Significance
Seasonal timing of biological events, phenology, is one of the strongest bioindicators of climate change. Our general understanding of phenological responses to climate change is based almost solely on the first day on which an event is observed, limiting our understanding of how ecological communities may be responding as a whole. Using a unique long-term record of flowering phenology from Colorado, we find that the number of species changing their flowering times likely has been underestimated and the magnitude of phenological change overestimated. In addition to earlier first flowering, we document a diverse assortment of other changes, such as delayed last flowering, as temperatures warm. This variety of species-level phenological shifts has ultimately reshaped various temporal components of the plant community.
Keywords: growing season, no-analogue community, phenological mismatch, phenology curve, species interactions
Abstract
Phenology—the timing of biological events—is highly sensitive to climate change. However, our general understanding of how phenology responds to climate change is based almost solely on incomplete assessments of phenology (such as first date of flowering) rather than on entire phenological distributions. Using a uniquely comprehensive 39-y flowering phenology dataset from the Colorado Rocky Mountains that contains more than 2 million flower counts, we reveal a diversity of species-level phenological shifts that bring into question the accuracy of previous estimates of long-term phenological change. For 60 species, we show that first, peak, and last flowering rarely shift uniformly and instead usually shift independently of one another, resulting in a diversity of phenological changes through time. Shifts in the timing of first flowering on average overestimate the magnitude of shifts in the timing of peak flowering, fail to predict shifts in the timing of last flowering, and underrepresent the number of species changing phenology in this plant community. Ultimately, this diversity of species-level phenological shifts contributes to altered coflowering patterns within the community, a redistribution of floral abundance across the season, and an expansion of the flowering season by more than I mo during the course of our study period. These results demonstrate the substantial reshaping of ecological communities that can be attributed to shifts in phenology.
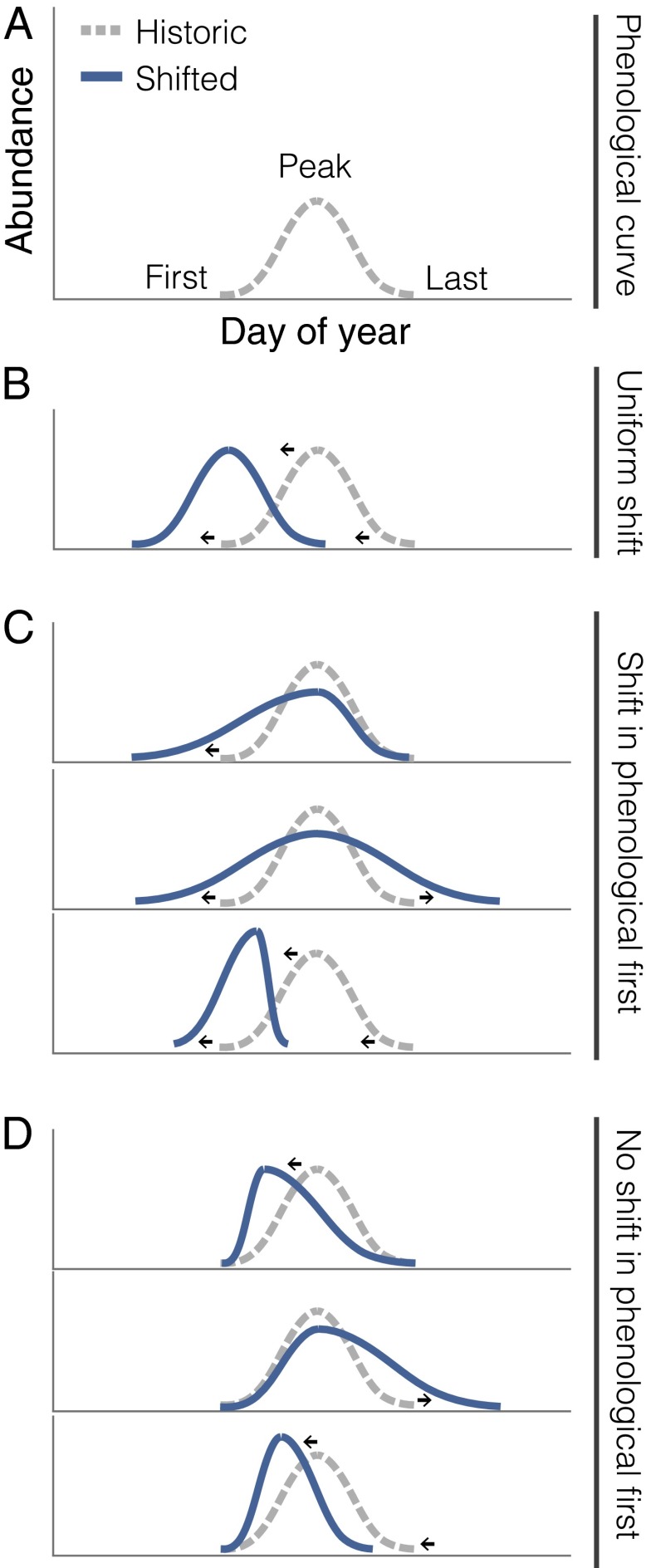
Phenology, the timing of biological events, is intimately tied to the reproduction and survival of organisms (1). Phenological events generally are occurring earlier in temperate environments in accordance with climate change, although several recent studies have emphasized species-specificity in the direction and magnitude of change (2–5). The great majority of these long-term datasets contain a single measure of phenology for individual species, most often the first day on which a biological event is observed (i.e., “phenological firsts” such as first flowering) (Fig. 1A). In addition to phenological firsts, basic components of an entire phenological response include the timing of the ending of a biological event and details of intermediate stages, such as the timing and magnitude of peak abundance or activity (Fig. 1A). Given that phenological firsts represent the early tail of a population-level response, most assessments of phenological change to date may provide an incomplete view of the magnitude of change, the number of responsive species, and how species-level shifts contribute to change at higher levels of biological organization.
Fig. 1.
Conceptual representation of shifts in multiple phenological measures for individual species through time. (A) Multiple measures of flowering phenology available for 60 species from a 39-y study of a plant community in the Colorado Rocky Mountains, USA. (B) If shifts in first flowering change at a rate similar to changes in other measures of phenology, then the distribution shifts forward uniformly through time. (C and D) In contrast, shifts in first flowering may be unrepresentative of both the direction and magnitude of changes in peak and last flowering (C), and peak and last flowering may shift while first flowering remains unchanged (D). Arrows indicate a shift in phenology. For simplicity of conceptual illustration, initial species’ distributions are represented as a Gaussian curve, and the area under the curve is held constant.
We have amassed a unique long-term record of flowering phenology that allows us to investigate complete phenological responses for a plant community. Over a 39-y period (1974–2012), we have sampled a montane site (2,900 m elevation) in Colorado, USA, counting the total number of flowers of 121 plant species across a series of permanent plots approximately every other day throughout the entire growing season [a map of the 30 permanent 2 × 2 m plots and description of the study site are published elsewhere (6, 7)]. Because flowering phenology is shaped by the abiotic environment as well as biotic interactions— plant–plant competition, attraction of mutualists, and avoidance of antagonists—changes in flowering phenology have broad implications for ecological interactions and their evolutionary consequences, including those among plants and with higher trophic levels (8). Here we report on 60 common plant species representative of the meadow communities at our site [mostly perennial herbs, excluding less-common species for which data are insufficient (9, 10)]. This portion of the phenology census yields more than 2 million flower counts from which we can assess (i) multiple aspects of changes in flowering phenology for individual species, (ii) how accurately shifts in first flowering predict shifts in peak and last flowering, and (iii) how species-specific shifts in first, peak, and last flowering, as well as changes in floral abundance, contribute to altered patterns of interaction potential among species and changes in community-level distribution of flowers across the season.
Results and Discussion
Here we focus on changes in flowering phenology that have occurred over our 39-y record, a timeframe during which summer air temperatures increased by 0.4 ± 0.1 °C per decade (June–August mean air temperature: R2 = 0.32, P = 0.0002) and the date of spring snowmelt advanced by 3.5 ± 2.0 d per decade (R2 = 0.07, P = 0.10) (10). Both temperature and the timing of snowmelt are strongly associated with shifts in flowering phenology in this study system (6, 7, 11–13), independently explaining on average 66% and 68% of the interannual variation in flowering phenology, respectively (10).
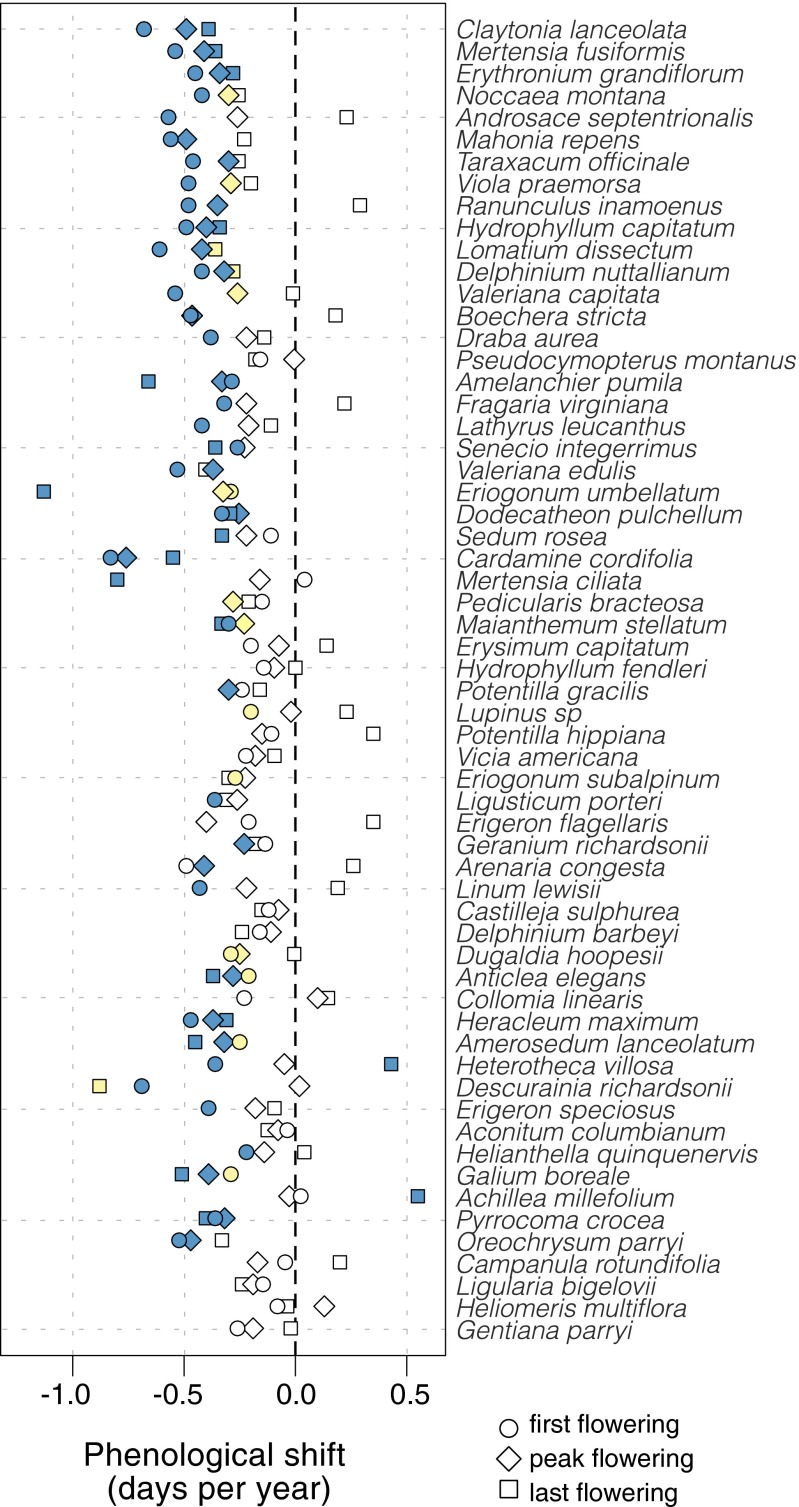
We find striking diversity in the phenological shifts of individual plant species through time. First flowering on average advanced by 3.3 ± 0.24 d per decade, peak flowering by 2.5 ± 0.20 d per decade, and last flowering by 1.5 ± 0.42 d per decade; significant shifts were observed in 50%, 38%, and 30% of species’ first, peak, and last flowering, respectively, and some form of change occurred in 68% of species (41/60). Thus, basing assessments of phenological change on a single measure of phenology underestimated the number of responsive species by 18–38%, depending on the measure (first, peak, or last flowering) used. Many species exhibit inconsistent shifts in first, peak, and last flowering (Fig. 2), resulting in changes in flowering duration for 27% of species (Table S1). Of all of the species exhibiting a significant change in flowering, only 17% (7/41) shifted all aspects of their phenology uniformly forward through time, as indicated by significant temporal advancements in first, peak, and last flowering (Figs. 1B and 2). In contrast, 56% (23/41) of these species showed earlier first flowering in combination with disparate changes in peak and last flowering (Figs. 1C and 2). Finally, 27% (11/41) exhibited significant changes in peak or last flowering with no significant change in first flowering (Figs. 1D and 2), indicating that classifications of species as responsive or nonresponsive based on phenological firsts can be inaccurate. Intraspecific variation in phenological sensitivity to changing abiotic conditions likely accounts for these independent shifts in first, peak, and last flowering at the population level. For example, the population-level pattern in Fig. 1C (Top) could result from the earliest-flowering individuals advancing their flowering at a faster rate than later-flowering individuals; alternatively, this pattern could result from several individuals advancing only their onset of flowering while maintaining open flowers for a longer timeframe. Although very little is known about intraspecific variation in phenological shifts, interspecific variation has emerged as a general pattern across the globe (2–5). Our results demonstrate a more nuanced type of species specificity than has been shown before. Observing this diversity of population-level responses dispels the overly simplistic notion that species’ composite phenologies are advancing, delaying, or not changing through time (Fig. 1).
Fig. 2.
Shifts in flowering phenology over 39 y (1974–2012). Each symbol represents a phenological shift as the slope of a line from simple linear regressions of first, peak, and last flowering by year (n = no. of years for each species). Significant shifts are represented in blue (P ≤ 0.05), marginally significant shifts in pale yellow (0.05 < P ≤ 0.10), and nonsignificant shifts in white (P > 0.10). Species are presented in order of mean date of first flowering throughout the growing season.
The probability of detecting phenological shifts in long-term data can be affected by changes in abundance (14). Indeed, one benefit of collecting abundance-based phenological data is the ability to examine evidence for biases in estimates of phenological shifts. For example, if a species’ floral abundance is increasing through time, its flowers are more likely to be observed both earlier and later in the season simply because there are more flowers to observe. Thus, apparent advances in first flowering and delays in last flowering potentially could reflect increased floral abundance instead of an actual phenological shift; the opposite would be expected with decreasing floral abundance. One third (20/60) of the species in our study exhibited significant changes in peak floral abundance over the timeframe of our study (Table S1). However, in only six cases (first flowering in two species and last flowering in four species) did we detect evidence that advanced phenology could be an artifact of changes in peak floral abundance (Table S2). The great majority of species showing a significant shift in flowering phenology did so independently of a change in peak floral abundance.
A paucity of long-term abundance-based phenological datasets has led to the implicit or explicit assumption that a single phenological measure represents an entire population-level phenological response (Fig. 1B) (13–15), but our results do not support this assumption. In this plant community, for every day that species-level first flowering advanced, the timing of peak flowering advanced by only 0.55 ± 0.09 d (R2 = 0.42, F1, 58 = 41.8, P < 0.0001). Furthermore, shifts in species-level first flowering failed to predict shifts in last flowering (R2 = 0.05, F1, 58 = 3.20, P = 0.079, slope = 0.40 ± 0.22 d). The ability of changes in first flowering to predict changes in peak and last flowering is nearly identical when phenological sensitivities to temperature or snowmelt are used in place of change through time (Table S3). Our results for 60 plant species, combined with information on first vs. mean arrival dates of three migratory bird species (16), are suggestive of a general pattern in which phenological firsts change at a faster rate than other measures of the same phenological event. Although additional abundance-based phenological studies will lend insight into the generality of our results, we can make recommendations for refining models of phenological change. Current predictive models based on phenological firsts are likely to exaggerate the magnitude of phenological change; to account for this potential source of bias, models should allow for a dampening of the response of the timing of peak abundance relative to phenological firsts. When modeling the end of life history events, variability in both the direction and magnitude of shifts in phenology should be incorporated.
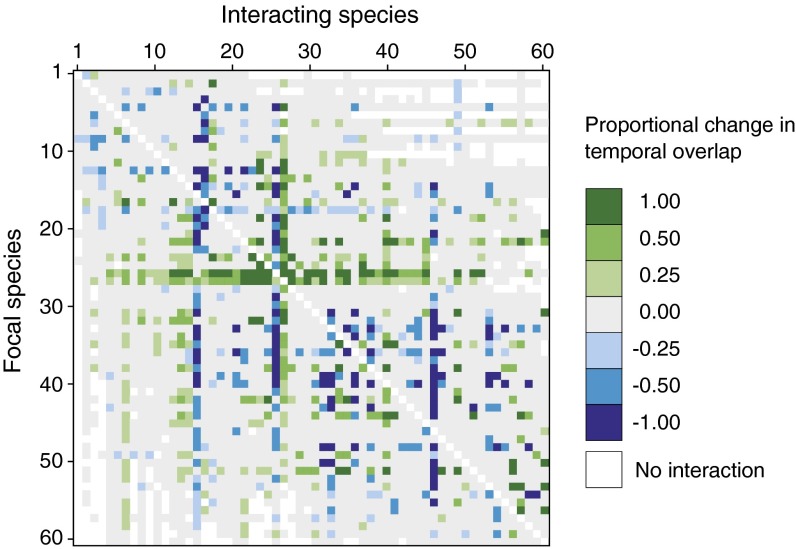
Species-specific phenological shifts are widely hypothesized to affect patterns of temporal overlap among species, but the extent of such changes for entire communities has remained elusive (17–19). We contend that the consequences of species-specific phenological change for interactions within trophic levels are generally underappreciated, especially in light of widespread concern about trophic mismatch (20–22). Interactions within trophic levels are important because they can affect community structure and stability (23–25) as well as regulate the response of ecological communities to climate change (26). In this study, 23.2% (725/3,119) of species’ pairwise coflowering interactions changed significantly over the 39-y record (of 3,540 possible pairwise coflowering interactions, 3,119 were realized) (Fig. 3). A total of 10.5% (329) of all plant species pairs increased in coflowering, and 12.7% (396) decreased (Fig. 3). A change in coflowering represents altered interaction potential (27), which can affect various ecological processes (19). Increased coflowering between plant species can exacerbate direct interspecific competition for abiotic resources (28) and can affect plant reproductive success indirectly via competition for or facilitation of pollination (29, 30). Because of its effect on plant reproduction, competition for pollination is thought to promote selection for sequential flowering (29, 31–33). Therefore it is probable that the changes in coflowering patterns shown here differ from those that have been shaped over longer timescales by natural selection.
Fig. 3.
Community-level change in interaction potential over 39 y. Each cell represents the proportional change in interaction potential, or coflowering overlap, between species pairs over the 39-y study period (1974–2012). Coflowering was calculated annually as the total number of flowers of every species pair that overlap in time, divided by the total number of flowers of the focal species (see main text for an example). To represent change in coflowering visually for all species, we multiplied each rate of change by 39 y; thus, a proportional change of 0.25 indicates a 25% increase in overlap of a focal species with an interacting species over the course of our study period. Proportional changes in overlap values are binned (e.g., 0.25 = 0.01–0.25). Colored cells indicate significant changes in interaction potential through time (P ≤ 0.05), gray cells indicate no change, and white cells indicate cases in which species pairs did not coflower in any years of the study. Species are ordered by mean first flowering date (as in Fig. 2).
Although climate-induced changes in community composition have been attributed mainly to species loss and colonization in association with shifting geographic ranges (34, 35), these coflowering shifts provide an example of an altered composition in the temporal community in the absence of species’ extinction or colonization. No-analog communities are defined as those with no contemporary analog, formed through the dissolution of contemporary species assemblages and the formation of new ones via species-specific range shifts (36, 37). Our results present a similar scenario over modern climate-change time scales in the temporal rather than the spatial dimension. Parallel to the way that species-specific range shifts can lead to novel patterns of spatial co-occurrence, species-specific phenological shifts can lead to novel patterns of temporal co-occurrence (Fig. 3).
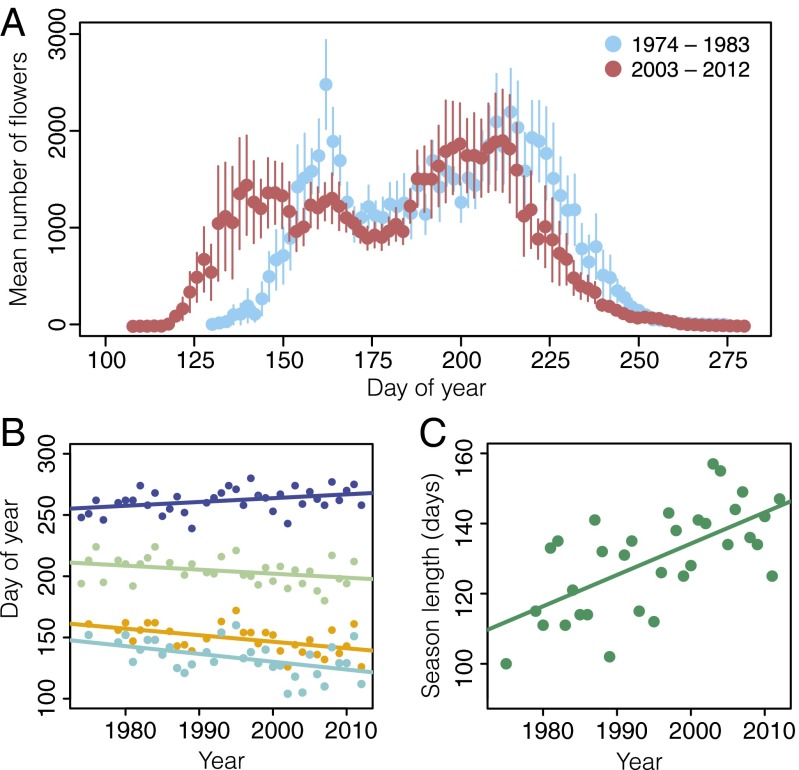
Our abundance-based phenology record also allows us to describe change in aggregate community-level phenology (i.e., a seasonal flowering curve for the entire community; Fig. 4A). Aggregate community-level responses are associated with the timing of snowmelt and air temperature, in directions consistent with climate change (Table S4). First flowering at the community level has advanced by 25.0 d over the course of this 39-y study at a rate of 6.4 ± 2.1 d per decade (R2 = 0.23, F1,31 = 9.13, P = 0.005) (Fig. 4B). The timing of the spring peak in floral abundance has advanced by 20.7 d, at a rate of 5.3 ± 1.7 d per decade, whereas the number of flowers composing this peak has remained constant (R2 = 0.26, F1,29 = 10.11, P = 0.0035 and R2 = 0.02, F1,29 = 0.70, P = 0.41, respectively) (Fig. 4B). Similarly, the timing of summer peak floral abundance has advanced by 12.9 d, at a rate of 3.3 ± 1.6 d per decade, with no change in the number of flowers composing this peak (R2 = 0.12, F1,31 = 4.3, P = 0.047 and R2 = 0.03, F1,31 = 0.81, P = 0.38, respectively) (Fig. 4B). In contrast, the date of community-level last flowering has delayed by 12.1 d at a rate of 3.1 ± 1.3 d per decade (R2 = 0.13, F1,35 = 5.37, P = 0.026) (Fig. 4B). The length of the flowering season has expanded by 34.7 d, at a rate of 8.9 ± 1.9 d per decade (R2 = 0.42, F1,31 = 22.72, P < 0.0001) (Fig. 4C), but total floral abundance across the season has remained constant (R2 < 0.001, F1,31 = 0.003, P = 0.96), indicating that the same number of flowers is spread across a longer growing season.
Fig. 4.
Aggregate community-level shifts in flowering phenology. (A) Comparison of the season-wide flowering curves for the first and last 10 y of the dataset; 10-y means were used to visualize the amount of change that has occurred in the community flowering curve. Each dot is the 10-y mean number of flowers; error bars are ±1 SEM. (B) Phenological shifts through time for first flowering of the community (cyan), last flowering for the community (dark blue), and timing of community-level spring peak (orange) and summer peak (green); each dot represents a community-level phenological measure in 1 y. (C) Change in the length of the flowering season; each dot represents the total number of days on which open flowers were present in each year.
Species-level changes underlie the month-long expansion of the flowering season. Earlier-flowering species advanced their first and peak flowering more rapidly than later-flowering species, a trend not exhibited in last flowering (first: R2 = 0.25, F1, 58 = 19.42, P < 0.0001; peak: R2 = 0.17, F1, 58 = 11.97, P = 0.001; last: R2 = 0.019, F1, 58 = 1.16, P = 0.29). Although the advancing onset of spring plays a clear role in the expansion of growing seasons in plant communities, the role of end-of-season events is less clear: hypothetically, the end of the season could advance at a slower rate than onset, could not change, or could be delayed (38). In our study, end-of-season flowering is delayed. The two species largely responsible for this delay of end-of-season flowering show significant delays in last flowering with either an advance or no change in first flowering (Figs. 1 C and D and 2), again highlighting how phenological firsts can misrepresent overall phenological change. The flowering season is brief in this subalpine plant community (9), so a month-long expansion represents an approximately 30% increase. Redistribution of flowers across this expanded flowering season likely has repercussions for community structure, interactions within and among trophic levels, and ecosystem function (Figs. 3 and 4) (39–41).
Here we show that the classification of species-specific phenological responses to climate change as advancing, delaying, or not changing is an oversimplification when such assessments are based solely on phenological firsts (Figs. 1 and 2). Assuming that phenological firsts represent overall phenological change can lead to inaccurate assessments of the magnitude of change and the number of responsive species within an ecological community, with implications for forecasting phenological shifts under future climate scenarios. We demonstrate that first, peak, and last flowering rarely shift uniformly but instead tend to shift independently of one another, resulting in a wide range of phenological changes through time in individual species. This diversity of species-level shifts in phenology ultimately leads to altered patterns of coflowering (Fig. 3), expansion of the flowering season, and community-level redistribution of floral abundance (Fig. 4). Our results highlight both the importance of considering phenology more broadly than first observations and the substantial reshaping of ecological communities that can be attributed to climate-induced shifts in phenology.
Methods
Study Site and Dataset.
This study was conducted at the Rocky Mountain Biological Laboratory (RMBL) in the Colorado Rocky Mountains, USA (38°57.5′N, 106°59.3′W, 2,900 m above sea level). For each of the 121 flowering plant species that occur in our thirty 2 × 2 m plots, either the number of flowers per stalk or the number of flowering inflorescences (for species with many small flowers) were counted every other day throughout the growing season from 1974–2012. Copies of the flowering phenology dataset and metadata are archived at www.rmbl.org and in the Digital Repository at the University of Maryland (http://drum.lib.umd.edu/). We limited the analysis to species that were present in at least half of the years of the dataset (19 y), leaving a total of 60 species that represent the meadow plant communities in and around the RMBL (see ref. 10 for more information about plant species). There was no census in 1978 and 1990. Thus, there was a maximum of n = 37 y for each species, and a minimum of n = 19 y because not all species flower in every year. Five of the 30 plots were added in later years: two in 1985 and three in 1998. The addition of five plots should not alter estimates of phenological change, because the magnitude of phenological change generally is not affected by changes in peak floral abundance (Table S2). Furthermore, we find no relationship between changes in peak floral abundance and shifts in the timing of peak flowering for the 60 species studied here (r = 0.038, n = 60 species). These five plots were excluded from analyses that used floral abundance: species-level change in peak floral abundance, coflowering patterns, and aggregate community-level responses. Records for the annual timing of snowmelt come from a permanent 5 × 5 m snow plot at the RMBL in which the first day of bare ground is recorded as the date of snowmelt. Mean temperatures used in analyses are the average of the daily minimum and maximum temperatures at the Crested Butte National Oceanic and Atmosphere Administration weather station (ca. 9 km south of the RMBL).
Species-Level Analyses.
For each species, the number of flowers was summed across all 30 plots on each census day to create one annual flowering distribution per species. First flowering was the first day on which a flower for that species was observed, and last flowering was the last day on which a flower was observed, taken from the across-plot sum. Peak flowering for individual species was the day on which 50% of the flowers were counted (following refs. 9 and 10). Peak floral abundance was the maximum number of flowers counted annually in one census. Years in which the census started late (1976, 1982, 1985, 1992, and 1994) were excluded from analysis when the response variable of interest was affected (first flowering and occasionally peak flowering for the earliest-flowering species). Linear regression was used to analyze change through time, with phenology or peak floral abundance as a response and year as a continuous predictor. We tested for temporal autocorrelation in the time series of species showing significant phenological change through time, using the Ljung–Box test with a lag time of 1 y. We found evidence of significant temporal autocorrelation in only three cases (Table S5). We reanalyzed these three cases with an autoregressive linear model, which allows the error structure to be correlated. The rates of change in these models were very similar to the rates of change in our simple linear regression analysis, and change through time was still significant in all three cases (Table S6). We therefore conclude that temporal autocorrelation in this dataset does not bias our results.
To determine whether phenological shifts could have been an artifact of changes in peak floral abundance (14), we ran correlation analyses for species showing significant shifts in both phenology and peak floral abundance (defined as the maximum number of flowers counted annually in a census for individual species). We looked for increasing peak floral abundance in correlation with earlier first flowering and later last flowering; we also looked for decreasing peak floral abundance in correlation with later first flowering and earlier last flowering. These relationships indicate the possibility of detecting what appears to be a phenological shift that actually is caused by a change in flower abundance (14). We assume that changes in peak floral abundance are indicative of changes in floral abundance because we do not track individual flowers through time. There is no mathematical reason to expect a change in peak abundance to alter the probability of detecting for shifts in the timing of peak flowering.
A thorough analysis of associations of temperature and snowmelt with first, peak, and last flowering has been presented elsewhere; interannual variation in temperature and the timing of snowmelt independently account for a significant amount of variation in first, peak, and last flowering in 93–98% of the species in this study, depending on the flowering response (10). To ensure that our conclusions about phenological predictions based on change through time are not affected by using sensitivities to climate variables in place of year, we used linear regression to assess how well sensitivity of first flowering to climate predicts sensitivity of peak and last flowering to climate (Table S3).
Interaction Potential.
For each year, coflowering was calculated as the number of flowers of every pair of species that overlap in time, weighted by the total number of flowers of the focal species. For each pair of species, the minimum number of open flowers of the two species was summed on each census day, representing the total number of flowers for the two species that were open at the same time. We then weighted this minimum value by the total number of flowers for each species in each year, so that coflowering values represent overlap relative to each species’ annual floral abundance. For example, the total number of Claytonia lanceolata and Mertensia fusiformis flowers that overlapped through time in 2012 was 633. A total of 3,909 C. lanceolata flowers were counted across all plots in this season, compared with 1,287 flowers of M. fusiformis. C. lanceolata’s flowering overlap score with M. fusiformis was 633/3,909 (0.162), and M. fusiformis’ overlap with C. lanceolata was 633/1,287 (0.492). These calculations resulted in 3,540 potential overlap scores for each year (a matrix of 60 species by 60 species, minus the diagonal of same-species interactions). Linear regression was run for each pair of species to examine the amount of change in coflowering overlap through time. We conducted a permutation test of 5,000 runs to obtain P values for each regression. Because we already have shown that species-specific phenological shifts are strongly associated with climate (10), we did not analyze the response of coflowering to climate.
Aggregate Community-Level Phenology.
Floral abundance was summed across all species and plots on each census day for each year to create an annual community-level phenology curve. Linear regression was used to assess changes in community phenology (first day of flowering, day of spring peak flowering, day of summer peak flowering, last day of flowering, and flowering duration) and community-level floral abundance (spring and summer maximum number of flowers, total number of flowers counted, and average number of flowers counted per census) through time. The onset of the flowering period was missed in 12 y because the census started after flowering had already begun in some of the earliest-flowering plots. In five of these years (1974, 1976–77, 1992, and 1994), peak abundance of the first species to flower and an important component of spring peak flowering, C. lanceolata was missed also. These 5 y were excluded from analysis of flowering season length, timing and abundance of spring peak, and start of the flowering season (similar to species-level analyses). For the remaining 7 y (1979, 1982–1983, 1985–1986, 1991, and 1993), we estimated the start of the flowering season based on the slope of a line of flower accumulation from years with known start dates and similar floral abundance. We applied the same procedure to estimate the end of the flowering season for 5 y in which the end of the flowering season was missed (1976–1977, 1984, and 1992–1993). We used ANCOVAs to verify that these estimations did not bias our results by comparing the slopes of regressions using estimated vs. missing values (Table S7).
Two community-level peaks in floral abundance were clearly evident in almost every year, with the exception of 4 y that were excluded from analysis (1985, 1987, 1994, and 2012) (Fig. 4A) (7). Additionally, in 5 y (1989, 1991–92, 2002, and 2007) there was some evidence of a third peak in floral abundance between the spring and summer peaks. We determined the summer peak in these years based on the species that typically compose the summer peak of floral abundance. There was virtually no turnover in species present in the first and last 10 y of the dataset (Fig. 4A), although Pedicularis bracteosa was absent in the last 10 y of the dataset. Because this species is relatively rare in this community, we do not expect its absence to affect the community-level patterns shown in Fig. 4A.
Supplementary Material
Acknowledgments
We thank field assistants for assistance with data collection; Billy Barr for snowmelt data; and members of the J. L. Bronstein laboratory, W. K. Petry, N. M. Waser, M. V. Price, A. E. Arnold, A. M. Tomescu, and anonymous reviewers for comments that improved the paper. This research was supported by National Science Foundation Grants DEB 75-15422, DEB 78-07784, BSR 81-08387, DEB 94-08382, IBN 98-14509, DEB 02-38331, and DEB 09-22080 (to D.W.I.) and DGE-1143953 (to P.J.C.). Additional funding came from research grants from the University of Maryland’s General Research Board and from Earthwatch and its Research Corps (to D.W.I.). Research facilities and access to study sites were provided by the Rocky Mountain Biological Laboratory and J. Tuttle.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The flowering phenology dataset and metadata are archived at www.rmbl.org and in the Digital Repository at the University of Maryland (http://drum.lib.umd.edu/).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323073111/-/DCSupplemental.
References
- 1.Forrest J, Miller-Rushing AJ. Toward a synthetic understanding of the role of phenology in ecology and evolution. Philos Trans R Soc Lond B Biol Sci. 2010;365(1555):3101–3112. doi: 10.1098/rstb.2010.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parmesan C. Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob Change Biol. 2007;13(9):1860–1872. [Google Scholar]
- 3.Cook BI, Wolkovich EM, Parmesan C. Divergent responses to spring and winter warming drive community level flowering trends. Proc Natl Acad Sci USA. 2012;109(23):9000–9005. doi: 10.1073/pnas.1118364109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ovaskainen O, et al. Community-level phenological response to climate change. Proc Natl Acad Sci USA. 2013;110(33):13434–13439. doi: 10.1073/pnas.1305533110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitter AH, Fitter RS. Rapid changes in flowering time in British plants. Science. 2002;296(5573):1689–1691. doi: 10.1126/science.1071617. [DOI] [PubMed] [Google Scholar]
- 6.Inouye DW. Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology. 2008;89(2):353–362. doi: 10.1890/06-2128.1. [DOI] [PubMed] [Google Scholar]
- 7.Aldridge G, Inouye DW, Forrest JRK, Barr WA, Miller-Rushing AJ. Emergence of a mid-season period of low floral resources in a montane meadow ecosystem associated with climate change. J Ecol. 2011;99(4):905–913. [Google Scholar]
- 8.Elzinga JA, et al. Time after time: Flowering phenology and biotic interactions. Trends Ecol Evol. 2007;22(8):432–439. doi: 10.1016/j.tree.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Iler AM, Høye TT, Inouye DW, Schmidt NM. Long-term trends mask variation in the direction and magnitude of short-term phenological shifts. Am J Bot. 2013;100(7):1398–1406. doi: 10.3732/ajb.1200490. [DOI] [PubMed] [Google Scholar]
- 10.Iler AM, Høye TT, Inouye DW, Schmidt NM. Nonlinear flowering responses to climate: Are species approaching their limits of phenological change? Philos Trans R Soc Lond B Biol Sci. 2013;368(1624):20120489. doi: 10.1098/rstb.2012.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price M, Waser NM. Effects of experimental warming on plant reproductive phenology in a subalpine meadow. Ecology. 1998;79(4):1261–1271. [Google Scholar]
- 12.Dunne JA, Harte J, Taylor KJ. Subalpine meadow flowering phenology responses to climate change: Integrating experimental and gradient methods. Ecol Monogr. 2003;73(1):69–86. [Google Scholar]
- 13.Diez JM, et al. Forecasting phenology: From species variability to community patterns. Ecol Lett. 2012;15(6):545–553. doi: 10.1111/j.1461-0248.2012.01765.x. [DOI] [PubMed] [Google Scholar]
- 14.Miller-Rushing AJ, Inouye DW, Primack RB. How well do first flowering dates measure plant responses to climate change? The effects of population size and sampling frequency. J Ecol. 2008;96(6):1289–1296. [Google Scholar]
- 15.Miller-Rushing AJ, Lloyd-Evans TL, Primack RB, Satzinger P. Bird migration times, climate change, and changing population sizes. Glob Change Biol. 2008;14(4):1959–1972. [Google Scholar]
- 16.Sparks TH, et al. Examining the total arrival distribution of migratory birds. Glob Change Biol. 2005;11(1):22–30. [Google Scholar]
- 17.Edwards MM, Richardson AJA. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature. 2004;430(7002):881–884. doi: 10.1038/nature02808. [DOI] [PubMed] [Google Scholar]
- 18.Forrest J, Thomson JD. Consequences of variation in flowering time within and among individuals of Mertensia fusiformis (Boraginaceae), an early spring wildflower. Am J Bot. 2010;97(1):38–48. doi: 10.3732/ajb.0900083. [DOI] [PubMed] [Google Scholar]
- 19.Burkle LA, Marlin JC, Knight TM. Plant-pollinator interactions over 120 years: Loss of species, co-occurrence, and function. Science. 2013;339(6127):1611–1615. doi: 10.1126/science.1232728. [DOI] [PubMed] [Google Scholar]
- 20.Visser ME, Both C. Shifts in phenology due to global climate change: The need for a yardstick. Proc Biol Sci. 2005;272(1581):2561–2569. doi: 10.1098/rspb.2005.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hegland SJ, Nielsen A, Lázaro A, Bjerknes A-L, Totland Ø. How does climate warming affect plant-pollinator interactions? Ecol Lett. 2009;12(2):184–195. doi: 10.1111/j.1461-0248.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- 22.Miller-Rushing AJ, Høye TT, Inouye DW, Post E. The effects of phenological mismatches on demography. Philos Trans R Soc Lond B Biol Sci. 2010;365(1555):3177–3186. doi: 10.1098/rstb.2010.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart AJ. Interspecific competition reinstated as an important force structuring insect herbivore communities. Trends Ecol Evol. 1996;11(6):233–234. doi: 10.1016/0169-5347(96)30009-8. [DOI] [PubMed] [Google Scholar]
- 24.Farrer EC, Goldberg DE, King AA. Time lags and the balance of positive and negative interactions in driving grassland community dynamics. Am Nat. 2010;175(2):160–173. doi: 10.1086/649584. [DOI] [PubMed] [Google Scholar]
- 25.Post E. Ecology of Climate Change. Princeton: Princeton Univ Press; 2013. [Google Scholar]
- 26.Suttle KB, Thomsen MA, Power ME. Species interactions reverse grassland responses to changing climate. Science. 2007;315(5812):640–642. doi: 10.1126/science.1136401. [DOI] [PubMed] [Google Scholar]
- 27.Encinas-Viso F, Revilla TA, Etienne RS. Phenology drives mutualistic network structure and diversity. Ecol Lett. 2012;15(3):198–208. doi: 10.1111/j.1461-0248.2011.01726.x. [DOI] [PubMed] [Google Scholar]
- 28.Veresoglou DS, Fitter AH. Spatial and temporal patterns of growth and nutrient uptake of five co-existing grasses. J Ecol. 1984;72(1):259–272. [Google Scholar]
- 29.Waser NM. Competition for hummingbird pollination and sequential flowering in two Colorado wildflowers. Ecology. 1978;59(5):934–944. [Google Scholar]
- 30.Ghazoul J. Floral diversity and the facilitation of pollination. J Ecol. 2006;94(2):295–304. [Google Scholar]
- 31.Stiles FG. Coadapted competitors: The flowering seasons of hummingbird-pollinated plants in a tropical forest. Science. 1977;198(4322):1177–1178. doi: 10.1126/science.198.4322.1177. [DOI] [PubMed] [Google Scholar]
- 32.Mosquin T. Competition for pollinators as a stimulus for the evolution of flowering time. Oikos. 1971;22:398–402. [Google Scholar]
- 33.Pleasants J. Competition for bumblebee pollinators in Rocky Mountain plant communities. Ecology. 1980;61(6):1446–1459. [Google Scholar]
- 34.Walther GR, et al. Ecological responses to recent climate change. Nature. 2002;416(6879):389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 35.Walther GR. Community and ecosystem responses to recent climate change. Philos Trans R Soc Lond B Biol Sci. 2010;365(1549):2019–2024. doi: 10.1098/rstb.2010.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graham RW, et al. Spatial response of mammals to late quaternary environmental fluctuations. Science. 1996;272(5268):1601–1606. doi: 10.1126/science.272.5268.1601. [DOI] [PubMed] [Google Scholar]
- 37.Williams JW, Jackson ST. Novel climates, no-analog communities, and ecological surprises. Front Ecol Environ. 2007;5:475–482. [Google Scholar]
- 38.Steltzer H, Post E. Ecology. Seasons and life cycles. Science. 2009;324(5929):886–887. doi: 10.1126/science.1171542. [DOI] [PubMed] [Google Scholar]
- 39.Olesen JM, Bascompte J, Elberling H, Jordano P. Temporal dynamics in a pollination network. Ecology. 2008;89(6):1573–1582. doi: 10.1890/07-0451.1. [DOI] [PubMed] [Google Scholar]
- 40.Cleland EE, Chuine I, Menzel A, Mooney HA, Schwartz MD. Shifting plant phenology in response to global change. Trends Ecol Evol. 2007;22(7):357–365. doi: 10.1016/j.tree.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Høye TT, Post E, Schmidt NM, Trøjelsgaard K, Forchhammer MC. Shorter flowering seasons and declining abundance of flower visitors in a warmer Arctic. Nature Climate Change. 2013;3(8):759–763. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.