Abstract
Purpose
This study was conducted to compare the efficacy and safety of naproxen 500 mg twice daily (BID) versus naproxen 500 mg as needed (PRN) for treatment of ankle sprain.
Methods
In this seven-day, randomized, parallel group trial, 135 patients with ankle sprain occurring less than 48 hours prior to the first dose of study medication were randomized to receive naproxen 500 mg BID (67 patients) and naproxen 500 mg as needed (PRN) (68 patients). The ankle pain was assessed at rest and on full weight bearing using Numeric Rating Scale (NRS) from 0 (no pain) to 10 (the worst imaginable pain). Ankle swelling was assessed as a 4-point scale ranging from 0 (no swelling) to 3 (severe swelling) rated by the investigator. The primary efficacy end point was the patient's assessment of ankle pain via NRS and the degree of swelling on day seven.
Results
Results showed a significant decrease in pain on weight bearing, pain at rest and the extent of swelling (P<0.001) in both groups, but there was no substantial difference between the two groups (P>0.05) after seven days. Assessing the safety profile of the two different dosing, 13.3% of the naproxen BID group and 6.7% of the as needed group had adverse events, showing that the as needed regimen was safer (P<0.001).
Conclusion
Results showed that naproxen as needed may reduce the pain and edema of the sprained ankle with no significant difference compared to the BID regimen, while it possesses better safety profile and lower total drug use.
Keywords: Ankle Sprain, Naproxen, Pain, Non-steroidal Anti-inflammatory Agents
INTRODUCTION
Ankle sprains are among the most common musculoskeletal injuries that occur in everyday life and sports related activities [1–3]. Approximately one ankle sprain occurs per 10000 person everyday [2, 4–6]. Inadequate and incorrect management of ankle sprain can result in prolonged complications such as decreased range of motion, chronic pain, early degenerative bony changes and chronic joint instability [7]. Ankle sprains are classified as mild (first-degree), moderate (second-degree), or severe (third-degree) according to the extent of pain, swelling, tenderness, joint instability, ecchymosis, functional loss and difficulty in walking [8–10].
Initial management goals are to limit inflammation and swelling and to restore normal function as much as possible [11]. Conventional treatment for ankle sprains includes the ‘rest, ice, elastic compression and limb elevation’ (RICE) protocol, protected weight bearing, early ambulation and use of analgesic and anti-inflammatory drugs [6, 8]. Non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, naproxen, diclofenac and piroxicam effectively reduce the pain and swelling associated with sprains [6–12]. No particular NSAID has superiority over the others for the treatment of ankle sprain [8]. NSAIDs also inhibit platelet aggregation, which has an important role in healing of the wound and gradual fading of the ecchymosis [10]. However, these medications have several adverse effects such as gastrointestinal upset, gastric and duodenal ulceration, perforation and hemorrhage [11]. NSAIDs’ adverse effects increase with upper dosage and duration of use. To the best of our knowledge, none of the published studies have compared the regular versus intermittent (as needed) dosing of such medications [7].
The aim of this study was to evaluate the efficacy and safety of the “as needed” dosing of naproxen compared to the twice daily dosing in the management of grade 1 and 2 acute ankle sprain.
METHODS AND SUBJECTS
This was an open label, randomized, outpatient, active-controlled, parallel-group, clinical trial conducted at emergency department (ED) of Imam hospital, Tehran, a general teaching hospital with an annual census of 40,000 visits. The study was conducted between May 2009 and October 2010. Inclusion criteria were as follows: the patients were aged ≥ 18 years and presented with an isolated unilateral mild to moderate soft tissue injury of the ankle (first- or second-degree ankle sprain), which had happened 48 hours prior to the first dose of medication. Also, the patients were included if they mentioned pain score of 4 or above on a verbally administered, 11-point numeric rating scale (NRS). All women of child bearing age had to have a negative urine pregnancy test, and not to be breast feeding.
Patients were excluded if any of the following criteria were present: preexisting ankle problems (including osteoarthritis, fracture, sprain, congenital deformity); a similar injury of the same joint within the past six months, presence of bilateral ankle sprain, third-degree sprain or ipsilateral knee injury; radiographic evidence of fracture or syndesmosis injury; known history of significant renal impairment (creatinine level >160 µmol/L) or hepatic insufficiency (aspartate aminotransferase >54 U/L or alanine aminotransferase >42 U/L); lower limb thrombosis; diabetes mellitus; chronic or toxic alcohol ingestion; known severe congenital or acquired coagulopathy; active gastrointestinal disease; history of esophageal, gastric or duodenal ulcer; sensitivity or allergy to NSAIDs; treatment with an intra-articular injection of a corticosteroid or hyaluronic acid in any joint within eight weeks of the first dose of study medication; treatment with any oral or intramuscular corticosteroid within 30 days of the first dose of study medication; use of non-COX-2 selective NSAIDs, COX-2 selective inhibitors (except aspirin ≤ 325 mg/day for cardiovascular prophylaxis), or other medications such as neuroleptics, tricyclic antidepressants, and lithium that could potentially confound the assessment of analgesia within 24 hours of the first dose of study drugs; pregnancy; history of current or past psychiatric disorders; or inability to return for follow-up.
All patients were informed of the nature and potential risks of the study and they provided written informed consent to participate. The research protocol was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Immediately after arrival at the ED, the patients were visited by research associates (emergency medicine residents or attending physicians). Before enrollment, patients underwent a baseline assessment including history and physical examination. The extent of ankle swelling, pain, and stability were evaluated by the same physician: The ankle pain was assessed at rest and on full weight bearing using NRS from 0 (no pain) to 10 (the worst imaginable pain). Ankle swelling was assessed as a 4-point scale ranging from 0 (no swelling), to 3 (severe swelling). In order to assess joint stability, clinical tests including ankle squeeze test (indicator of injury to anterior talo-fibular ligament), external rotation stress test (representing injury to syndesmosis), anterior drawer test and talar tilt test were performed. Based on the above parameters on physical examination, investigators classified the ankle sprain as first-degree, second-degree or third-degree. First-degree ankle sprain is defined as partial tearing of the lateral ligaments complex, while the stability and the structure of the joint are completely preserved. On physical examination, the pain on weight bearing is little and not severe, thus the patient has full weight bearing; while the edema is rare and ecchymosis does not occur. In second-degree ankle sprain, tearing of the anterior talo-fibular ligament occurs, while the calcaneo-fibular ligament is preserved. In addition to point tenderness and partial instability of the joint, the pain is moderate to severe on weight bearing. Ecchymoses occur frequently and the edema is also moderate to severe. In third-degree ankle sprain, the patient is disabled while the joint capsule is disintegrated and the joint is unstable. The reliability of this clinical grading system for the sprained ankle has been previously approved [2]. After initial assessment, inclusion and exclusion criteria were applied and eligible patients were enrolled. Baseline data were collected (including patients’ name, gender, age, occupation, mechanism of injury, and time since the injury occurred) and recorded in the first visit form.
After completing assessment, the research associates randomly assigned patients to one of the two treatment groups to receive either naproxen 500 mg twice daily (BID) or naproxen 500 mg as needed (pro re nata, PRN) for pain but not more than twice daily. Block randomization was applied using a computer generated random sequence of numbers in 4-digit blocks. Treatment duration was seven days, after which the patients were re-assessed for pain severity and ankle swelling as well as for occurrence of any adverse events by the same physician. Both groups received 14 naproxen tablets (Iran Najo Co., pharmaceutical hygienic and cosmetic, Tehran, IR Iran) at the first visit and were asked to return the remaining pills for the follow up visit. They were instructed when and how to use the drug according to the study protocol and were asked not to use other analgesic medications during the study period. The ‘rest, ice, and limb elevation’ protocol was recommended for all patients. For immobilization, a short-leg splint was applied for all patients. Patients were requested to keep their splints until the follow-up visit and elevate the injured leg for 48 hours. Non-weight bearing was recommended until they could walk with a normal gait and no pain.
In the follow-up visit, set on day seven, assessments by the same clinician included ankle pain scores at rest and on full weight bearing, and ankle swelling. Participants were enquired about the adverse effects of the study medications. With regard to adverse effects of naproxen, prevalent major and minor adverse effects such as gastrointerstinal bleeding or upset were investigated. Data were recorded in the follow-up visit form. The patient's compliance was calculated using this formula:
Where “n” was the number of pills returned.
The primary measures of efficacy included changes in pain during weight bearing and at rest from baseline visit to the follow-up visit and changes in ankle swelling. Secondary measures were the proportion of patients whose pain score decreased by at least two on the NRS at the follow-up visit, as well as the rate of adverse events.
The sample size of about 67 patients for each group was determined according to the day seven rest pain NRS score (difference of less than two) to show comparable clinical effect of two different dosing schedules.
Data were analyzed by SPSS Windows 10.0.5 (SPSS, Chicago, IL). Significance level was considered to be < 0.05. Parametric Student's t-test was used to compare the characteristics of the patients in the two groups. The Mann–Whitney test compared differences in ankle swelling and the paired t-test was used to compare differences in rating of pain at rest and on weight-bearing within each group and between day 0 and 7. Differences in the rate of drug adverse events between the two groups were compared using Fisher's exact test.
Since the medication (i.e. naproxen) was the same in both groups and the study intervention was different dosing of the medications, the patients could not be blinded to the study intervention. Before the allocation of a patient to a study protocol his or her data were collected and recorded, so blinding of the investigator was not needed for the first visit. For the follow-up visit, the parameters were assessed with the same, most of which were objective measures.
RESULTS
Subjects
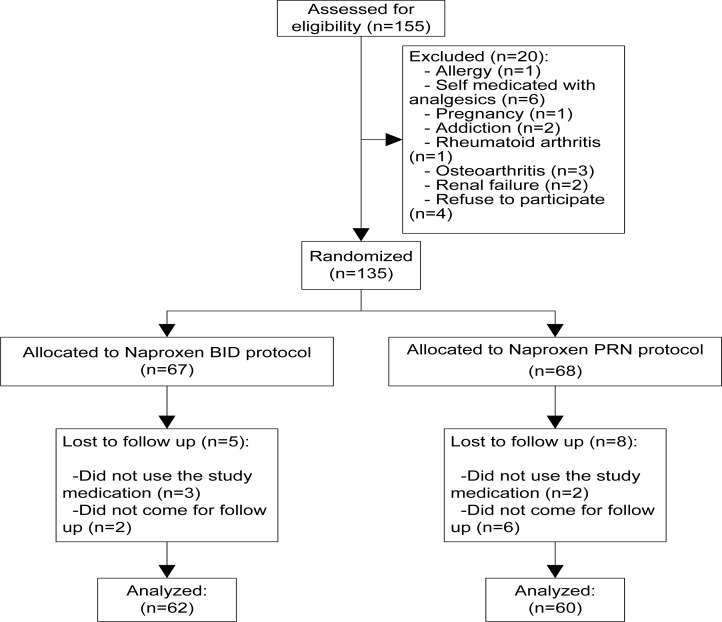
Of the 155 patients screened, 135 were considered eligible for enrollment and were randomized to receive either naproxen 500 mg BID (67 patients) or naproxen 500 mg as needed (PRN) for pain (68 patient). Five patients from BID group and eight patients from PRN group did not take the study medication or did not refer for follow up and eventually 122 patients completed the study protocol and were included in our analysis (Fig. 1).
Figure 1.
The sampling scheme of participants
Baseline characteristics
There was no significant difference in baseline characteristics between the two study groups (Table 1). According to Table 2, there was no significant difference between the two groups in baseline pain score measured through NRS at rest, baseline pain in daily activities (on weight bearing) and ankle swelling.
Table 1.
Baseline characteristics of the participants
| Baseline characteristics | BID group | PRN group |
|---|---|---|
| Age (Mean ± SD) | 29.8 (10.7) | 34.08 (15.07) |
| Sex (male%) | 40 (64.5) | 33 (55) |
| Causative Mechanism (Exercise%) | 42 | 36 |
| Injury type (Inversion%) | 82 | 74 |
| Time last from the injury (≤ 12 hr.%) | 58 | 64 |
BID: twice daily; PRN: as needed; SD: Standard Deviation
Table 2.
Ankle pain and swelling severity on the first and follow-up day
| Parameter | BID group | PRN group | P. value | ||
|---|---|---|---|---|---|
| Pain on weight bearing (NRS, Mean ± SD) | Baseline | 5.9 (1.86) | 5.88 (2.21) | - | |
| Follow up | 1.07 (1.28) | 1.32 (1.57) | - | ||
| Decrease | 4.83 (1.78) | 4.56 (2.31) | 0.5 | ||
| Pain at rest (NRS, Mean ± SD) | Baseline | 4.2 (3.09) | 4.93 (3.39) | - | |
| Follow up | 0.33 (0.71) | 0.42 (1.01) | - | ||
| Decrease | 3.87 (2.9) | 4.52 (3.59) | 0.4 | ||
| Swelling (% of total) | Baseline | Mild | 2 (3.3%) | 3 (5%) | |
| Moderate | 40 (66.7%) | 35 (58.3%) | - | ||
| Severe | 18 (30%) | 22 (36.7%) | |||
| Follow up | Mild | 44 (73.3%) | 36 (60%) | 0.4 | |
| Moderate | 16 (26.7%) | 24 (40%) | |||
| Severe | 0 | 0 |
NRS: Numeric Rating Scale; Mod: moderate; SD: Standard Deviation
Primary measures of efficacy
Results from the follow-up visit showed a significant decrease in pain on weight-bearing on day seven with mean decrease in pain scores of 4.8 from the baseline (P<0.001). The overall pain decrease on weight bearing as well as the decrease in pain at rest was not significantly different between groups. Swelling decreased significantly during the seven days trial (P<0.03 in BID group and 0.003 in as needed group) but there was no substantial difference between the two groups (Table 2).
Planed analysis showed that naproxen 500 mg as needed was not inferior to naproxen BID in reducing the baseline primary measures of efficacy.
Assessing the safety profile of the two different naproxen dosings (Table 3), the as needed regimen was safer (P<0.001) compared to the BID regimen. There were no serious adverse events (i.e. gastrointestinal bleeding) or death (Table 3).
Table 3.
Adverse event rates between the two groups
| Adverse effects | BID group | PRN group | P. value |
|---|---|---|---|
| Gastrointestinal upset (%) | 8 (13.3%) | 4 (6.7%) | - |
| No adverse events (%) | 54 (87.7%) | 56 (93.3%) | <0.001 |
| Gastrointestinal bleeding (%) | 0 | 0 | - |
BID: twice daily; PRN: as needed
The mean numbers of tablets returned by BID and as needed groups were 3.5 and 9.4 tablets, respectively. In this case, we observed a significant difference between adherence to therapy in both groups (P<0.01).
DISCUSSION
This study used ankle sprain as a model of acute musculoskeletal pain to evaluate the safety and efficacy of Naproxen 500 mg BID or Naproxen 500 mg as needed for the management of acute musculoskeletal pain. We used the ‘Rest, Ice, elastic Compression and limb Elevation’ (RICE) protocol for all of the patients in both treatment groups. Grade 1 and 2 ankle sprains show a good clinical response to non-operative management (RICE protocol) [13, 14]. However, the best conservative treatment, both in costs and clinical outcome is not clarified in present clinical trials.
Non-steroidal anti-inflammatory drugs (NSAIDs) are more effective than placebo for the initial treatment of ankle sprain [15, 16]. Naproxen 500 mg BID is effective in the treatment of ankle sprain and other soft tissue injuries, and has a good safety profile [9, 17]. Cukiernik and her colleagues compared acetaminophen versus naproxen in the treatment of ankle sprain in children[9]. Both drugs were equally effective in reducing pain and disability. They suggested that as needed dosing of NSAIDs in the management of soft tissue musculoskeletal injuries should be further studied [9].
In our trial, also, naproxen BID proved to be effective in reducing ankle swelling, pain on weight bearing and pain at rest with no serious adverse events. However, 8% of patients treated with naproxen BID reported some minor complications mostly gastrointestinal upset. This was lower than the report by Kyle et al (23%). They compared naproxen 500 mg BID with lumiracoxib in the treatment of acute musculoskeletal pain, and concluded that both drugs were similarly effective in reducing pain intensity during the five- and seven-day periods [18].
Patients treated with naproxen as needed, showed the same clinical result as naproxen BID group, but adverse events reported by this group and the number of pills used were significantly lower. To our knowledge, higher doses of NSAIDs increase the risk of GI complication. Warner and Mitchell performed a systematic review and demonstrated a correlation between NSAIDs use and hospital admission due to perforation or hemorrhage [19]. They found that lower GI complications risk of ibuprofen is due to the lower dosage of the drug used in general practice. They concluded that “Use of low risk drugs in low dosage as first line treatment would substantially reduce the morbidity and mortality due to serious gastrointestinal toxicity from these drugs”[19]. Rodríguez and his colleagues conducted an analysis of users and non-users of acetaminophen and NSAIDs. They concluded that acetaminophen doses higher than two grams and sustained-release formulation of NSAIDs were associated with higher incidence of GI complications [20].
Our study has several limitations; the study participants were mostly among the low to middle socioeconomic population group and accordingly, we had lost several patients to follow-up. A significant proportion of patients did not fill the diary about their pain and time they used the analgesic medication, so we could not conclude that in which group the pain free situation had occurred sooner. Furthermore, the fact that our patients had different adherence to therapy –as a secondary measure of our study- might have indirectly influenced the decrease in pain scores. With regard to adherence, we could not collect data regarding the amount of pills consumed on each day after injury in the PRN group, which might have considerably influenced the extent of pain decrease among the patients. Additionally, we did not consider the use of RICE protocol by patients prior to their referral to our center. Also, it would be better to perform at least two or three follow-ups in order to evaluate the back-to-work time interval of patients. We suggest future studies consider a third control group which would receive no treatments other than RICE.
CONCLUSION
Our study showed that naproxen as needed may reduce the pain and edema of the sprained ankle with no significant difference compared to the BID regimen, while it possesses better safety profile and lower total drug use; therefore, we recommend physicians to prescribe naproxen as needed for pain instead of the regular twice daily doses.
ACKNOWLEDGMENTS
This study was approved and financially supported as the project no. 8732136 by Tehran University of Medical Sciences. We would also like to thank the electronic library of Tehran University of Medical Sciences for providing us with the full text of articles.
Conflict of interests: None
REFERENCES
- 1.Coudreuse JM, De Vathaire F. Effect of a plaster containing DHEP and heparin in acute ankle sprains with oedema: a randomized, double-blind, placebo-controlled, clinical study. Curr Med Res Opin. 2010;26:2221–8. doi: 10.1185/03007995.2010.508020. [DOI] [PubMed] [Google Scholar]
- 2.Adamson C, Cymet T. Ankle sprains: evaluation, treatment, rehabilitation. Md Med J. 1997;46:530–7. [PubMed] [Google Scholar]
- 3.Dalton JD, Jr, Schweinle JE. Randomized controlled noninferiority trial to compare extended release acetaminophen and ibuprofen for the treatment of ankle sprains. Ann Emerg Me. 2006;48:615–23. doi: 10.1016/j.annemergmed.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Dizon JM, Reyes JJ. A systematic review on the effectiveness of external ankle supports in the prevention of inversion ankle sprains among elite and recreational players. J Sci Med Sport. 2010;13:309–17. doi: 10.1016/j.jsams.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Cardenas-Estrada E, Oliveira LG, Abad HL, et al. Efficacy and safety of celecoxib in the treatment of acute pain due to ankle sprain in a Latin American and Middle Eastern population. J Int Med Res. 2009;37:1937–51. doi: 10.1177/147323000903700632. [DOI] [PubMed] [Google Scholar]
- 6.Nadarajah A, Abrahan L, Lau FL, et al. Efficacy and tolerability of celecoxib compared with diclofenac slow release in the treatment of acute ankle sprain in an Asian population. Singapore Med J. 2006;47:534–42. [PubMed] [Google Scholar]
- 7.Popovic N, Gillet P. [Ankle sprain. Management of recent lesions and prevention of secondary instability] Rev Med Liege. 2005;60(10):783–8. [PubMed] [Google Scholar]
- 8.Petrella R, Ekman EF, Schuller R, et al. Efficacy of celecoxib, a COX-2-specific inhibitor, and naproxen in the management of acute ankle sprain: results of a double-blind, randomized controlled trial. Clin J Sport Med. 2004;14:225–31. doi: 10.1097/00042752-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Cukiernik VA, Lim R, Warren D, et al. Naproxen versus acetaminophen for therapy of soft tissue injuries to the ankle in children. Ann Pharmacother. 2007;41(9):1368–74. doi: 10.1345/aph.1H596. [DOI] [PubMed] [Google Scholar]
- 10.Rogers NV, Rowland K. An alternative to oral NSAIDs for acute musculoskeletal injuries. J Fam Pract. 2011;60:147–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Tamblyn R, Eguale T, Buckeridge DL, et al. The effectiveness of a new generation of computerized drug alerts in reducing the risk of injury from drug side effects: a cluster randomized trial. J Am Med Inform Assoc. 2012;19:635–43. doi: 10.1136/amiajnl-2011-000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witjes S, Gresnigt F, Van Den Bekerom MP, et al. The ANKLE TRIAL (ankle treatment after injuries of the ankle ligaments): what is the benefit of external support devices in the functional treatment of acute ankle sprain? A randomised controlled trial. BMC Musculoskelet Disord. 2012;13:21. doi: 10.1186/1471-2474-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Todd KH, Funk KG, Funk JP, et al. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27(4):485–9. doi: 10.1016/s0196-0644(96)70238-x. [DOI] [PubMed] [Google Scholar]
- 14.Hockberger RS, Binder LS, Graber MA, et al. The model of the clinical practice of emergency medicine. Ann Emerg Med. 2001;37:745–70. doi: 10.1067/mem.2001.115495. [DOI] [PubMed] [Google Scholar]
- 15.Polzer H, Kanz KG, Prall WC, et al. Diagnosis and treatment of acute ankle injuries: development of an evidence-based algorithm. Orthop Rev (Pavia) 2012;4:e5. doi: 10.4081/or.2012.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheng SS, Xing GX. [Operative treatment of III degree injuries without fracture of ankle joint ligaments] Zhongguo Gu Shang. 2009;22:136. [PubMed] [Google Scholar]
- 17.Pearson RL. Re: Efficacy of celecoxib, a COX-2-specific inhibitor, and naproxen in the management of acute ankle sprain: results of a double-blind, randomized controlled trial. Clin J Sport Med. 2005;15:196. doi: 10.1097/01.jsm.0000157659.78146.7c. [DOI] [PubMed] [Google Scholar]
- 18.Kyle C, Zachariahz J, Kasangra M, et al. Lumiracoxib 400 mg once daily is comparable to naproxen 500 mg twice daily for treatment of acute muscular pain following soft tissue injury. Ann Rheum Dis. 2006;65:241. [Google Scholar]
- 19.Warner TD, Mitchell JA. COX-2 selectivity alone does not define the cardiovascular risks associated with non-steroidal anti-inflammatory drugs. Lancet. 2008;371:270–3. doi: 10.1016/S0140-6736(08)60137-3. [DOI] [PubMed] [Google Scholar]
- 20.Garcia Rodriguez LA, Lagergren J, Lindblad M. Gastric acid suppression and risk of oesophageal and gastric adenocarcinoma: a nested case control study in the UK. Gut. 2006;55:1538–44. doi: 10.1136/gut.2005.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]