Significance
By using a series of cell-type–restricted Adeno-Cre vectors, we show that expression of mutant K-Ras in different cell types in mouse lungs can give rise to adenocarcinomas. Moreover, the cell-of-origin appears to be a determining factor in the histopathological characteristics of the resulting tumor. This is most apparent in the early stages of tumor development, whereby different routes of tumor development are taken when either Clara cells or alveolar type 2 cells serve as the initiating cell type. Both the physical site of onset and the marker expression of the early lesions are distinct. Future studies should reveal whether this affects other tumor characteristics, such as the mutation spectrum and response to treatment.
Keywords: non small cell lung cancer, NSCLC, bronchioalveolar stem cells, BASCs
Abstract
Much controversy surrounds the cell-of-origin of mutant K-Ras (K-RasG12D)–induced lung adenocarcinoma. To shed light on this issue, we have used technology that enables us to conditionally target K-RasG12D expression in Surfactant Protein C (SPC)+ alveolar type 2 cells and in Clara cell antigen 10 (CC10)+ Clara cells by use of cell-type–restricted recombinant Adeno-Cre viruses. Experiments were performed both in the presence and absence of the tumor suppressor gene p53, enabling us to assess what effect the cell-of-origin and the introduced genetic lesions have on the phenotypic characteristics of the resulting adenocarcinomas. We conclude that both SPC-expressing alveolar type 2 cells and CC10-expressing Clara cells have the ability to initiate malignant transformation following the introduction of these genetic alterations. The lungs of K-Raslox–Stop–lox–G12D/+ and K-Raslox–Stop–lox–G12D/+;tumor suppressor gene Trp53F/F mice infected with Adeno5–SPC–Cre and Adeno5–CC10–Cre viruses displayed differences in their tumor spectrum, indicating distinct cellular routes of tumor initiation. Moreover, using a multicolor Cre reporter line, we demonstrate that the resulting tumors arise from a clonal expansion of switched cells. Taken together, these results indicate that there are multiple cellular paths to K-RasG12D–induced adenocarcinoma and that the initiating cell influences the histopathological phenotype of the tumors that arise.
Non-small-cell lung cancer (NSCLC) includes a broad range of tumors. There is significant heterogeneity within this subclass of lung tumors encompassing squamous cell carcinomas and adenocarcinomas as the major subtypes. In line with this heterogeneity, different mutations have been found that give rise to this disease. Oncogenic mutation in the K-RAS gene is found in ∼25% of human adenocarcinomas (1).
Much controversy surrounds the identity of the cell-of-origin of K-RasG12D–induced lung cancer. For many years human adenocarcinomas were thought to arise from transformed alveolar type 2 cells (AT2) cells, as a hallmark feature of this tumor subtype is the expression of Surfactant Protein C (pro-SPC or Sftpc), a well-characterized marker of AT2 cells. However, recent studies in mouse models suggest that this may not be the case. In fact a very rare cell population residing at the bronchioalveolar duct junction (BADJ), a well-established stem cell niche (2), has been proposed to be the target cell of K-Ras–driven lung cancer (3–6). Loss of the tumor suppressors p27 and Pten also results in lung tumors that might originate from these cells (7, 8). This rare cell population was shown to coexpress Clara cell antigen 10 (CC10) and surfactant protein C (SPC) (4, 9). However, using alternative genetic approaches, Xu et al. (10) more recently concluded that AT2 cells, but not Clara cells, are the predominant cancer-initiating cells of K-RasG12D–induced lung adenocarcinoma.
Emerging studies have highlighted the importance of specific genetic alterations and how these aberrations may be able to drive different cell types down the same lineage. An insertional mutagenesis screen in which transposon mutagenesis targeted to different T-cell progenitors selects for different sets of mutations to give rise to T-cell acute lymphoblastic leukemia (T-ALL) (11) elegantly exemplifies this. These findings indicate that cells at different positions along a lymphoid differentiation pathway can be turned into a T-ALL–initiating cell when supplied with the right set of oncogenic lesions. Likely this can also occur in the lung with its diversity of cell types and range of progenitors needed to secure maintenance of this complex organ structure. However, some cell types are more likely to serve as the cell-of-origin of a cancer than others, dictated by the probability to acquire and accumulate the necessary mutations to cause tumorous growth.
To investigate the cell-of-origin of lung adenocarcinoma and the impact of different genetic modifications on the oncogenic transformation of a specific cellular compartment, we used two well-characterized mouse models of human NSCLC (9, 12). Activation of K-RasG12D either alone or in combination with Trp53 loss was carried out in different epithelial cells in the lung using several cell-type–restricted Adeno5 (Ad5)–Cre viruses (13). Using this technology we provide evidence that both CC10+ Clara cells and SPC+ AT2 cells can form adenocarcinomas in response to K-RasG12D activation. Moreover, using the R26R-Confetti multicolor Cre reporter mouse, we demonstrate that the lesions that form in the alveolar duct area of K-raslox–Stop–lox–G12D/+ mice following infection with the Clara cell-specific Ad5–CC10–Cre virus arise from the clonal expansion of CC10+ cells resident at the BADJ. In addition, we show that the additional loss of Trp53 gives rise to invasive and metastatic tumors in the vast majority of the cases.
Results
K-RasG12D Activation and Trp53 Loss in Specific Lung Cell Types Using Cell-Type–Restricted Ad5–Cre Recombinant Vectors.
To determine what influence the cell-of-origin and the specific genetic alterations have on the properties of the tumor, we induced lung tumors in mice harboring a LSL–K-RasLSL-G12D/+ knock-in allele or mice harboring the K-RasLSL-G12D/+ allele in conjunction with Trp53 conditional alleles (K-RrasLSL-G12D/+;Trp53F/F) (9, 12–14). Cre-recombinase was delivered via intratracheal administration of recombinant adenoviral vectors that express Cre-recombinase specifically in Clara cells (CC10) or AT2 cells (9, 12–14). We chose to focus on targeting CC10- and SPC-expressing cells, as rare cells that express both of these markers have been hypothesized to be the cell-of-origin of K-RasG12D–driven adenocarcinoma formation (4, 10). Our recently described cell-type–restricted Ad5–Cre vectors exhibit a high level of cell-type selectivity and have proven valuable tools in assessing the cell-of-origin of small-cell lung cancer (13). The histopathology of the lesions observed in K-RasLSL-G12D/+ and K-RasLSL-G12D/+;Trp53F/F mice following infection with cell-type–restricted Ad5–Cre viruses revealed a process of tumorigenesis from hyperplasia to adenoma and finally to adenocarcinoma.
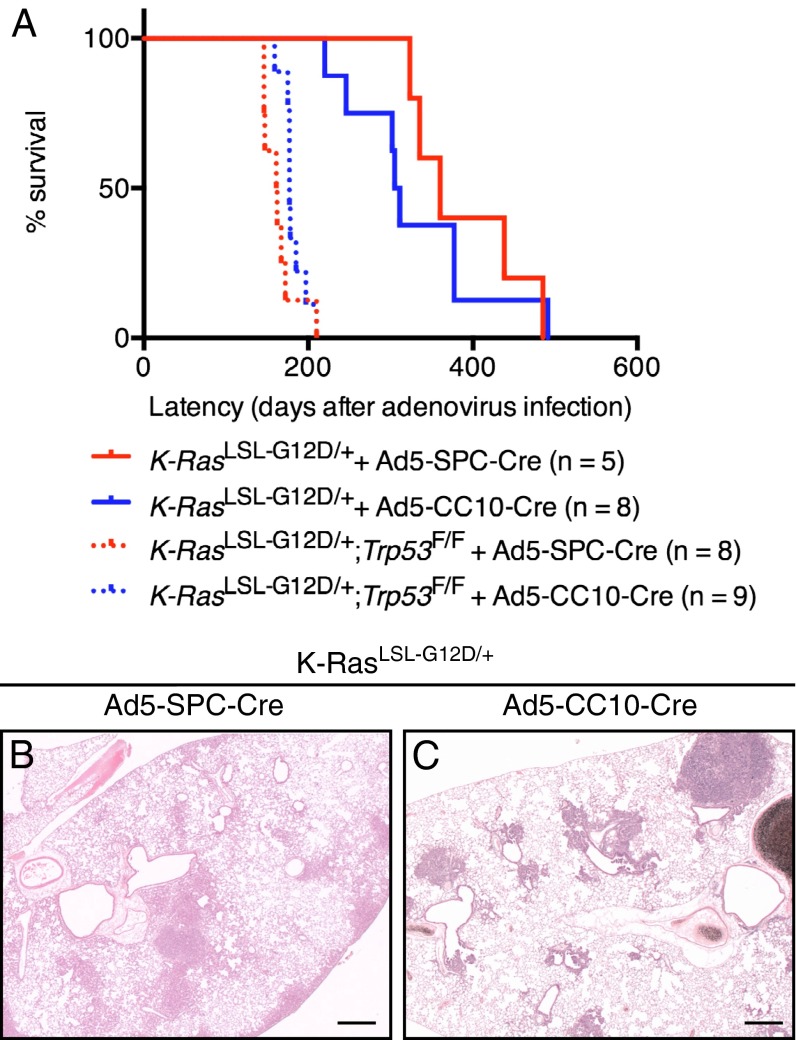
The median survival rate of K-RasLSL-G12D/+ animals infected with the Ad5–SPC–Cre virus (median 360 d, n = 5) was similar to that observed following Ad5–CC10–Cre infection (median 308 d, n = 8) (Fig. 1A). Thirty-two weeks following Ad5–SPC–Cre infection K-RasLSL-G12D/+ mice showed adenomas and adenocarcinomas that were distributed throughout the alveolar space (Fig. 1B). In contrast, the lesions found in Ad5–CC10–Cre-infected K-RasLSL-G12D/+ mice were generally associated with the BADJ (Fig. 1C). In line with previous findings, loss of Trp53 strongly accelerated the progression of K-RasG12D–induced lung tumors (12), with K-RasLSL-G12D/+;Trp53F/F animals exhibiting a similarly reduced latency following infection with either Ad5–SPC–Cre or Ad5–CC10–Cre (Ad5–SPC–Cre, 162 d, n = 8; Ad5–CC10-Cre, 177 d, n = 9) (Fig. 1A). These findings indicate that the tumor progression capacity of both SPC- and CC10-expressing cells is very similar.
Fig. 1.
Using a cell-type–restricted adenoviral infection approach to identify the cell-of-origin of K-RasG12D–induced lung adenocarcinomas. (A) Kaplan Meier survival curves of K-RasLSL-G12D/+ mice (solid lines) and K-RasLSL-G12D/+;Trp53F/F mice (dashed lines) infected with Ad5–SPC–Cre virus (red curve) or Ad5–CC10–Cre virus (blue curve). The median survival of K-RasLSL-G12D/+ and K-RasLSL-G12D/+;Trp53F/F mice infected with Ad5–SPC–Cre virus is 360 d (n = 5) and 162 d (n = 8), respectively. The median survival of K-RasLSL-G12D/+ and K-RasLSL-G12D/+;Trp53F/F mice infected with Ad5–CC10–Cre virus is 308 d (n = 8) and 177 d (n = 9), respectively. (B) A representative H&E section from a K-RasLSL-G12D/+ animal examined 32 wk following Ad5–SPC–Cre infection. The majority of lesions arise in the alveolar compartment at the periphery of the lung. (C) A representative H&E section from a K-RasLSL-G12D/+ animal 32 wk following Ad5–CC10–Cre infection. The majority of lesions observed are associated and/or in close proximity to the BADJ. (Scale bar in B and C, 500 μM.)
Tumor Spectrum Observed in K-RasLSL-G12D/+ and K-RasLSL-G12D/+;Trp53F/F Mice Varies Depending on the Cell-of-Origin.
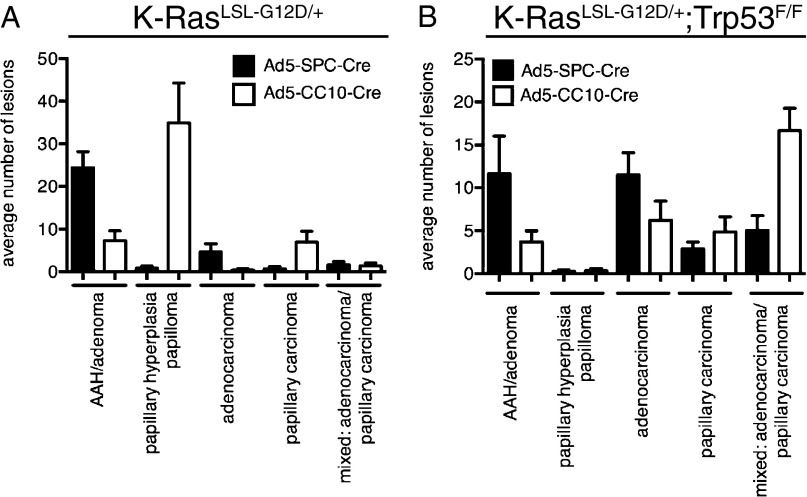
There is emerging evidence in other solid tumors that the target cell of transformation has an important influence on tumor cell fate and tumor pathology (reviewed in ref. 15). To determine if this is also the case for lung adenocarcinomas, we classified the histopathology of lesions observed in K-RasLSL-G12D/+ and K-RasLSL-G12D/+;Trp53F/F mice following infection with Ad5–SPC–Cre and Ad5–CC10–Cre viruses. This classification, shown in Fig. 2 A and B, provides us with information about the progression states of different lesions in the lungs. Morphological changes in the peripheral area of the lung, such as alveolar hyperplasia and alveolar adenomatous hyperplasia (AAH), were far more pronounced in Ad5–SPC–Cre-infected K-RasLSL-G12D/+ animals with a fraction of these lesions progressing to adenocarcinomas (Fig. 2A). In contrast, lesions observed at the terminal bronchioles, such as papillary hyperplasia and papilloma, observed in Ad5–CC10–Cre-infected K-RasLSL-G12D/+ mice were never seen in K-RasLSL-G12D/+ mice following Ad5–SPC–Cre infection (Fig. 2A). In line with this finding, a higher number of papillary carcinomas were observed in the lungs of Ad5–CC10–Cre-infected K-RasLSL-G12D/+ mice compared with animals infected with Ad5–SPC–Cre (Fig. 2A).
Fig. 2.
Distinct cells-of-origin of K-RasG12D–induced lung adenocarcinoma. (A) Quantification of lesions observed in the lungs of K-RasLSL-G12D/+ following infection with Ad5–SPC–Cre virus (black bars; n = 5; 323–485 d) and Ad5–CC10–Cre virus (white bars; n = 8; 220–491 d). (B) Quantification of lesions observed in the lungs of K-RasLSL-G12D/+;Trp53F/F mice following infection with Ad5–SPC–Cre virus (black bars; n = 8; 146–210 d) and Ad5–CC10–Cre virus (white bars; n = 9; 159–197 d). The bar graphs represent the average number of lesions per lung: AAH/adenomas, papillary hyperplasia/papilloma, adenocarcinoma, papillary carcinoma, mixed carcinoma:adenocarcinoma/papillary carcinoma, per mouse examined. Data represent means ± SEM.
Consistent with previous reports (12, 15), poorly differentiated carcinomas were more frequently observed in K-RasLSL-G12D/+;Trp53F/F mice following infection with both Ad5–Cre viruses (Fig. 2B). These tumors contained areas of trabecular arrangement, spindle cell transformation, and desmoplastic stroma (Fig. S1A). Moreover, these histological characteristics are closely associated with the immunohistochemical expression pattern of Nkx2-1 and Hmga2 (16). High expression of Nkx2-1 is observed in well- to moderate-differentiated areas (Fig. S1B), whereas high Hmga2 expression is detected in more poorly differentiated areas (Fig. S1C). In tumors from K-RasLSL-G12D/+;Trp53F/F mice, high Hmga2 expression has been implicated in the progression of lung adenocarcinomas to an invasive and metastatic state (15) Tumors induced by Ad5–CC10–Cre tended to be more invasive and metastatic than tumors induced in Ad5–SPC–Cre-infected K-RasLSL-G12D/+;Trp53F/F, although in both cases loss of Trp53 appeared to be an important driver of tumor progression. In line with the observations made by Winslow et al. (16), all invading tumors (Fig. S1 D and G) exhibited an absence of Nkx2-1 expression (Fig. S1 E and H) and high expression levels of Hmga2 (Fig. S1 F and I).
Taken together, the tumor spectrum observed in both K-RasLSL-G12D/+ and K-RasLSL-G12D/+;Trp53F/F mice support a different cell-of-origin of these lesions.
Unique Tumor Initiation and Development Pattern Following Oncogenic K-Ras in Distinct Cell Types.
To more closely trace both tumor initiation and progression within individual cellular compartments, we examined the lungs of K-RasLSL-G12D/+ mice at defined time- points following adenovirus infection. Lesions were examined and characterized with respect to their cellular characteristics, organization, and spatial location.
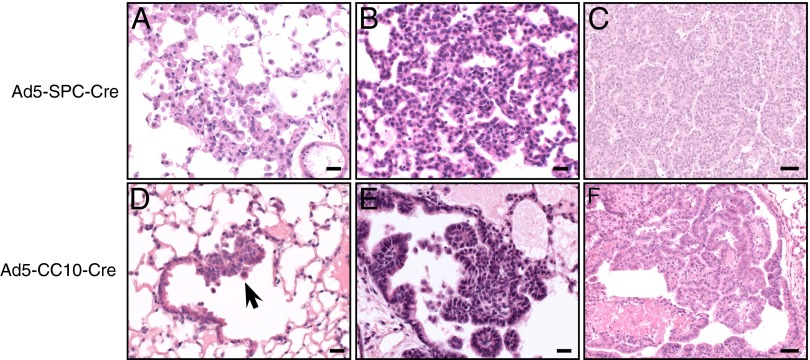
Histological examination of K-RasLSL-G12D/+ lungs taken for necropsy 8–12 wk following Ad5–SPC–Cre infection revealed the presence of focal hyperplasia of the AT2 cells (Fig. 3A). These lesions were characterized by an increase in cellularity and were often seen as single-layered regions along the preexisting alveolar septa. These lesions developed into AAH, characterized by a further increase in cellularity of the AT2 cells. As shown in Fig. 3B, the alveolar structures within the AAH lesions are still recognizable. Histological analysis of lungs collected from Ad5–SPC–Cre-infected K-RasLSL-G12D/+ mice killed when they became moribund revealed the presence of adenocarcinomas (Fig. 3C). These tumors were composed of small cuboidal cells with solid or papillary-like structures and exhibited a moderate differentiated appearance, atypia, necrosis, and/or hemorrhages. Importantly, no abnormalities were observed in cells lining the bronchioles at all time points examined.
Fig. 3.
Tumor initiation and progression occurs in distinct cell types and locations following K-RasG12D activation. (A–C) Microphotographs of H&E-stained sections from K-RasLSL-G12D/+ mice following Ad5–SPC–Cre infection showing (A) alveolar hyperplasia, (B) AAH, and (C) adenocarcinoma. (D–F) H&E stained sections from K-RasLSL-G12D/+ mice following Ad5–CC10–Cre infection showing (D) focal hyperplasia of bronchiolar epithelial cells residing at the terminal bronchiole (arrow), (E) papillary hyperplasia of bronchiolar epithelial cells at the terminal bronchiole, and (F) papillary carcinoma in the lumen of a dilated bronchiole. (Scale bar in A, B, D, and E, 20 μM; C and F, 50 μM.)
Activation of K-RasG12D in CC10-expressing cells resulted in lesions that could be readily distinguished from the initial lesions observed in Ad5–SPC–Cre-infected K-RasLSL-G12D/+ mice. Focal hyperplasia of the bronchiolar epithelial cells was detected in K-RasLSL-G12D/+ mice as early as 8 wk following Ad5–CC10–Cre infection, and almost exclusively restricted to the terminal bronchioles (Fig. 3D). No abnormalities were detected at this early stage in the alveolar septa. At 18 wk, the hyperplasia at the BADJ became more pronounced, consisting of papillary proliferations of the epithelial cells lining the bronchioles that projected into the airway lumen (Fig. 3E). Finally, papillary carcinomas were observed in the lungs of Ad5–CC10–Cre-infected K-RasLSL-G12D/+ mice killed when they became moribund. These large nodular or irregular lesions present in the bronchioles (Fig. 3F) as well as in the alveolar spaces exhibited malignancy features such as atypia and necrosis.
CC10/SPC Dual-Positive Cells Are Not the Primary Cell-of-Origin of K-RasG12D–Induced Lung Tumors.
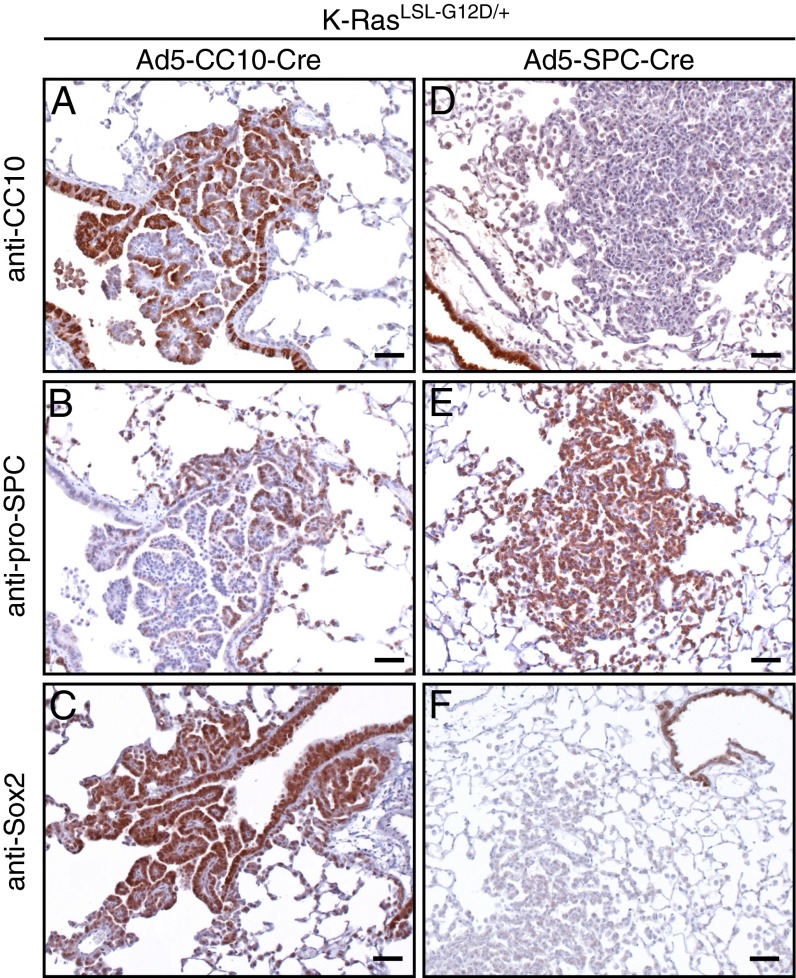
A rare cell type localized at the BADJ and characterized by coexpression of CC10 and SPC has been suggested to be the cell-of-origin of K-RasG12D–induced lung tumors (4). Therefore, we were interested in examining the expression pattern of CC10 and SPC in the lungs of K-RasLSL-G12D/+ mice following Ad5–SPC–Cre and Ad5–CC10–Cre infection.
Ad5–CC10–Cre infection of K-RasLSL-G12D/+ lungs resulted in areas of papillary hyperplasia at the terminal bronchioles with a heterogeneous immunostaining pattern (Fig. 4). Immunohistochemical staining of 4 μm serial sections revealed that the vast majority of the hyperplastic cells stained positive for CC10 (Fig. 4A), whereas a small number were positive for the AT2 cell marker, SPC (Fig. 4B). Expression of Sox2 was recently demonstrated to differentiate K-RasG12D lesions that were derived from CC10- or SPC-expressing cells (10). Consistent with these findings, lesions located at the terminal bronchiole were strongly positive for Sox2 (Fig. 4C), confirming that these lesions are derived from transformed CC10-expressing cells. Sox2 expression was partially lost in more progressed lesions (10).
Fig. 4.
Immunohistochemical heterogeneity of papilloma and AAH at the terminal bronchiole and alveoli. (A–C) Sections (4 μm) of papilloma observed in K-RasLSL-G12D/+ mice following Ad5–CC10–Cre infection at the terminal bronchiole, (A) showing positive staining for the Clara cell marker, CC10. (B) A number of hyperplasic cells (CC10+) at the terminal bronchiole stain positive for the AT2 cell marker, pro-SPC. (C) Sox2-positive staining of cells lining bronchiolar and hyperplasic cells at the terminal bronchiole. (D–F) Representative images of AAH and adenoma observed in K-RasLSL-G12D/+ mice following Ad5–SPC–Cre infection. (D) Adenomas are negative for the Clara cell marker, CC10. (E) AAH stain positive for the AT2 marker, pro-SPC, but are negative for Sox2 (F). (Scale bar in A–F, 50 μM.)
To determine whether individual cells within these lesions were expressing both CC10 and SPC, we performed dual immunofluorescence analysis. As shown in Fig. S2A, and previously by others (4, 10), hyperplasic cells that stained positive for CC10 and SPC were observed at the BADJ following K-RasG12D activation in CC10-expressing cells. Papillary hyperplasic regions at the BADJ consisted of a small number of CC10/SPC dual-positive cells, in addition to single CC10+ and single SPC+ cells (Fig. S2B). Advanced lesions found in the alveoli, juxtaposed to the CC10-positive hyperplastic areas, were SPC+ but had partially lost CC10 (Fig. S2C) and Sox2 expression (Fig. S3).
Areas of alveolar hyperplasia, AAH, adenomas, and adenocarcinomas observed in the alveolar septa following K-RasG12D activation induced by Ad5–SPC–Cre virus were found to be negative for CC10 (Fig. 4D) but strongly positive for SPC (Fig. 4E and Fig. S2 D–F). Moreover, the hyperplasia and adenomas that form following induction of K-RasG12D in SPC-expressing cells are Sox2 negative (Fig. 4F). Taken together, these results indicate that these lesions arose from transformed AT2 cells. No abnormalities were observed at the BADJ in Ad5–SPC–Cre-infected K-RasLSL-G12D/+ mice, and in line with this finding, we observed no increase in the number of CC10/SPC dual-positive cells in the lungs of these animals (Fig. S2 D–F). The lack of any hyperplasia in the BADJ region upon inoculation with Ad5–SPC–Cre virus suggests that either expression of the Ad5–SPC–Cre virus in CC10/SPC dual-positive cells is too low to activate the conditional K-RasLSL-G12D/+ allele or that the CC10/SPC double-positive cells are not the cell-of-origin of these lesions. The double-positive cells we do observe in the lesions induced by Ad5–CC10–Cre virus may therefore represent CC10 single-positive cells that have become temporarily double-positive during their transition to SPC single-positive adenomas/adenocarcinomas.
K-RasG12D Tumors Arise from Clonal Expansion of Switched Cells.
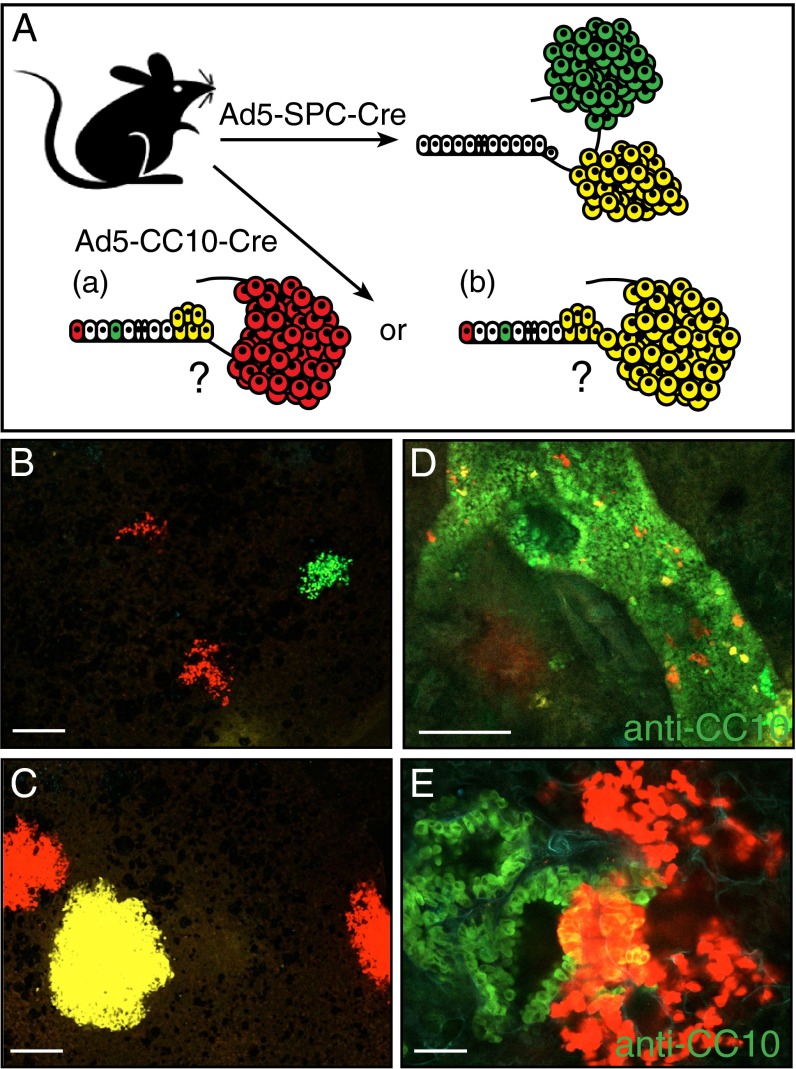
To investigate the clonality of K-RasG12D–induced lesions, we exploited the multicolor Cre reporter mouse, R26R-Confetti, whereby Cre-mediated recombination results in the expression of one of four different fluorescent proteins (17). Infection of K-RasLSL-G12D/+;R26R-Confetti mice with either Ad5–SPC–Cre or Ad5–CC10–Cre virus allowed us to lineage trace the cancer-initiating cells and determine the clonality of the resulting lesions (Fig. 5A). In particular, we were interested in establishing the relationship between lesions observed at the BADJ and adjacent alveolar compartment in K-RasLSL-G12D/+ mice following Ad5–CC10–Cre infection. K-RasLSL-G12D/+;R26R-Confetti animals were infected with either Ad5–SPC–Cre or Ad5–CC10–Cre, and lung tissue was examined 12–18 wk following adenovirus infection. Both initial and more progressed lesions in the K-RasLSL-G12D/+;R26R-Confetti mice were indistinguishable from those observed in K-RasLSL-G12D/+ mice, indicating that expression of the confetti reporter did not modulate the tumor phenotype. Areas of hyperplasia that expressed a uniform Confetti color were observed following Ad5–SPC–Cre infection (Fig. 5 B and C). Interestingly, we did not observe single Confetti+ cells in the alveoli of Ad5–SPC–Cre-infected K-RasLSL-G12D/+;R26R-Confetti mice. However, we suspect that this is due to their lack of visibility upon confocal imaging, as in studies using LacZ reporters single stained cells were easily observed upon histochemical staining (13, 18). Taken together, these results imply that the lesions form as a result of clonal expansion of a single Cre-recombined SPC-expressing cell. We observed a distinct Confetti expression pattern in the lungs of K-RasLSL-G12D/+;R26R-Confetti animals following Ad5–CC10–Cre infection. A large number of single Confetti+ cells were detected in addition to clusters of Confetti+ cells with the same color. Immunofluorescence staining of whole-mount lung tissue sections with an anti-CC10 antibody revealed the bronchiolar structure in lung tissue sections. Virtually all Confetti+ cells were contained within the bronchiolar epithelium. Interestingly, not all Confetti+ “Cre-recombined” cells were capable of K-RasG12D–induced expansion, indicating that only a subfraction of the CC10-expressing cells can undergo transformation by K-RasG12D expression. As depicted in Fig. 5D, early hyperplasic lesions (Confetti red) consisted of cells that costained with CC10 (green) and cells that did not costain with CC10. More progressed lesions extending into the alveoli from the BADJ also contain CC10+ and CC10− cells, while exhibiting a single Confetti color (Fig. 5E), implying a clonal relationship between lesions observed at the BADJ and in the alveoli.
Fig. 5.
Clonal outgrowth of CC10+ and SPC+ cells following K-RasG12D activation. (A) Schematic representation of the genetic strategy to trace CC10- and SPC-expressing cells in K-RasG12D lung lesions. Regarding the lesions observed in K-RasLSL-G12D/+ mice following Ad5–CC10–Cre infection, (a) do the lesions in the alveoli arise from CC10-expressing cells present in the alveoli, or (b) do the lesions in the alveoli arise from transformed cells present at the BADJ? (B and C) Confocal imaging of multiple areas of alveolar hyperplasia (B) and AAH/adenomas (C) present in the lungs of K-RasLSL-G12D/+;R26R-Confetti animals following Ad5–SPC–Cre infection. Each lesion is marked uniformly by a single Confetti color. (D and E) Confocal imaging of CC10-stained lung tissue sections from a K-RasLSL-G12D/+;R26R-Confetti mouse following Ad5–CC10–Cre infection. (D) Single Confetti+ switched cells resident on the bronchiolar wall stained positive with anti-CC10 immunofluorescence staining. This immunofluorescence staining pattern indicates that not all K-RasG12D–expressing (Confetti+) switched cells are capable of further expansion/proliferation. (E) Areas of clonal hyperplasia (red Confetti color) located at the terminal bronchiole contain cells that coexpress the Clara cell marker, CC10 (yellow). (Scale bar in B–D, 100 μM; E, 500 μM.)
Discussion
In the present study, we have used lung cell-type–restricted Ad5–Cre viruses to target oncogenic K-RasG12D expression with and without the additional loss of Trp53 to SPC- and CC10-expressing cells in the adult mouse lung. Our data show that contrary to previous findings both target cell populations can give rise to adenocarcinomas when K-RasG12D is activated. Moreover, Trp53 loss concomitant with K-RasG12D activation leads to an overall faster tumor progression in both Ad5–SPC–Ce- and Ad5–CC10–Cre-induced tumors.
The cell-of-origin of K-RasG12D–induced lung tumors has been previously studied (4). Using an Ad5–CMV–Cre virus, which resulted in expression of K-RasG12D in a wide variety of lung epithelial cell types, Kim et al. (4) observed expansion of CC10+ SPC+ cells at the BADJ. In this model this expansion was taken as evidence that CC10+ SPC+ cells are the cells-of-origin of K-RasG12D–induced tumors. Use of our cell-type–restricted Ad5–Cre viruses (13) permitted us to define more specifically the cell-of-origin of K-RasG12D–induced lung cancer. The absence of tumor formation at the BADJ in K-RasLSL-G12D/+ mice following Ad5–SPC–Cre infection argues against bronchioalveolar stem cells (BASCs) being the cells giving rise to adenocarcinomas and supports the findings made by Xu et al. (10). We identify AT2 cells as the predominant cell-of-origin of NSCLCs induced by K-RasG12D activation. The complete absence of Sox2, a characteristic of early lesions induced in CC10-expressing cells (10), in Ad5–SPC–Cre-induced lesions argues in favor of a CC10 single-positive cell as the cell-of-origin of tumors arising in the BADJ region. The fact that we did not see switched cells at the BADJ following Ad5–SPC–Cre infection is in line with this observation, although we cannot formally exclude that our Ad5–SPC–Cre virus does not direct sufficiently high expression of Cre in the CC10/SPC double-positive cells identified in this region. To definitively assess whether CC10/SPC double-positive cells can give rise to these tumors, methodologies need to be developed that can unequivocally demonstrate the targeting of specifically this dual-positive cell population.
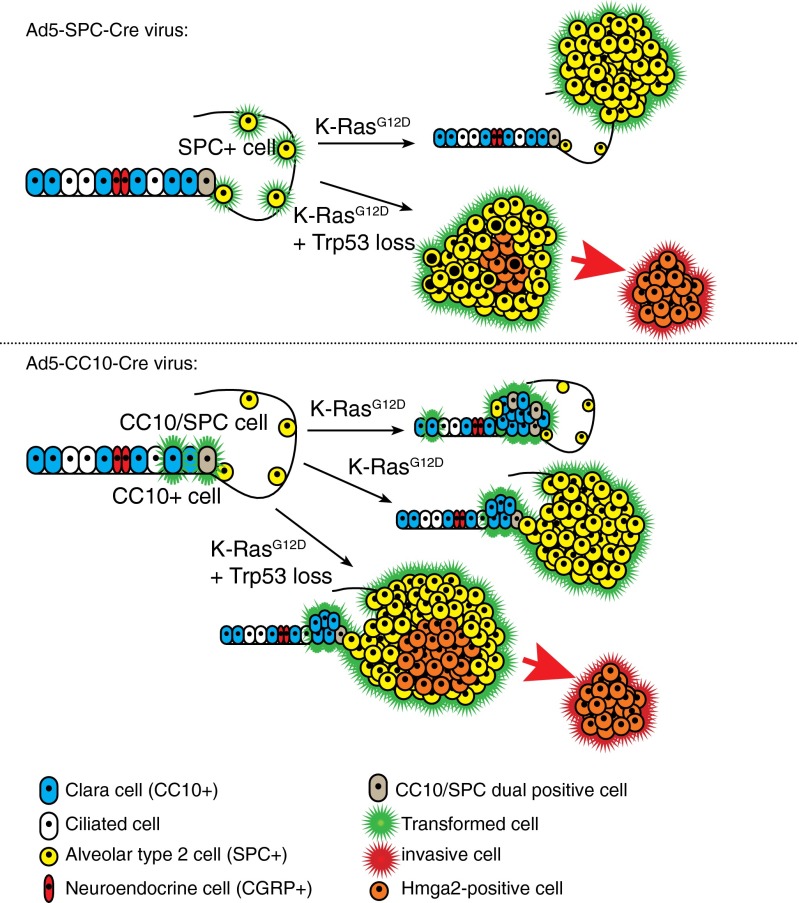
Our results show that CC10-expressing cells display different responses to K-RasG12D activation. Although K-RasG12D is activated in many Clara cells that line the larger bronchi and bronchioles, we do not frequently observe hyperplasia or tumor formation in this region. These observations are consistent with those made by Xu et al. (10) and imply a level of heterogeneity within CC10+ Clara cell compartment. We did, however, observe hyperplasia and subsequent tumor formation in CC10-expressing cells resident at the terminal bronchiole (Fig. 6).
Fig. 6.
Schematic representation of the consequences of K-RasG12D activation and Trp53 loss in distinct lung epithelial cells. (Upper) Ad5–SPC–Cre virus directs Cre-recombinase activity to SPC-expressing AT2 cells (yellow). Activation of K-RasG12D in these cells (green halo) results in adenoma and adenocarcinoma formation in the alveoli. Cells resident in the bronchiolar lining and BADJ are normal. The additional loss of Trp53 in Ad5–SPC–Cre-infected K-RasLSL-G12D/+;Trp53F/F animals results in moderate to poorly differentiated adenocarcinomas. Well- to moderately differentiated areas of the tumors stain positive for are Nkx2-1, whereas Hmga2-positive tumor cells (orange) are more poorly differentiated. (Lower) In a wild-type lung, Clara cells (blue) lining the bronchiolar and CC10+SPC+ BASC cells (brown) resident at the BADJ are targeted by the Ad5–CC10–Cre virus. Infection of K-RasLSL-G12D/+ mice with Ad5–CC10–Cre results in papillary hyperplasia at the BADJ that involve not only CC10+SPC+ BASCs but also Clara cells. Moreover, some hyperplasic cells lose expression of CC10, but gain expression of the AT2 cell marker, SPC, resulting in SPC+ adenomas in Ad5–CC10–Cre-infected K-RasLSL-G12D/+ animals, and these are generally associated with the BADJ. The additional loss of Trp53 in K-RasLSL-G12D/+;Trp53F/F mice results in adenocarcinomas with pleomorphism and atypia. These tumors express Hmga2 (orange cells) and have the propensity to invade (red arrow).
In an elegant lineage-tracing experiment, Rawlins et al. (19) has reported the existence of rare cells residing in the alveoli that express the CC10 lineage mark following four consecutive applications of tamoxifen (tmx) in CC10–CreER mice. In subsequent use of this mouse line to address the cell-of-origin of K-RasG12D–induced lung cancer, Xu et al. concluded that these CC10+ AT2 cells give rise to the adenomas/adenocarcinomas they observed in CC10–CreER;K-RasLSL-G12D/+ animals (10). Unlike the observations reported by Rawlins et al. (19), we do not observe any Cre-recombined cells in the alveoli of Rosa26R–lacZ reporter mice following Ad5–CC10–Cre infection (13). The first lesions that we observe in the lungs of K-RasLSL-G12D/+ mice following Ad5–CC10–Cre infection are focal areas of bronchiole hyperplasia at the BADJ (Fig. 3D). In these lesions SPC+ cells can be detected (Fig. 4B), suggesting that Ras pathway activation facilitates differentiation of Clara cells toward SPC-expressing cells in the BADJ area. Lineage-tracing experiments performed in steady-state conditions and in response to lung injury clearly demonstrated that CC10+SPC+ putative BASCs are unable to give rise to SPC+ alveolar cells (19). However, under specific conditions (such as K-RasG12D expression as we report here) as well as under inflammatory stress as reported by the group of Jianzhu Chen (20), such transdifferentiation might nevertheless occur. Occasionally, we observed apparently normal alveolar cells in the vicinity of BADJ regions of Ad5–CC10–Cre switched K-RasLSL-G12D/+ mice, suggesting that K-RasG12D expression in a subpopulation of CC10+ cells resident in the BADJ region can permit cells to undergo AT2 cell differentiation, possibly facilitated by an inflammatory response induced by the adenovirus. Using the multicolor R26R-Confetti reporter mouse (17) to assess the clonal relationship between hyperplasia at the BADJ and the adenomas found adjacent in the alveolar space following K-RasG12D activation in CC10-expressing cells, we also observed a transition from CC10+SPC− toward CC10−SPC+ cells during tumor development. Examination of whole-mount lung tissue isolated from these animals demonstrated a clonal relationship between CC10-expressing switched cells at the BADJ and the subsequent expansion of CC10− hyperplasic cells in this region (Fig. 5), which in parallel experiments were demonstrated to be SPC+ positive. Therefore, we favor a sequence of events in which expression of K-RasG12D in CC10-positive cells in the BADJ area results in local hyperplasia of CC10-positive cells that progress to CC10+SPC+ cells and finally to CC10−SPC+ adenomas (Fig. 6). The capacity of K-rasLSL-G12D/+ to facilitate “transdifferentiation” of CC10+ cells into SPC+ cells, as we observe here, might reconcile the apparent discrepancy between lineage-tracing experiments performed in wild-type (19) and K-RasLSL-G12D/+ mutant mice (4).
In conclusion, our observations show that multiple cell types in lung can give rise to adenocarcinomas. Dependent on the cell-of-origin, the phenotypic characteristics differ and this will likely also have ramifications for other tumor-specific characteristics, possibly including their response to therapy.
Materials and Methods
Mouse Strains.
All experiments involving animals comply with local and international regulations and ethical guidelines and have been authorized by our local experimental animal committee at The Netherlands Cancer Institute. K-RasLSL-G12D/+ (9) and Trp53F2-10/F2-10 (14) are maintained on a C57BL/6J background. R26R-Confetti (17) mice have been previously described.
Intratracheal Adeno-Cre Virus Administration.
Seven-week-old mice were treated with cyclosporine A (Novartis) orally in the drinking water, 1 wk before adenovirus administration and 2–3 wk following adenovirus infection. Mice were intratracheally intubated with 20 μL of 1 × 1010 pfu/mL purified Adeno-Cre viruses: Ad5–SPC–Cre and Ad5–CC10–Cre virus. Experimental animals were killed at defined time points following Ad5–Cre infection (Table S1 and Table S2). Briefly, K-RasLSL-G12D/+ mice were killed 8, 12, 18, 24, and 32 wk and K-RasLSL-G12D/+;Trp53F/F mice were killed 4, 8, and 18 wk following Ad5–Cre infection. A cohort of K-RasLSL/G12D/+ and K-RasLSL-G12D/+;Trp53F/F mice were also killed when they showed signs of illness (breathing abnormalities and weight loss).
Immunohistochemistry Staining.
For histological analysis, lungs were inflated with ethanol–acetic acid–formalin (EAF) and fixed for 24 h in EAF. Fixed tissues were subsequently dehydrated, embedded in paraffin and sections (2 μm), prepared, and stained with hematoxylin and eosin (H&E). For immunohistochemistry, tissue sections were rehydrated, blocked in BSA containing PBS, and sequentially incubated with specific primary antibodies and with biotinylated secondary antibodies (DAKO). We carried out immunohistochemistry for anti-CCSP (goat polyclonal, 1:5,000), anti–pro-SPC (rabbit polyclonal, 1:2,000, Chemicon), and anti-Sox2 (rabbit polyclonal, 1:1,000, Millipore). Streptavidin-peroxidase (DAKO) or Powervision Poly-HRP (Leica Microsystems) was used for visualization and diaminobenzidine as a chromagen (DAKO).
K-RasLSL-G12D/+;R26R-Confetti Lung Tissue Preparation for Confocal Analysis.
For semithick sectioning of K-RasLSL-G12D/+;R26R-Confetti tissue, lungs were inflated and fixed in 4% (vol/vol) paraformaldehyde at room temperature for 1 h, and washed briefly in PBS. Lung tissue sections (150–200 μm) were cut manually and embedded directly in Vectashield (Vector Laboratories). For whole-mount staining, K-RasLSL-G12D/+;R26R-Confetti lung tissue were permeablized with 0.2% Triton X-100 in PBS for at least 1 h at room temperature. Sections were incubated with goat anti-CC10 antibody (1:200, Santa Cruz) in blocking solution overnight at 4 °C. After washing, sections were incubated with Alexa Fluor 488 diluted 1:400 in PBS for 1 h at room temperature and then washed and mounted directly in Vectashield (Vector Laboratories). Images were captured on a Leica SP5C Spectral Confocal Laser Scanning Microscope.
Supplementary Material
Acknowledgments
We thank E. Riem and J. van Ooij for their expert technical assistance, the personnel of the animal facility for this excellent animal husbandry, P. Krimpenfort and J. Vissers for critically reading the manuscript, and R. van Amerongen for advice regarding the imaging of R26R-Confetti multicolor reporter animals. We thank Tyler Jacks for providing the K-RasLSL-G12D/+ mice. We acknowledge B. Stripp for providing us with the anti-CCSP antibody. K.D.S. was a recipient of a National Health and Medical Research Council of Australia Overseas-based Biomedical Training Fellowship (No. 516781). This work was supported by a program grant of the Dutch Cancer Society and by funds provided by the European Platform for Translational Cancer Research consortium.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 4745.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1319963111/-/DCSupplemental.
References
- 1.Mao C, et al. KRAS mutations and resistance to EGFR-TKIs treatment in patients with non-small cell lung cancer: A meta-analysis of 22 studies. Lung Cancer. 2010;69(3):272–278. doi: 10.1016/j.lungcan.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 2.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161(1):173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dovey JS, Zacharek SJ, Kim CF, Lees JA. Bmi1 is critical for lung tumorigenesis and bronchioalveolar stem cell expansion. Proc Natl Acad Sci USA. 2008;105(33):11857–11862. doi: 10.1073/pnas.0803574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim CF, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121(6):823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Ventura JJ, et al. p38alpha MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat Genet. 2007;39(6):750–758. doi: 10.1038/ng2037. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, et al. Phosphatidylinositol 3-kinase mediates bronchioalveolar stem cell expansion in mouse models of oncogenic K-ras-induced lung cancer. PLoS ONE. 2008;3(5):e2220. doi: 10.1371/journal.pone.0002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besson A, et al. Discovery of an oncogenic activity in p27Kip1 that causes stem cell expansion and a multiple tumor phenotype. Genes Dev. 2007;21(14):1731–1746. doi: 10.1101/gad.1556607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanagi S, et al. Pten controls lung morphogenesis, bronchioalveolar stem cells, and onset of lung adenocarcinomas in mice. J Clin Invest. 2007;117(10):2929–2940. doi: 10.1172/JCI31854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson EL, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15(24):3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu X, et al. Evidence for type II cells as cells of origin of K-Ras-induced distal lung adenocarcinoma. Proc Natl Acad Sci USA. 2012;109(13):4910–4915. doi: 10.1073/pnas.1112499109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berquam-Vrieze KE, et al. Cell of origin strongly influences genetic selection in a mouse model of T-ALL. Blood. 2011;118(17):4646–4656. doi: 10.1182/blood-2011-03-343947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson EL, et al. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res. 2005;65(22):10280–10288. doi: 10.1158/0008-5472.CAN-05-2193. [DOI] [PubMed] [Google Scholar]
- 13.Sutherland KD, et al. Cell of origin of small cell lung cancer: Inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell. 2011;19(6):754–764. doi: 10.1016/j.ccr.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Jonkers J, et al. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29(4):418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 15.Visvader JE. Cells of origin in cancer. Nature. 2011;469(7330):314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- 16.Winslow MM, et al. Suppression of lung adenocarcinoma progression by Nkx2-1. Nature. 2011;473(7345):101–104. doi: 10.1038/nature09881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snippert HJ, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143(1):134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Guerra C, et al. Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell. 2003;4(2):111–120. doi: 10.1016/s1535-6108(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 19.Rawlins EL, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4(6):525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin L, et al. Spatiotemporal quantification of cell dynamics in the lung following influenza virus infection. J Biomed Opt. 2013;18(4):046001. doi: 10.1117/1.JBO.18.4.046001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.