Abstract
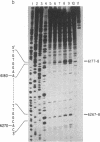
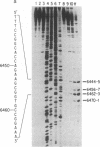
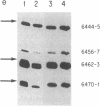
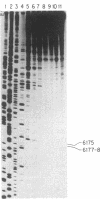
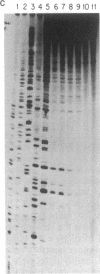
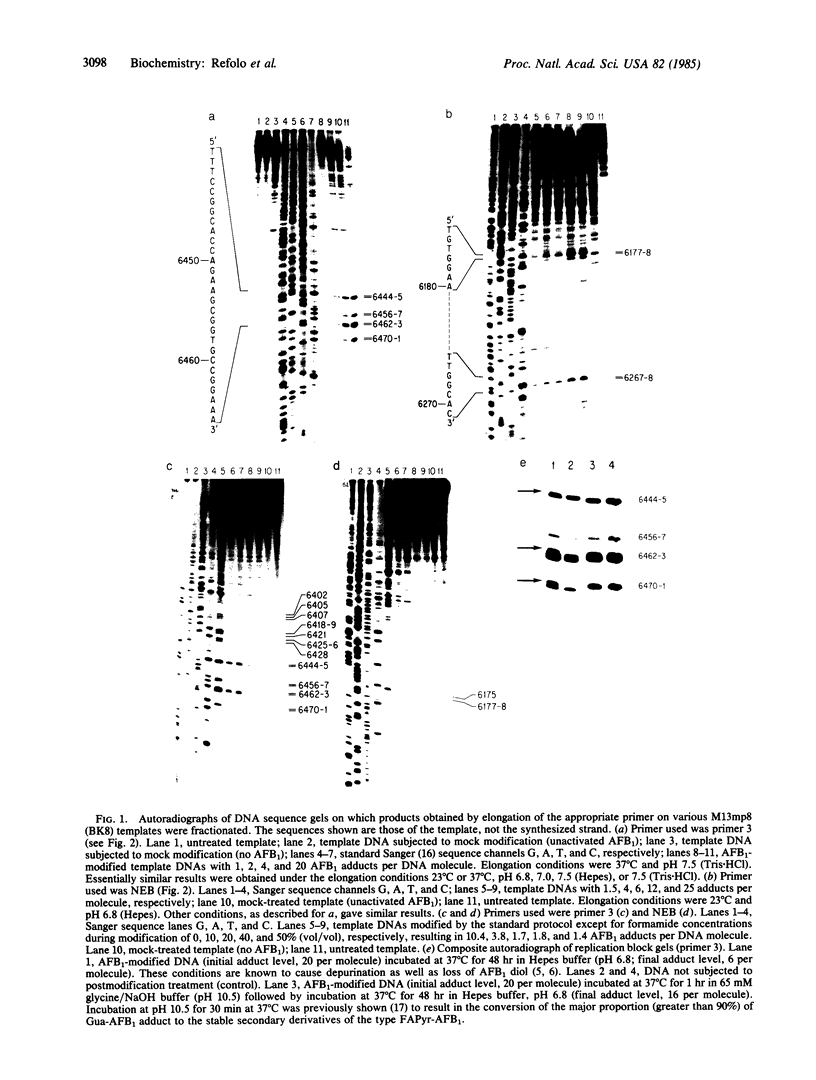
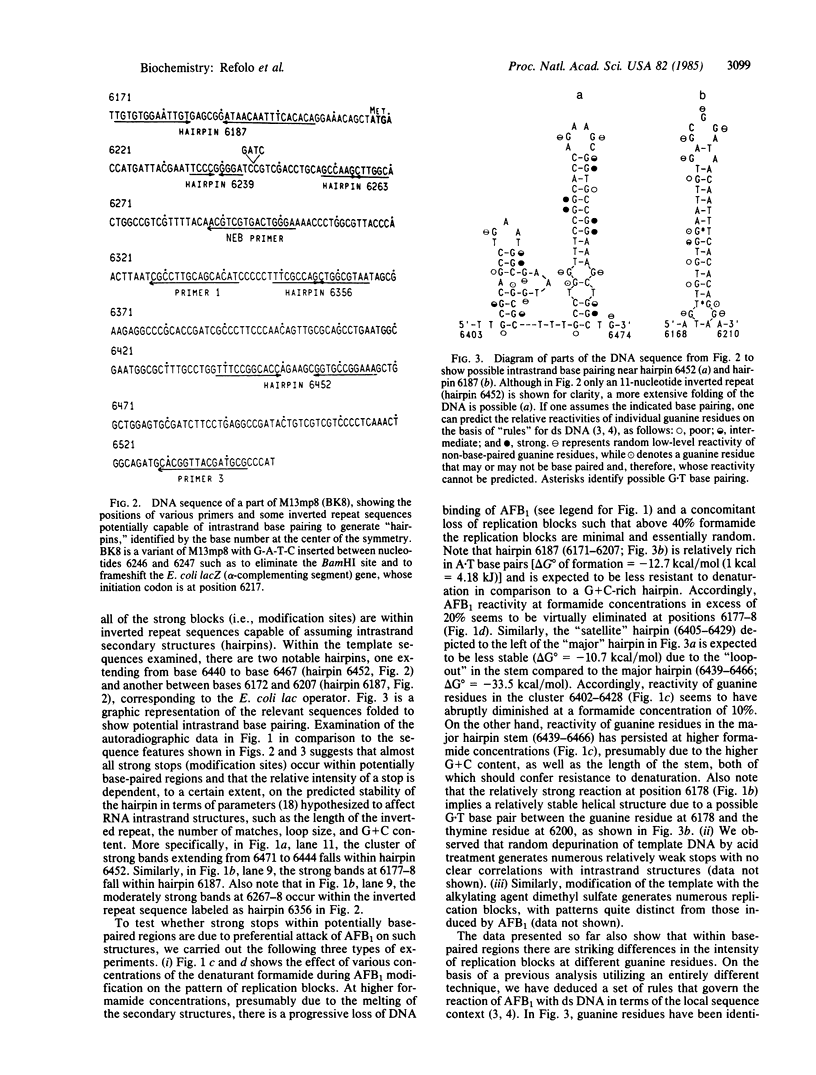
The genotoxic effects of the potent mutagenic carcinogen aflatoxin B1 (AFB1) are believed to be mediated by its reaction with the N-7 atom of guanine residues in DNA. We have analyzed the effect of AFB1-induced chemical modification on the template function of single-stranded DNA in vitro. The experimental strategy involves the elongation of a primer on a modified template by Escherichia coli DNA polymerase I (large fragment) and analysis of the products by high-resolution gel electrophoresis. Our data show that (i) AFB1 induces specific replication blocks one nucleotide 3' to the sites of occurrence of guanine residues on template DNA; (ii) AFB1-induced replication blocks occur predominantly at sequences capable of participation in intrastrand base pairing; (iii) within the intrastrand base-paired regions there are strong sequence context effects, in accordance with the previously described [Muench, K. F., Misra, R. P. & Humayun, M. Z. (1983) Proc. Natl. Acad. Sci. USA 80, 6-10] specificity "rules" that apply to the reaction of AFB1 with guanine residues in double-stranded DNA; (iv) there is evidence that the (7-guanyl)-AFB1 adducts as well as secondary derivatives such as the formamidopyrimidine-AFB1 act as replication blocks. In summary, these data suggest that previously observed inhibition of DNA replication and transcription by AFB1 is directly attributable to (7-guanyl)-AFB1 adducts or their secondary reaction products.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu Y. H., Saffhill R. Errors in DNA synthesis induced by aflatoxin B1 modification of poly(dC-dG). Carcinogenesis. 1983;4(5):643–646. doi: 10.1093/carcin/4.5.643. [DOI] [PubMed] [Google Scholar]
- Groopman J. D., Croy R. G., Wogan G. N. In vitro reactions of aflatoxin B1-adducted DNA. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5445–5449. doi: 10.1073/pnas.78.9.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humayun Z., Jeffrey A., Ptashne M. Completed DNA sequences and organization of repressor-binding sites in the operators of phage lambda. J Mol Biol. 1977 May 15;112(2):265–277. doi: 10.1016/s0022-2836(77)80143-5. [DOI] [PubMed] [Google Scholar]
- Irvin T. R., Wogan G. N. Quantitation of aflatoxin B1 adduction within the ribosomal RNA gene sequences of rat liver DNA. Proc Natl Acad Sci U S A. 1984 Feb;81(3):664–668. doi: 10.1073/pnas.81.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. N., Garner R. C. Aflatoxin B -oxide generated by chemical or enzymic oxidation of aflatoxin B1 causes guanine substitution in nucleic acids. Nature. 1977 Jun 30;267(5614):863–865. doi: 10.1038/267863a0. [DOI] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra R. P., Muench K. F., Humayun M. Z. Covalent and noncovalent interactions of aflatoxin with defined deoxyribonucleic acid sequences. Biochemistry. 1983 Jul 5;22(14):3351–3359. doi: 10.1021/bi00283a008. [DOI] [PubMed] [Google Scholar]
- Moore P. D., Bose K. K., Rabkin S. D., Strauss B. S. Sites of termination of in vitro DNA synthesis on ultraviolet- and N-acetylaminofluorene-treated phi X174 templates by prokaryotic and eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1981 Jan;78(1):110–114. doi: 10.1073/pnas.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. D., Rabkin S. D., Osborn A. L., Strauss B. S. Interactions between DNA polymerase and aminofluorene adducts that affect the recognition and possibly the mutagenicity of the lesions. Biochimie. 1982 Aug-Sep;64(8-9):757–762. doi: 10.1016/s0300-9084(82)80125-9. [DOI] [PubMed] [Google Scholar]
- Muench K. F., Misra R. P., Humayun M. Z. Sequence specificity in aflatoxin B1--DNA interactions. Proc Natl Acad Sci U S A. 1983 Jan;80(1):6–10. doi: 10.1073/pnas.80.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Hao W. M., Wogan G. N., Rich A. Salt-induced conversion of B-DNA to Z-DNA inhibited by aflatoxin B1. Science. 1983 Mar 25;219(4591):1434–1436. doi: 10.1126/science.6402818. [DOI] [PubMed] [Google Scholar]
- Smith A. J. DNA sequence analysis by primed synthesis. Methods Enzymol. 1980;65(1):560–580. doi: 10.1016/s0076-6879(80)65060-5. [DOI] [PubMed] [Google Scholar]
- Strauss B., Rabkin S., Sagher D., Moore P. The role of DNA polymerase in base substitution mutagenesis on non-instructional templates. Biochimie. 1982 Aug-Sep;64(8-9):829–838. doi: 10.1016/s0300-9084(82)80138-7. [DOI] [PubMed] [Google Scholar]
- Swenson D. H., Miller J. A., Miller E. C. The reactivity and carcinogenicity of aflatoxin B1-2,3-dichloride, a model for the putative 2,3-oxide metabolite of aflatoxin B1. Cancer Res. 1975 Dec;35(12):3811–3823. [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wang T. V., Cerutti P. Spontaneous reactions of aflatoxin B1 modified deoxyribonucleic acid in vitro. Biochemistry. 1980 Apr 15;19(8):1692–1698. doi: 10.1021/bi00549a027. [DOI] [PubMed] [Google Scholar]
- Yu F. L. Preferential binding of aflatoxin B1 to the transcriptionally active regions of rat liver nucleolar chromatin in vivo and in vitro. Carcinogenesis. 1983;4(7):889–893. doi: 10.1093/carcin/4.7.889. [DOI] [PubMed] [Google Scholar]
- Yu F. L. Studies on the mechanism of aflatoxin B1 inhibition of rat liver nucleolar RNA synthesis. J Biol Chem. 1981 Apr 10;256(7):3292–3297. [PubMed] [Google Scholar]