligand-dependent and ligand-independent manners and thus anti-HER3 therapy may need to inhibit both of these dimer types. In this regard, LJM716 stands out as it was designed to inhibit both ligand-dependent and ligand-independent activation. In support of this type of activity, LJM716 has shown growth inhibitory effects inHER2+ and NRG-1-expressing models as measured by cell proliferation assays as well as xenograft models (42, 45) with over 80% growth inhibition in HER2+ BT474 xenografts. However, thus far, LJM716 has mainly been demonstrated to have tumor regression efficacy in vivo against ligand driven models like FaDu (42).
Preclinical data on U3-1287showsmore limited ability to induce tumor regressions as a single agent in xenograft studies featuring bothHER2+ and NRG-1-driven models, including A459 and FaDu cells (46, 47). With the A459 model, U3-1287 was able to achieve tumor stasis; however with the FaDu model the antibody only partially inhibited growth of the tumor cells potentially indicating increased effectiveness in HER2+/EGFR+ amplified models over NRG-1-driven models. Interestingly, MM-121 showed little benefit in HER2+ models suggesting HER2 amplification as a mechanism of resistance to antibodies, such as MM-121, which are specifically relevant in competing away ligand-binding (44). MM-121 did significantly reduce tumor growth in multiple xenograft models including ovarian (OVCAR8), prostate (DU145) and kidney (ACHN) cancer but did not lead to tumor regressions (32, 41, 44). In these contexts, the lack of tumor regression observed may have been attributable to a lack dependence of the model on HER3 or a lack of potency of the compound against activated HER3. Sheng and colleagues attempted to address this key issue by characterizing the importance of a neuregulin/Her3 autocrine loop in certain models of ovarian cancer (32). They showed that both MM-121 as well as HER3-targeted shRNA slowed growth of OVCAR8 cells in vitro and in vivo. One of the shRNAs could indeed induce regression whileMM-121 treatment did not appear to do so suggesting that single-agent treatment with MM-121 may be insufficient in the clinical setting despite the importance of the Her3/neuregulin loop in select tumors types.
Given the limited single agent activity of the HER3 targeting antibodies and the key role of HER3 as a mechanism of feedback regulation and resistance, therapeutic targeting of HER3 in combination with primary drivers of the tumor has been evaluated in multiple contexts. For instance, we showed that combining an AKT inhibitor with either lapatinib or an inhibitor against the chaperone protein HSP90 prevented the feedback-mediated induction of phosphorylated HER3 and led to significantly greater anti-tumor effects than did either drug alone in BT474 xenografts (21). Even though the use of lapatinib or the HSP90 inhibitor provide indirect inhibition of HER3 (Figure 2), their effectiveness in combination with the AKT inhibitor validates the idea that targeted therapy against HER3 will prove to be synergistic with multiple PI3K and possibly even MAPK pathway inhibitors. Recently, more direct evidence has come in the form of a study examining the benefit of LJM716 in combination with trastuzumab and lapatinib. In this setting, feedback induction of HER3 would be expected to limit the efficacy of the anti-HER2 antibody doublet. Addition of LJM716 was shown to significantly improve survival of mice when added to the doublet and synergistically induce cell death when given in combination with the PI3K inhibitor, BYL719 (45). These data support the addition of a pure HER3 antagonist to a disease where HER3 is well established to mediate the driver oncoprotein signal and also be the key node of feedback regulation.
Overall, the data on the activity of HER3 selective drugs may be viewed as modest. Despite several reagents developed to target the protein, tumor regressions in mice and single agent activity in humans has not been robustly demonstrated. There appears to be more than one reason for this. First, unlike other RTKs that have been successfully targeted in the clinic, HER3 is predominantly serving as a scaffold with little enzymatic activity. Antibody targeting has the potential to lower surface expression and block selected sets of dimers, but is less likely to achieve rapid and potent down regulation. Second and perhaps more significantly, the lack of a suitable biomarker for activated HER3 remains a major hurdle in moving forward with HER3 targeted therapy. Most of the studies on the HER3 antibodies thus far have focused on tumors with either HER2 amplification or neuregulin expression, both of which are only surrogates for actual HER3 activity. Fortunately, there exist modalities that may enable us to better gauge where HER3 is activated. For example, using either the Collaborative Enzyme Enhanced Reactive (CEER) immunoassay (Prometheus) or reverse-phase protein micro arrays (RPPA) with antibodies against phosphorylated HER3, we may be able to quantitate levels of active HER3 in patient samples. Additionally, the development of radioactively or fluorescently labeled probes against activated HER3 may facilitate a noninvasive method for ascertaining where the receptor is important. Beyond these methods, a more rigorous assessment of genetic contexts where we anticipate HER3 to be essential (HER3 ECD mutants, HER2 amplification, in combination with PI3K inhibitors in PI3K driven tumors) is likely to prove fruitful.
Acknowledgments
Funding: SC is funded by NIH K08CA134833 and a Damon Runyon Clinical Investigator Award
Footnotes
Conflict of interests: Sarat Chandarlapaty consults for GlaxoSmithKline. The authors declare no other conflict of interests
REFERENCES
- 1.Tebbutt N, Pedersen MW, Johns TG. Targeting the ERBB family in cancer: couples therapy. Nature reviews Cancer. 2013;13:663–673. doi: 10.1038/nrc3559. [DOI] [PubMed] [Google Scholar]
- 2.Jura N, Shan Y, Cao X, Shaw DE, Kuriyan J. Structural analysis of the catalytically inactive kinase domain of the human EGF receptor 3. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21608–21613. doi: 10.1073/pnas.0912101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho HS, Leahy DJ. Structure of the extracellular region of HER3 reveals an interdomain tether. Science. 2002;297:1330–1333. doi: 10.1126/science.1074611. [DOI] [PubMed] [Google Scholar]
- 4.Fedi P, Pierce JH, di Fiore PP, Kraus MH. Efficient coupling with phosphatidylinositol 3-kinase, but not phospholipase C gamma or GTPase-activating protein, distinguishes ErbB-3 signaling from that of other ErbB/EGFR family members. Molecular and cellular biology. 1994;14:492–500. doi: 10.1128/mcb.14.1.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soltoff SP, Carraway KL, 3rd, Prigent SA, Gullick WG, Cantley LC. ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Molecular and cellular biology. 1994;14:3550–3558. doi: 10.1128/mcb.14.6.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prigent SA, Gullick WJ. Identification of c-erbB-3 binding sites for phosphatidylinositol 3'-kinase and SHC using an EGF receptor/c-erbB-3 chimera. The EMBO journal. 1994;13:2831–2841. doi: 10.1002/j.1460-2075.1994.tb06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339–345. [PubMed] [Google Scholar]
- 8.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–1149. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 11.Choi BK, Fan X, Deng H, Zhang N, An Z. ERBB3 (HER3) is a key sensor in the regulation of ERBB-mediated signaling in both low and high ERBB2 (HER2) expressing cancer cells. Cancer medicine. 2012;1:28–38. doi: 10.1002/cam4.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaught DB, Stanford JC, Young C, Hicks DJ, Wheeler F, Rinehart C, Sanchez V, Koland J, Muller WJ, Arteaga CL, et al. HER3 is required for HER2-induced preneoplastic changes to the breast epithelium and tumor formation. Cancer research. 2012;72:2672–2682. doi: 10.1158/0008-5472.CAN-11-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alimandi M, Romano A, Curia MC, Muraro R, Fedi P, Aaronson SA, et al. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 1995;10:1813–1821. [PubMed] [Google Scholar]
- 14.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer research. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 15.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vu T, Claret FX. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Frontiers in oncology. 2012;2:62. doi: 10.3389/fonc.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer cell. 2002;2:127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 18.Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer research. 2004;64:2343–2346. doi: 10.1158/0008-5472.can-03-3856. [DOI] [PubMed] [Google Scholar]
- 19.Verma S, Joy AA, Rayson D, McLeod D, Brezden-Masley C, Boileau JF, et al. HER Story: The Next Chapter in HER-2-Directed Therapy for Advanced Breast Cancer. The oncologist. 2013;18:1153–1166. doi: 10.1634/theoncologist.2013-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Mello RA, Marques AM, Araujo A. HER2 therapies and gastric cancer: A step forward. World journal of gastroenterology : WJG. 2013;19:6165–6169. doi: 10.3748/wjg.v19.i37.6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E, et al. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer discovery. 2011;1:248–259. doi: 10.1158/2159-8290.CD-11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakrabarty A, Sanchez V, Kuba MG, Rinehart C, Arteaga CL. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2718–2723. doi: 10.1073/pnas.1018001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30:2547–2557. doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amin DN, Sergina N, Ahuja D, McMahon M, Blair JA, Wang D, et al. Resiliency and vulnerability in the HER2-HER3 tumorigenic driver. Science translational medicine. 2010;2:16ra7. doi: 10.1126/scitranslmed.3000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sanchez V, Chakrabarty A, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5021–5026. doi: 10.1073/pnas.1016140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montero-Conde C, Ruiz-Llorente S, Dominguez JM, Knauf JA, Viale A, Sherman EJ, et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer discovery. 2013;3:520–533. doi: 10.1158/2159-8290.CD-12-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abel EV, Basile KJ, Kugel CH, 3rd, Witkiewicz AK, Le K, Amaravadi RK, et al. Melanoma adapts to RAF/MEK inhibitors through FOXD3-mediated upregulation of ERBB3. The Journal of clinical investigation. 2013;123:2155–2168. doi: 10.1172/JCI65780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fattore L, Marra E, Pisanu ME, Noto A, de Vitis C, Belleudi F, et al. Activation of an early feedback survival loop involving phospho-ErbB3 is a general response of melanoma cells to RAF/MEK inhibition and is abrogated by anti-ErbB3 antibodies. Journal of translational medicine. 2013;11:180. doi: 10.1186/1479-5876-11-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell MR, Amin D, Moasser MM. HER3 comes of age: new insights into its functions and role in signaling, tumor biology, and cancer therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:1373–1383. doi: 10.1158/1078-0432.CCR-09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheng Q, Liu X, Fleming E, Yuan K, Piao H, Chen J, et al. An activated ErbB3/NRG1 autocrine loop supports in vivo proliferation in ovarian cancer cells. Cancer cell. 2010;17:298–310. doi: 10.1016/j.ccr.2009.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeong EG, Soung YH, Lee JW, Lee SH, Nam SW, Lee JY, et al. ERBB3 kinase domain mutations are rare in lung, breast and colon carcinomas. International journal of cancer Journal international du cancer. 2006;119:2986–2987. doi: 10.1002/ijc.22257. [DOI] [PubMed] [Google Scholar]
- 36.Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–873. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- 37.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.TCGA. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.TCGA. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaiswal BS, Kljavin NM, Stawiski EW, Chan E, Parikh C, Durinck S, et al. Oncogenic ERBB3 mutations in human cancers. Cancer cell. 2013;23:603–617. doi: 10.1016/j.ccr.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Schoeberl B, Pace EA, Fitzgerald JB, Harms BD, Xu L, Nie L, et al. Therapeutically targeting ErbB3: a key node in ligand-induced activation of the ErbB receptor-PI3K axis. Science signaling. 2009;2:ra31. doi: 10.1126/scisignal.2000352. [DOI] [PubMed] [Google Scholar]
- 42.Garner AP, Bialucha CU, Sprague ER, Garrett JT, Sheng Q, Li S, et al. An Antibody That Locks HER3 in the Inactive Conformation Inhibits Tumor Growth Driven by HER2 or Neuregulin. Cancer research. 2013;73:6024–6035. doi: 10.1158/0008-5472.CAN-13-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garrett JT, Sutton CR, Kuba MG, Cook RS, Arteaga CL. Dual blockade of HER2 in HER2-overexpressing tumor cells does not completely eliminate HER3 function. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:610–619. doi: 10.1158/1078-0432.CCR-12-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoeberl B, Faber AC, Li D, Liang MC, Crosby K, Onsum M, et al. An ErbB3 antibody, MM-121, is active in cancers with ligand-dependent activation. Cancer research. 2010;70:2485–2494. doi: 10.1158/0008-5472.CAN-09-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garrett JT, Sutton CR, Kurupi R, Bialucha CU, Ettenberg SA, Collins SD, et al. Combination of Antibody That Inhibits Ligand-Independent HER3 Dimerization and a p110alpha Inhibitor Potently Blocks PI3K Signaling and Growth of HER2+ Breast Cancers. Cancer research. 2013;73:6013–6023. doi: 10.1158/0008-5472.CAN-13-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mol Cancer Ther Meeting Abstr. 2009:B161–B171. [Google Scholar]
- 47.Mol Cancer Ther Abstr. 2011:A182. [Google Scholar]