Significance
Type 2 diabetes mellitus (T2DM) has become a common disease, and long-term effective drugs have become a necessity. In recent years, dipeptidyl peptidase 4 (DPP4) inhibitors have been commercialized due to its ability to inhibit glucagon-like peptide 1 degradation, a hormone important for enhancing insulin secretion. Despite the availability of efficient drugs, the success of treatment is limited by patients’ inconsistent drug intake and the economic burden included in a lifelong treatment required for T2DM. To alleviate these limitations, in this study an affordable and effective immunotherapeutic method was developed and assessed for T2DM treatment. We selected and designed the appropriate peptide sequences that induce the anti-DPP4 antibody that effectively improves the diabetic phenotype without an adverse autoimmune response.
Abstract
The increasing prevalence of type 2 diabetes mellitus is associated with a significant economic burden. We developed a dipeptidyl peptidase 4 (DPP4)-targeted immune therapy to increase glucagon-like peptide 1 hormone levels and improve insulin sensitivity for the prevention and treatment of type 2 diabetes mellitus. Immunization with the DPP4 vaccine in C57BL/6J mice successfully increased DPP4 titer, inhibited plasma DPP4 activity, and induced an increase in the plasma glucagon-like peptide 1 level. Moreover, this elevated titer was sustained for 3 mo. In mice fed a high-fat diet, DPP4 vaccination resulted in improved postprandial glucose excursions and insulin sensitivity and, in the diabetic KK-Ay and db/db mice strains, DPP4 vaccination significantly reduced glucose excursions and increased both plasma insulin and pancreatic insulin content. Importantly, T cells were not activated following challenge with DPP4 itself, which suggests that this vaccine does not induce cell-mediated autoimmunity. Additionally, no significant immune-mediated damage was detected in cells and tissues where DPP4 is expressed. Thus, this DPP4 vaccine may provide a therapeutic alternative for patients with diabetes.
Type 2 diabetes mellitus (T2DM) is increasingly recognized as a major cause of morbidity and mortality worldwide. In addition, the prevalence of T2DM is expected to increase to 439 million by 2030 (1). Glucagon-like peptide 1 (GLP-1), one of the incretin hormones, is critical for glucose homeostasis and represents a therapeutic target for T2DM (2). GLP-1 increases insulin secretion and improves insulin sensitivity (3–5) but is rapidly degraded by the enzyme dipeptidyl peptidase 4 (DPP4) (6–8). DPP4 inhibitors, such as sitagliptin, vildagliptin, and saxagliptin, are currently being used clinically in patients with T2DM (9–11), and can improve glucose homeostasis due to their ability to inhibit GLP-1 degradation and enhance insulin secretion (12). Nevertheless, treatment success is limited by inconsistent drug intake and the economic burden associated with lifelong treatment. To alleviate the compliance issue and improve therapeutic outcomes for patients, we developed and assessed an immunotherapeutic method for T2DM treatment.
Vaccines are a common method for the prevention of infectious diseases, and they have been recently expanded to treat chronic diseases, such as hypertension and Alzheimer’s disease, by targeting self-antigens (13–16). Immunization may provide a low-cost alternative to conventional therapy due to vaccines’ relatively long-lasting effects and lack of daily dosage requirements (17, 18).
We selected three suitable regions in DPP4 as candidate targets for a vaccine and conjugated these peptides, termed E1, E2, and E3, to keyhole limpet hemocyanin (KLH), which presents a variety of T-cell epitopes to induce helper T-cell responses. We then assessed the efficacy of this vaccine in two animal models: high-fat diet (HFD)–fed C57BL/6J mice and the diabetic KK-Ay and db/db mouse strains, which are commonly used as rodent models of insulin resistance and early-onset T2DM, respectively (19–21). We also evaluated the safety of the DPP4 vaccine by focusing on T-cell activation.
Results
Selection and Screening of the Appropriate Antigen Sequence for the DPP4 Vaccine.
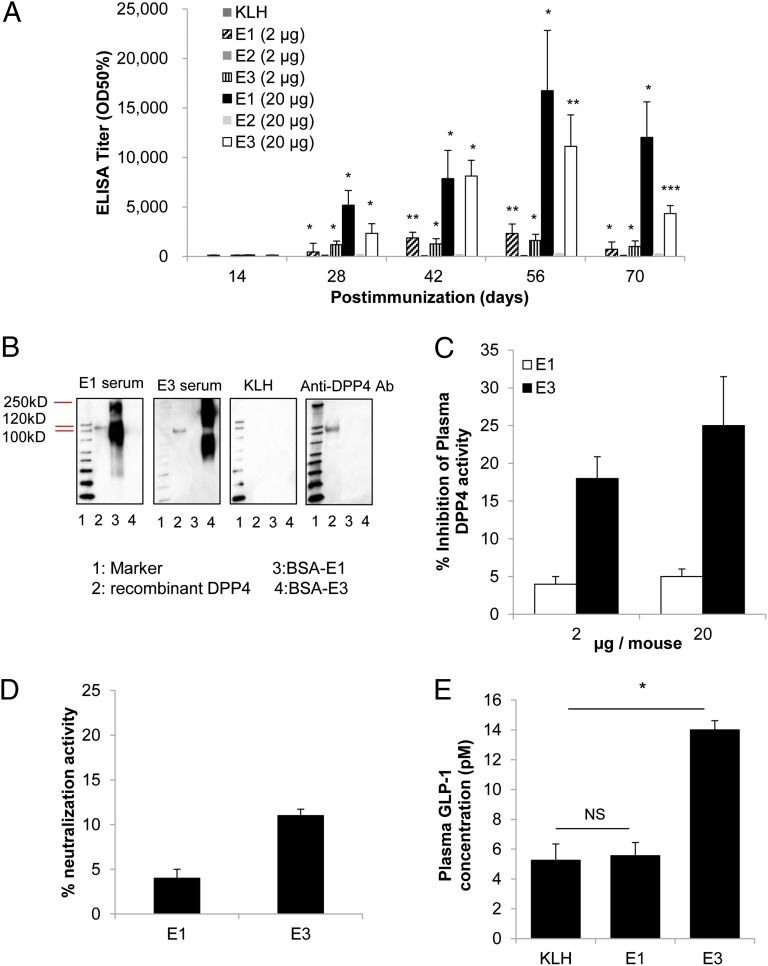
According to the three-dimensional structure of DPP4 (22), we selected three peptides as antigens (E1, 29–40 aa; E2, 48–57 aa; E3, 89–97 aa) based on their positions that overlap the enzymatic active site, thus increasing the likelihood of neutralizing antibody production. The three epitopes within DPP4 (E1, E2, and E3) were conjugated to KLH as a carrier protein, and the vaccines were administered to mice a total of three times with 2-wk intervals between injections. Either a low (2 μg peptide per mouse) or high dose (20 μg peptide per mouse) was administered in combination with Freund’s adjuvant to male C57BL/6J mice (8 wk of age; n = 6). The antibody titer against DPP4 was increased in a dose-dependent manner on day 28 in mice immunized with the E1 and E3 antigens but not with E2 (Fig. 1A). The antibody titer was further increased on days 42 and 56 but gradually decreased by day 70. Moreover, the antibody titer was approximately sixfold greater in the high-dose mice than in the low-dose mice on day 56 after immunization (Fig. 1A). We further investigated the ability of the induced antibodies from the E1 and E3 vaccine groups to recognize and bind to the DPP4 protein compared with induced antibodies from the control group (mice immunized only with the carrier protein KLH). The antibodies present in the E1 and E3 sera, but not in the KLH control group, recognized recombinant DPP4 protein and BSA-conjugated E1 or E3 by Western blot (Fig. 1B).
Fig. 1.
Screening of three different peptide sequences for DPP4 vaccination in male C57BL/6J mice. (A) Normal diet-fed mice were immunized with three candidate peptides (E1, E2, or E3) at either a low (2 μg peptide per mouse) or high dose (20 μg peptide per mouse) or with KLH only. The titer is expressed as the dilution of serum giving half-maximal binding (optical density: OD50%) ± SE of the mean. (B) After the third immunization, sera were collected from the E1 (high-dose) and E3 (high-dose) groups. Antibodies that specifically bound to recombinant DPP4 (lane 2) were assessed by Western blot. Sera from KLH control mice did not demonstrate any binding, and commercially available anti-DPP4 antibody (anti-DPP4 Ab) showed binding to only recombinant DPP4 and not BSA-conjugated E1 or E3 (lanes 3 and 4, respectively). (C) The inhibition of plasma DPP4 activity was investigated on day 56 after immunization in the low- and high-dose E1 and E3 groups. (D) Neutralization activity was measured on day 56 in the high-dose E1- and E3-immunized groups. The data are expressed as the percent neutralization activity, calculated as % neutralization activity = 100[1 − (Vi/Vc)], where Vi is the rate of reaction of the immunized sample and Vc is the rate of reaction of the control sample. (E) After overnight fasting (16 h) and 10 min after the meal challenge (2 g CHO/kg), the plasma GLP-1 concentration was measured on day 70 in the E1, E3, and KLH groups by ELISA. All data are expressed as the mean ± SEM. NS, not significant; *P < 0.05, **P < 0.01, and ***P < 0.001 versus the KLH control group.
To evaluate the neutralizing activity of these antibodies, plasma DPP4 activity was measured in the E1 and E3 groups on day 56. The plasma DPP4 activity was significantly decreased in mice immunized with E3 peptide at doses of both 2 μg and 20 μg per mouse (18 ± 2.9% and 25 ± 6.6%; P < 0.05) (Fig. 1C), but this reduction in plasma DPP4 activity was not detected in the E1 group. Moreover, the inhibition of plasma DPP4 activity was elevated on days 28, 42, and 56, and was significantly correlated with the increased antibody titer in the E3 vaccine group (Fig. S1A).
We further assessed the neutralizing ability of anti-DPP4 antibodies induced by the DPP4 vaccine using a neutralization assay in vitro (Fig. 1D). The addition of sera from mice immunized with E3 peptide, but not E1 peptide, resulted in the neutralization of recombinant DPP4 on day 56 (13 ± 0.7%; P < 0.05). The neutralization ability of antibodies induced by E3 peptide was also observed using sera from days 28, 42, and 56, and this neutralization correlated with the increased antibody titer in the E3 group (Fig. S1B). Thus, these results suggested that E3 vaccine-induced antibodies effectively inhibited DPP4 function in vivo.
To further confirm the effect of the DPP4 vaccine, intact plasma GLP-1 was measured in mice immunized with the E1 and E3 peptides as well as in mice that received the KLH control. Because it is known that plasma GLP-1 levels peak at 10 min after oral glucose administration (23), we administered 2 g carbohydrate (CHO) per kilogram of body weight of the immunized mice and measured the GLP-1 levels in the plasma after 10 min. The results indicated that the GLP-1 level was significantly higher in the E3 group compared with the E1 group or KLH control group (Fig. 1E).
Improvement in Insulin Resistance Following DPP4 Vaccination in Mice Fed a High-Fat Diet.
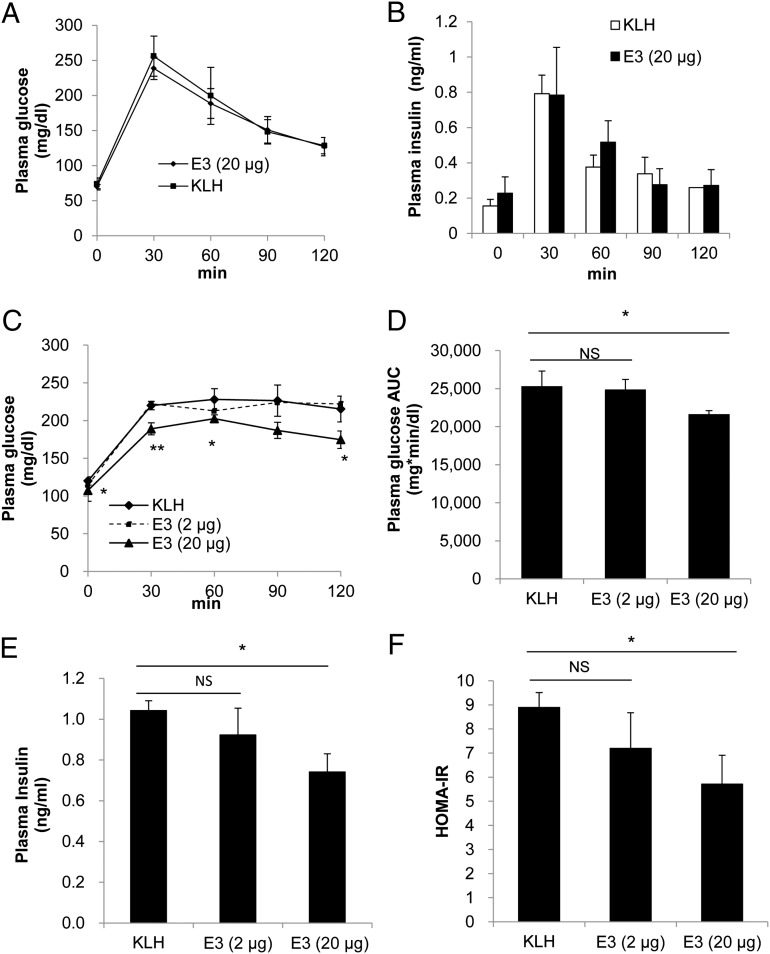
To evaluate the effect of the DPP4 (E3) vaccine on glucose metabolism, a meal tolerance test (MTT) was performed instead of a glucose tolerance test, as it was reported that the secretion of GLP-1 is more strongly induced by complex nutrients than by glucose (24). Male mice (8 wk of age; n = 6) immunized with the E3 vaccine and fed a normal diet showed no change in glucose and insulin levels compared with the KLH control mice (Fig. 2 A and B), which indicated that the DPP4 vaccine did not induce hypoglycemia in mice fed a normal diet.
Fig. 2.
Evaluation of the effectiveness of the E3 vaccine to improve glucose metabolism in HFD-induced insulin-resistant mice. (A) The meal tolerance test (2 g CHO/kg) was administered following an overnight fast (16 h) to normal diet-fed mice on day 56 after E3 (20 μg per mouse) or KLH immunization. (B) The plasma insulin level was measured on day 56 following the MTT. (C) After fasting the HFD-fed mice overnight, the blood sugar level was measured after meal loading in mice from the E3 groups (high dose, 20 μg per mouse; low dose, 2 μg per mouse) and KLH control group. (D) The glucose AUC was determined (time 0–120 min) during the MTT. (E) The fasting plasma insulin level in overnight-fasted and HFD-fed mice was measured on day 105 after E3 (2 or 20 μg per mouse) or KLH immunization. (F) HOMA-IR values (fasting plasma insulin [μU/mL] × fasting plasma glucose [mg/dL]/405) were calculated on day 105 after E3 (2 and 20 μg per mouse) or KLH immunization. All data are expressed as the mean ± SEM. *P < 0.05 and **P < 0.01 versus the KLH control group.
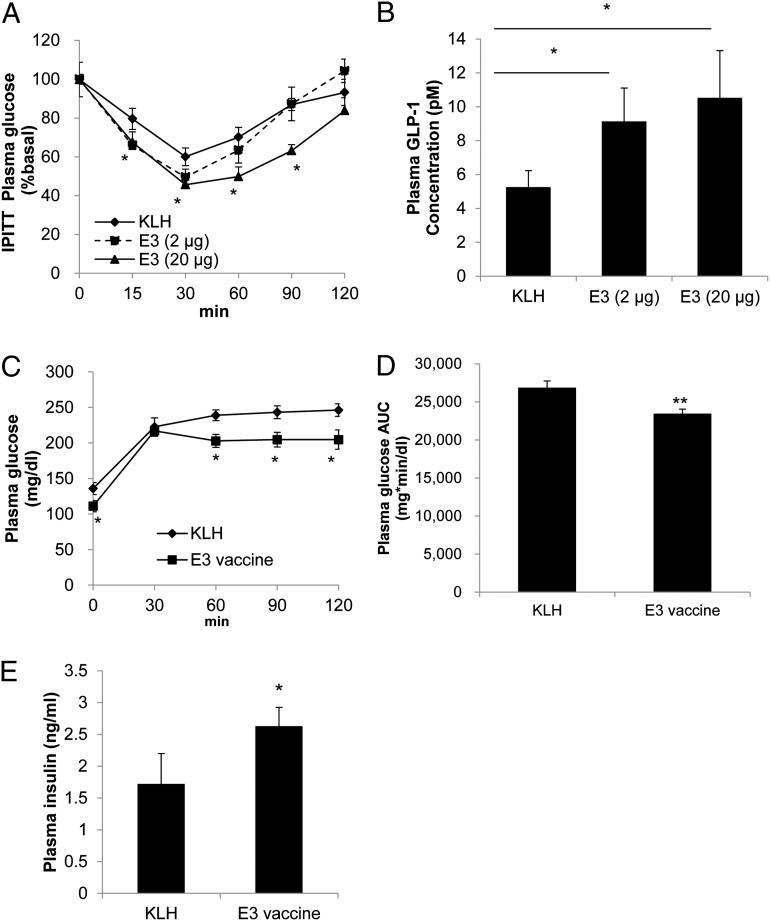
In mice with high-dose E3 vaccination (8 wk of age; n = 6) and fed an HFD beginning 1 wk after the immunization protocol (Fig. S2A), glucose levels were significantly lower after oral MTT performed on day 105 compared with the low-dose E3 group and KLH control group (Fig. 2 C and D). The increased plasma insulin levels in mice fed an HFD were also decreased following E3 high-dose vaccination (Fig. 2E). Moreover, in the homeostasis model assessment for insulin resistance (HOMA-IR), a method used to quantitate insulin sensitivity (25), the high-dose E3 group revealed significantly improved insulin sensitivity levels compared with the KLH control group (Fig. 2F). In accordance, in the insulin challenge test, high-dose E3 vaccination significantly improved the rate of blood glucose reduction compared with the KLH control group (Fig. 3A). Furthermore, the plasma GLP-1 level was significantly increased following E3 vaccination in a dose-dependent manner (Fig. 3B). The levels of DPP4 antigen were also significantly reduced in the high-dose E3 vaccine group (Fig. S2C), and there were no significant changes in weight gain or diet consumption compared with the KLH control group (Fig. S3 A and B).
Fig. 3.
Improvement in insulin sensitivity in HFD-fed mice and the effect of the E3 vaccine on HFD-induced diabetic model mice. (A) In 6 h-fasted HFD-fed mice, an i.p. insulin tolerance test (IPITT) was performed on day 112 after E3 (2 or 20 μg per mouse) or KLH immunization. (B) At the end of the study (day 133), the intact plasma GLP-1 concentration in overnight-fasted HFD-fed mice was determined by ELISA. (C and D) In HFD-induced therapeutic model mice, the MTT (2 g CHO/kg) was performed on day 112 after E3 or KLH immunization (C), and at the same time the AUC (time 0–120 min) for the blood sugar level was calculated (D). (E) Plasma insulin secretion was determined at 30 min after meal administration (2 g CHO/kg) during the above MTT. All data are expressed as the mean ± SEM. *P < 0.05 and **P < 0.01 versus the KLH control group.
Assessment of the DPP4 Vaccine in Therapeutic Models.
To further investigate the efficacy of the E3 vaccine (20 μg per mouse), we used the previously described HFD-induced early-onset T2DM model (19). As shown in Fig. S3C, mice (8 wk of age; n = 6) were fed an HFD for 5 wk prior to the first high-dose E3 vaccination. DPP4 activity decreased (18 ± 3.8%; P < 0.05; Fig. S3D) in E3 vaccine-immunized mice compared with the KLH control mice, and the E3 vaccine group exhibited improved glucose excursions following the MTT (Fig. 3 C and D) and enhanced plasma insulin secretion on day 112 (Fig. 3E). These data suggested that the E3 vaccine not only improved insulin resistance but also ameliorated the early stages of T2DM in mice.
DPP4 Vaccination Delayed the Onset of Diabetes in Diabetic Mice.
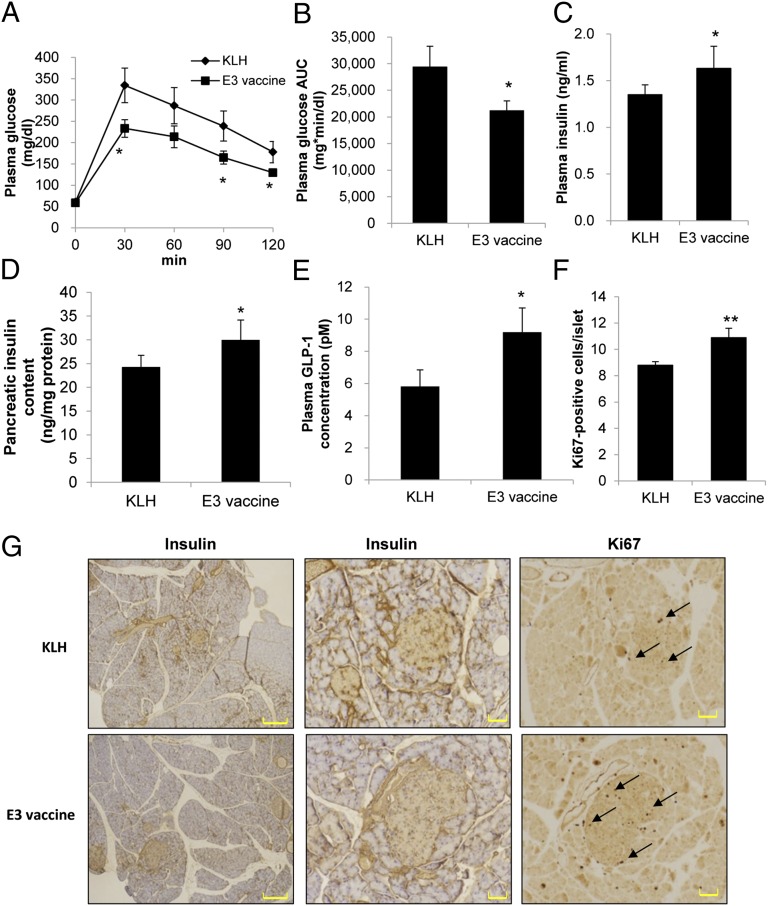
To evaluate the effect of the E3 vaccine on glucose metabolism and pancreatic pathology, we used a spontaneous diabetic model: yellow KK mice that carry the obsess gene Ay and develop obesity, hyperglycemia, and hyperinsulinemia. It has also been reported that the administration of DPP4 inhibitor in these mice effectively attenuates the hyperglycemia (26). The MTT was performed on day 28 after E3 vaccine immunization, and the immunized mice exhibited significant improvement in postprandial blood glucose levels (Fig. 4 A and B) and tended to have increased plasma insulin secretion and pancreatic insulin content compared with the KLH control mice (Fig. 4 C and D). Furthermore, to investigate the mechanism of the E3 vaccine on insulin secretion in KK-Ay mice, we examined the plasma level of intact GLP-1, and the results indicated that the E3 vaccine increased the GLP-1 level compared with the KLH control group (Fig. 4E). To examine whether the E3 vaccine enhanced insulin secretion and improved the proliferation of pancreatic β-cells, a morphological examination was performed 42 d after immunization. Islets from E3-immunized mice were larger and detected in greater numbers compared with those from the KLH control mice (Fig. 4G). E3 vaccine immunization also resulted in a dramatic increase in β-cell proliferation (Fig. 4 F and G), as analyzed by Ki67 expression. These results indicate that the E3 vaccine prevented the loss and destruction of β-cell mass compared with the KLH control treatment.
Fig. 4.
Evaluation of E3 vaccine effectiveness against the onset of diabetes in KK-Ay mice. (A) In overnight-fasted KK-Ay mice (n = 6), the MTT was performed on day 28. (B) The AUC for glucose (time 0–120 min) was calculated during the MTT. (C) At 30 min after meal loading (2 g CHO/kg), plasma insulin level and pancreatic insulin content were measured on day 28. (D) Pancreatic insulin content was assessed on day 63. (E) After overnight fasting (16 h) and meal loading (2 g CHO/kg), the plasma GLP-1 concentration also was measured. (F) The proliferation rates of β-cells were determined by dividing the Ki67-positive β-cell number by the total islet number on day 42. (G) Pancreatic sections from KLH- or E3-immunized mice were obtained on day 42 for insulin or Ki67 immunostaining. [Scale bars, 400 μm (Left) and 50 μm (Center and Right).] All data are expressed as the mean ± SEM. *P < 0.05 and **P < 0.01 versus the KLH control group.
To more directly examine the effects of the E3 vaccine on the development of diabetes, we used another diabetic mouse model (young db/db mice, 6 wk of age; n = 5). In MTTs performed after high-dose E3 vaccination, immunized mice showed a decrease in plasma glucose area under the curve (AUC) compared with the KLH control mice (Fig. S4 A and B). In addition, E3 vaccination caused a significant increase in plasma insulin level and pancreatic insulin content compared with the KLH control group (Fig. S4 C and D). In the morphological analysis, the size and number of islets in the immunized mice were greater than in the KLH control mice and nontreated mice (Fig. S5A). Furthermore, the E3 vaccine increased the GLP-1 level and decreased the DPP4 level compared with the KLH control group (Fig. S5 B and C). Collectively, these results suggested that the increased level of endogenous GLP-1 in E3-vaccinated mice caused by the decrease in DPP4 was able to stimulate pancreatic insulin secretion and ultimately reduce blood glucose excursions.
Evaluation of T-Cell Activation in DPP4-Immunized Mice.
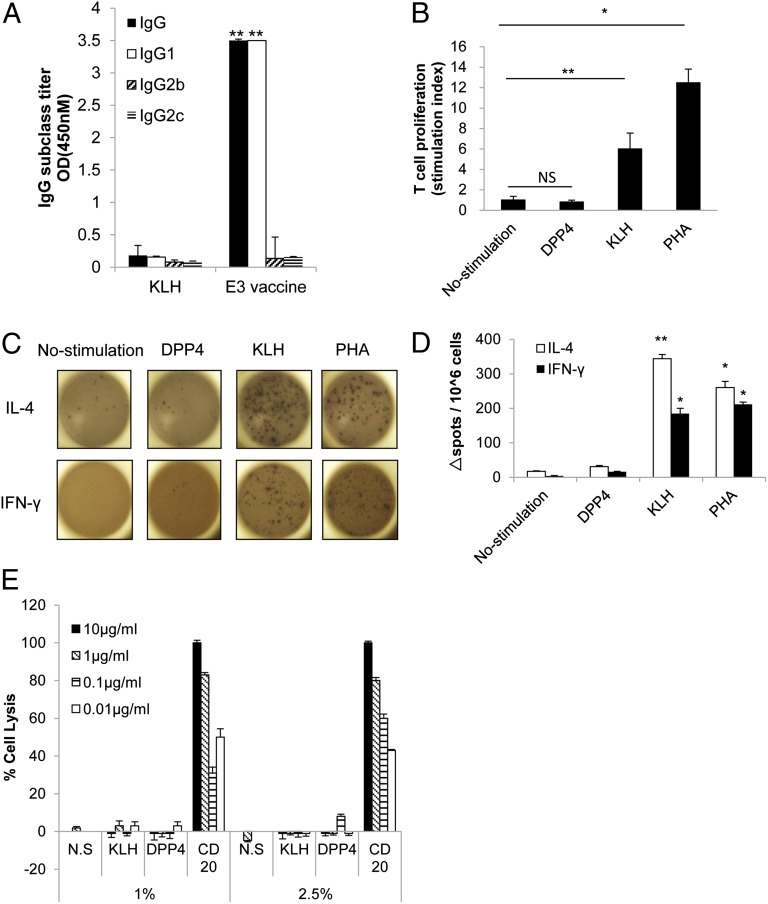
We next investigated T-cell activation in E3-immunized mice using an IgG subclass ELISA, a T-cell proliferation assay, and an enzyme-linked immunosorbent spot (ELISPOT) assay as described previously (27). In the IgG subclass ELISA, the IgG1:IgG2b ratio in the anti-DPP4 antibody pool was greater than 1.0 in the E3 vaccine group (dilution 1:500), indicating that the E3 vaccine induced a primarily T helper (Th)2-type response (IgG1) (Fig. 5A). In the T-cell proliferation assay, stimulation with KLH significantly induced the proliferation of splenocytes isolated from E3-vaccinated mice, but stimulation with the DPP4 peptide did not induce proliferation compared with the unstimulated control (Fig. 5B). We also examined the production of IFN-γ and IL-4 cytokines associated, respectively, with Th1 and Th2 responses in splenocytes from E3-immunized mice by ELISPOT. Stimulation with KLH induced the production of IFN-γ and IL-4, generating significant numbers of splenocytes that produced both cytokines similar to the splenocytes treated with the T-cell mitogen phytohemagglutinin (PHA) as positive control. However, the DPP4 peptide did not elicit a significant response in comparison with the unstimulated and KLH control groups (Fig. 5 C and D). These results indicated that KLH contains adequate T-cell epitopes to induce T-cell activation in immunized mice, whereas DPP4 was unable to elicit T-cell activation even after immunization with E3. And because most of the T cells were differentiated into Th2-type cells after E3 vaccination, these data suggested that this level of T-cell activation was sufficient to promote antibody production but was not capable of inducing an autoimmune response.
Fig. 5.
Evaluation of T-cell activity after E3 vaccination. (A) The total IgG, IgG1 (Th2), IgG2b (Th1), and IgG2c (Th1) profiles of the humoral immune response in mice immunized with E3 (20 μg per mouse) or KLH only were determined on day 56. The sera for ELISAs were pooled and diluted 1:500. All data are expressed as the mean ± SEM. **P < 0.01 versus the KLH control group. (B) The T-cell proliferation assay was performed on day 70. Splenocytes (106 cells per well) from E3-vaccinated mice were cultured in RPMI medium 1640 and were stimulated with E3 peptide, KLH, or PHA at 10 μg/mL. After an 8-h incubation with [3H]thymidine, the extent of uptake was determined. The stimulation index is expressed as the ratio of stimulation to no stimulation. All data are expressed as the mean ± SEM. (C) In the ELISPOT assay, splenocytes (106 cells per well) from E3-immunized mice were stimulated with E3 peptide, KLH, or PHA at 10 μg/mL. The production of IL-4 or IFN-γ by splenocytes was detected as black spots. (D) The number of spots was quantified in triplicate wells of each group. (E) The CDC activity was assessed (n = 3) in the presence of complement serum at 1 and 2.5%. Splenocytes, as the target cell, were incubated with purified IgG from E3-immunized mice (DPP4), the KLH control group, or anti-CD20 antibody at various concentrations from 0.01 to 10 μg/mL, and cell death was evaluated as the percentage of cell lysis (total cell death). N.S., no stimulation with antibody. All data are expressed as the mean ± SEM. **P < 0.01 versus no stimulation, *P < 0.05 versus no stimulation.
We also examined whether the immunized plasma would cause damage to cells expressing DPP4 through complement-dependent cell cytotoxicity (CDC)-mediated or antibody-dependent cellular cytotoxicity (ADCC)-mediated processes. For both cytotoxic assays, anti-CD20 antibody was used as a positive control (28) and splenocytes were selected as target cells, because both CD20 and DPP4 are expressed in cultured cells obtained from spleen, as confirmed by Western blot (Fig. S6A). In the CDC assay, anti-CD20 antibody (0.01, 0.1, 1, 10 μg/mL) caused cell death with cotreatment of complement serum (1 or 2.5%). However, the purified IgG from mice immunized with KLH or the E3 vaccine (Fig. S6B) did not cause cell death in the same optimized condition (Fig. 5E). Similarly, in the ADCC assay, lymphocytes were used as effector cells and the ratio of effector cells and target cells was optimized under the treatment of anti-CD20 antibody (0.01, 0.1, 1, 10 μg/mL). Indeed, treatment with CD20 antibody (1, 10 μg/mL) significantly induced cell death in the optimized condition (effector/target cell ratios 10:1 and 1:1). However, as shown in Fig. S6C, the purified IgG from mice immunized with KLH or the E3 vaccine did not cause cell death in the same optimized condition. These results suggest that immunized plasma does not cause death in cells expressing DPP4. Furthermore, we also evaluated immune-mediated damage in tissues such as jejunum, liver, and kidney, where endothelial DPP4 is expressed at a high level (29). Similarly, no clear tissue injury or leukocyte accumulation was observed at the end of the study period in E3-immunized mice compared with the KLH control group (Fig. S7). These results suggested that the E3 vaccine did not have the potential to stimulate an autoimmune response.
Discussion
In this study, we developed a DPP4 peptide vaccine to increase GLP-1 levels and improve insulin sensitivity. This experimental study has developed a vaccine for T2DM, and we have demonstrated its efficacy in improving the diabetic phenotype without causing adverse autoimmune responses.
Oral DPP4 inhibitors are safe and effective for the treatment of T2DM, but as a medication of daily administration, they may exhibit peak variation throughout the day (18) and represent an economic burden for the patient. The significance of this study is the possibility of an alternative approach to effectively target DPP4 using a DNA vaccine to prevent the disease and treat T2DM patients in the future.
Previous studies reported that the incretin hormone GLP-1 not only stimulates glucose-induced insulin secretion but enhances glycogen synthesis, glucose oxidation, and utilization in skeletal muscle, liver, and adipose tissue (3, 30, 31). Moreover, in mice lacking the gene encoding DPP4, elevated GLP-1 effectively increased insulin sensitivity and reduced hyperinsulinemia after a high-fat diet (32). It has also been reported that GLP-1 may mediate its effects on glucose control independent of insulin secretion through activation of peripheral sensors linked to enhanced glucose disposal (33). Accordingly, in our study, the E3 vaccine significantly improved insulin sensitivity in an HFD-induced insulin resistance mouse model as assessed by insulin tolerance and HOMA-IR (Figs. 2F and 3A), which might be correlated with increased GLP-1 secretion due to sustained inhibition of DPP4 activity. The further mechanisms underlying the improvement in insulin sensitivity by the E3 vaccine remain elusive.
On the other hand, in a severe diabetic model (i.e., db/db mice or therapeutic model for HFD), the effect of GLP-1 is not restricted to improving insulin sensitivity but also increases insulin secretion. This is more likely because in type 2 diabetic stages, decreasing levels of circulating insulin and hyperglycemia will gradually stall due to exhaustion of insulin-positive β-cells. In our study, these defects were attenuated in the HFD-induced therapeutic model and young diabetic db/db mice after immunization with the E3 vaccine compared with the KLH control group, leading to increased insulin secretion. In addition, we used KK-Ay mice, because the administration of DPP4 inhibitor effectively attenuated the hyperglycemia in KK-Ay mice (26). The severity of KK-Ay mice as a diabetic model is less than that of db/db mice, and insulin action can be improved by the inhibitor (26). In addition, we postulate that the E3 vaccine improved insulin secretion due to increased islet mass and β-cell proliferation. These results suggest that the main action of induced anti-DPP4 antibody may differ according to the severity of the diabetic model. Overall, in this study, the E3 vaccine may present an opportunity for improving insulin resistance and delaying the development of T2DM.
Peptide vaccines as immunotherapy against self-antigens carry the risk of causing adverse effects. Also, studies show that previous vaccines against amyloid-β led to T cell-mediated aseptic meningoencephalitis (34–36). Therefore, a more detailed evaluation of T-cell activation is warranted to support the clinical application of future vaccines. The effectiveness and safety of vaccines depend on the appropriate activation of T and B cells by specific epitopes within the peptide sequences of the target and carrier proteins. The target peptide is conjugated to a carrier protein and, as a complex, is captured by antigen-presenting cells, particularly dendritic cells (DCs). The conjugate is then cleaved and presented to the T cell. After cleavage, the carrier protein also stimulates DCs to promote T-cell activation. Thus, the carrier protein is essential for inducing the activation processes leading to CD4+ and CD8+ T-cell differentiation (e.g., cytotoxic T-cell activation). CD4+ T cells can be further differentiated into Th1 and Th2 cells, depending on predominant signals during activation. Th1 cells produce IFN-γ and IL-2 and activate cytotoxic T cells, thereby eliciting a cell-mediated response. Th2 cells produce IL-4 and IL-10 and stimulate B-cell activation (humoral response). After receiving costimulatory signals from DC- and Th2-derived cytokines, B cells undergo clonal expansion and differentiate into antibody-secreting plasma cells (37).
The strategy of using a peptide from a self-antigen conjugated to an appropriate carrier protein has been shown to successfully break tolerance and produce antibodies against the self-antigen. We also evaluated the potential autoimmune response against DPP4, as B- and T-cell epitopes were present in the E3 vaccine. Concerning B-cell epitopes, the E3 vaccine produced a strong titer of antibodies against DPP4 after immunization. For T-cell epitopes, based on the ELISPOT and T-cell proliferation assay results, T cells were differentiated toward the Th2 type and supported the humoral response rather than the generation of cytotoxic T cells, thereby showing no potential to elicit an autoimmune response.
In summary, the DPP4 vaccine markedly improved insulin resistance and delayed the development of T2DM by increasing GLP-1 secretion without eliciting an autoimmune response. Thus, the DPP4 vaccine could provide an affordable and effective alternative for T2DM treatment.
Materials and Methods
Full experimental procedures and associated references are available in SI Materials and Methods.
Vaccine Design and Synthesis.
With the aim of generating neutralizing antibodies against DPP4, we selected three peptides: E1, which spans a site in the N-terminal sequence of DPP4 (epitope 1, 29–40 aa); E2 (48–57 aa); and E3, which spans the 89–97 aa sequence near the opening of the DPP4 active site. The N termini of the candidate peptides were conjugated to KLH (Wako), and the synthetic peptides were purified by reverse-phase HPLC (>98% purity) (Peptide Institute).
Animal Study and Immunization.
The experiments were approved by the Ethical Committee for Animal Experiments of the Osaka University Graduate School of Medicine. Eight-week-old male C57BL/6J mice, 4-wk-old male KK-Ay mice, and 6-wk-old male db/db mice were purchased from Oriental Yeast and housed in a temperature- and light cycle-controlled facility with free access to food and water. For HFD treatment, C57BL/6J mice received HFD60 chow (18.2% protein, 62.2% fat, and ca. 19.6% CHO; Oriental Yeast). The peptide solutions were mixed with an equal volume of complete/incomplete Freund’s adjuvant (Wako) before immunization. Groups of mice (n = 6) were injected s.c. on days 0, 14, 28, 84, and 119 with either 2 or 20 μg of the DPP4 peptide. Control groups were injected with an equal quantity of KLH mixed with an equal volume of Freund’s adjuvant. Serum was collected from the tail vein, and antibody titers against the immunizing peptide were determined by ELISA.
DPP4 Assay.
DPP4 activity was measured in the plasma 15 min after meal challenge on days 28, 42, and 56 as previously described (38). Briefly, 5 μL of serum was mixed with DPP4-GloTM solution (DPP4-Glo Protease Assay; Promega) and assay buffer (100 mM Hepes, pH 7.6, 0.1 mg/mL BSA) in a total reaction volume of 60 μL. To assess the neutralizing ability of anti-DPP4 antibodies, 1 μL of serum was placed at 37 °C for 6 h and then incubated with recombinant DPP4 (R&D Systems) for 1 h at 4 °C, followed by addition of the DPP4-GloTM reagent solution. The released luminescence was recorded every minute for 30 min using a luminometer Spectra Fluor (SH-9000; Corona Electric). The plasma DPP4 level was measured by ELISA according to the manufacturer’s instructions (Mouse DPP4 ELISA Kit; R&D Systems), and the absorbance was detected using a microplate reader (Bio-Rad).
MTT, Insulin Tolerance Test, and Plasma Parameters.
Mice were fasted overnight (16 h), and then a meal (consisting of 14% protein, 31.5% fat, and 54.5% CHO; Ensure H; Meiji) was administered orally at a dose of 2 g CHO/kg. Following the meal challenge, blood glucose levels were determined by performing tail bleeds at 0, 30, 60, 90, and 120 min using a glucometer (Sanwa Kagaku Kenkyusho). The blood glucose excursion profile from t 0 to 120 min was used to integrate the AUC (AUC 0–2 h). The plasma insulin concentration (Mouse ELISA Insulin Kit; Morinaga), plasma active GLP-1 concentration (GLP-1 ELISA Kit, 96-well plate; Linco Research), and blood glucose levels were measured at each indicated time point. The insulin-sensitizing effects were evaluated according to the HOMA-IR method (fasting plasma insulin [μU/mL] × fasting plasma glucose [mg/dL]/405). For the i.p. insulin tolerance test, following the injection of 1 U/kg insulin, blood samples were collected at 0, 15, 30, 60, 90, and 120 min from 6 h-fasted mice. The data are expressed as the percentage of basal fasting glucose.
ELISPOT Assay.
These assays were performed as described previously (27). Briefly, 96-well ELISPOT plates (Millipore) were coated with anti-mouse IFN-γ capture antibody or anti-mouse IL-4 capture antibody overnight at 4 °C. After incubation, the plates were washed with PBS containing 0.05% Tween 20 (PBS-T) solution and then blocked with 1% BSA and 5% (wt/vol) sucrose in PBS. Splenocyte suspensions from immunized mice were added to the plates (106 cells per well) and stimulated with 10 μg/mL DPP4 peptide, KLH, and PHA at 37 °C for 48 h. The plates were washed with PBS-T after incubation with biotinylated anti-mouse IFN-γ or IL-4 antibody overnight at 4 °C. After washing, streptavidin-alkaline phosphatase was added to each well and incubated for 2 h at room temperature. After washing with PBS-T, the plates were incubated with 5-Bromo-4-chloro-3 indolylphosphate P-toluidine salt and Nitro Blue Tetrazolium solution for 30 min at room temperature. Finally, the plates were rinsed with water and air-dried at room temperature, and colored spots were counted using a dissecting microscope (LMD6500; Leica).
CDC Assay.
IgG antibodies from immunized mice were purified using a kit according to the manufacturer’s instructions (Melon Gel IgG Spin Purification Kit; Thermo Scientific) and assessed by Western blot analysis. The complement-dependent cell cytotoxicity assay was performed using a CytoTox 96 Nonradioactive Cytotoxicity Assay Kit (Promega). Splenocytes were cultured on a 96-well plate at 104 cells per well, and then treated with a positive-control antibody (anti-mouse CD20 functional grade-purified; 16-0201; eBioscience) or purified IgG antibodies from E3-immunized or KLH control mice at various concentrations from 0.01 to 10 μg/mL at 37 °C for 30 min. Then, mouse complement serum (Sigma) was added to the individual wells at 1 and 2.5%. After a 4-h incubation at 37 °C, the lactate dehydrogenase release in the supernatant was determined using a Spectra Fluor at 490 nm (SH-9000; Corona Electric). Cell death was evaluated as the percentage of total cell lysis.
Statistical Analysis.
All data are expressed as the mean ± SEM. Differences between two groups were assessed using the unpaired two-tailed Student t test. Datasets involving more than two groups were assessed with Tukey’s post hoc test using Prism version 5.01 (GraphPad Software). A difference was considered statistically significant when P < 0.05.
Supplementary Material
Acknowledgments
The authors thank Ms. Hizuki Hamada and Ms. Yoko Horiguchi for their technical assistance and Ms. Satoe Kitabata for her help in office procedures.
Footnotes
Conflict of interest statement: The Department of Clinical Gene Therapy is financially supported by AnGes MG, Daiichi-Sankyo, Novartis, Shionogi, Boehringer, and Rohto. The Division of Vascular Medicine and Epigenetics is financially supported by Bayer.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322009111/-/DCSupplemental.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Drucker DJ, Nauck MA. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 3.Sandhu H, et al. Glucagon-like peptide 1 increases insulin sensitivity in depancreatized dogs. Diabetes. 1999;48(5):1045–1053. doi: 10.2337/diabetes.48.5.1045. [DOI] [PubMed] [Google Scholar]
- 4.Young AA, et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: Studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta) Diabetes. 1999;48(5):1026–1034. doi: 10.2337/diabetes.48.5.1026. [DOI] [PubMed] [Google Scholar]
- 5.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and β-cell function in type 2 diabetes: A parallel-group study. Lancet. 2002;359(9309):824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 6.Pauly RP, et al. Investigation of glucose-dependent insulinotropic polypeptide-(1-42) and glucagon-like peptide-1-(7-36) degradation in vitro by dipeptidyl peptidase IV using matrix-assisted laser desorption/ionization-time of flight mass spectrometry. A novel kinetic approach. J Biol Chem. 1996;271(38):23222–23229. doi: 10.1074/jbc.271.38.23222. [DOI] [PubMed] [Google Scholar]
- 7.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136(8):3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 8.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 9.Aschner P, et al. Sitagliptin Study 021 Group Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29(12):2632–2637. doi: 10.2337/dc06-0703. [DOI] [PubMed] [Google Scholar]
- 10.Deacon CF, Holst JJ. Saxagliptin: A new dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. Adv Ther. 2009;26(5):488–499. doi: 10.1007/s12325-009-0030-9. [DOI] [PubMed] [Google Scholar]
- 11.Burkey BF, et al. Acute and chronic effects of the incretin enhancer vildagliptin in insulin-resistant rats. J Pharmacol Exp Ther. 2005;315(2):688–695. doi: 10.1124/jpet.105.087064. [DOI] [PubMed] [Google Scholar]
- 12.Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: Preclinical biology and mechanisms of action. Diabetes Care. 2007;30(6):1335–1343. doi: 10.2337/dc07-0228. [DOI] [PubMed] [Google Scholar]
- 13.Morgan D, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408(6815):982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 14.Schenk D. Amyloid-β immunotherapy for Alzheimer’s disease: The end of the beginning. Nat Rev Neurosci. 2002;3(10):824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- 15.Ferrer I, Boada Rovira M, Sánchez Guerra ML, Rey MJ, Costa-Jussá F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathol. 2004;14(1):11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, et al. Effectiveness and safety of a therapeutic vaccine against angiotensin II receptor type 1 in hypertensive animals. Hypertension. 2013;61(2):408–416. doi: 10.1161/HYPERTENSIONAHA.112.201020. [DOI] [PubMed] [Google Scholar]
- 17.Burnier M. Long-term compliance with antihypertensive therapy: Another facet of chronotherapeutics in hypertension. Blood Press Monit. 2000;5(Suppl 1):S31–S34. [PubMed] [Google Scholar]
- 18.Ambühl PM, et al. A vaccine for hypertension based on virus-like particles: Preclinical efficacy and phase I safety and immunogenicity. J Hypertens. 2007;25(1):63–72. doi: 10.1097/HJH.0b013e32800ff5d6. [DOI] [PubMed] [Google Scholar]
- 19.Winzell MS, Ahrén B. The high-fat diet-fed mouse: A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl 3):S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Brubaker PL. Glucagon-like peptide-1 treatment delays the onset of diabetes in 8 week-old db/db mice. Diabetologia. 2002;45(9):1263–1273. doi: 10.1007/s00125-002-0828-3. [DOI] [PubMed] [Google Scholar]
- 21.Diani AR, et al. The KKAy mouse: A model for the rapid development of glomerular capillary basement membrane thickening. Blood Vessels. 1987;24(6):297–303. doi: 10.1159/000158706. [DOI] [PubMed] [Google Scholar]
- 22.Engel M, et al. The crystal structure of dipeptidyl peptidase IV (CD26) reveals its functional regulation and enzymatic mechanism. Proc Natl Acad Sci USA. 2003;100(9):5063–5068. doi: 10.1073/pnas.0230620100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology. 1999;140(4):1687–1694. doi: 10.1210/endo.140.4.6643. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki K, et al. Comparison of efficacies of a dipeptidyl peptidase IV inhibitor and alpha-glucosidase inhibitors in oral carbohydrate and meal tolerance tests and the effects of their combination in mice. J Pharmacol Sci. 2007;104(1):29–38. doi: 10.1254/jphs.fp0061376. [DOI] [PubMed] [Google Scholar]
- 25.Matthews DR, et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, et al. The DPP-4 inhibitor MK0626 and exercise protect islet function in early pre-diabetic kkay mice. Peptides. 2013;49:91–99. doi: 10.1016/j.peptides.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Cribbs DH, et al. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int Immunol. 2003;15(4):505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pievani A, et al. Enhanced killing of human B-cell lymphoma targets by combined use of cytokine-induced killer cell (CIK) cultures and anti-CD20 antibodies. Blood. 2011;117(2):510–518. doi: 10.1182/blood-2010-06-290858. [DOI] [PubMed] [Google Scholar]
- 29.Fukasawa KM, et al. Immunohistochemical localization of dipeptidyl aminopeptidase IV in rat kidney, liver, and salivary glands. J Histochem Cytochem. 1981;29(3):337–343. doi: 10.1177/29.3.6787113. [DOI] [PubMed] [Google Scholar]
- 30.Villanueva-Peñacarrillo ML, Alcántara AI, Clemente F, Delgado E, Valverde I. Potent glycogenic effect of GLP-1(7-36)amide in rat skeletal muscle. Diabetologia. 1994;37(11):1163–1166. doi: 10.1007/BF00418382. [DOI] [PubMed] [Google Scholar]
- 31.Gedulin BR, et al. Exenatide (exendin-4) improves insulin sensitivity and beta-cell mass in insulin-resistant obese fa/fa Zucker rats independent of glycemia and body weight. Endocrinology. 2005;146(4):2069–2076. doi: 10.1210/en.2004-1349. [DOI] [PubMed] [Google Scholar]
- 32.Conarello SL, et al. Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100(11):6825–6830. doi: 10.1073/pnas.0631828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Orgogozo JM, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61(1):46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 35.Nicoll JA, et al. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: A case report. Nat Med. 2003;9(4):448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 36.Broytman O, Malter JS. Anti-Abeta: The good, the bad, and the unforeseen. J Neurosci Res. 2004;75(3):301–306. doi: 10.1002/jnr.10876. [DOI] [PubMed] [Google Scholar]
- 37.Nakagami F, et al. Decrease in blood pressure and regression of cardiovascular complications by angiotensin II vaccine in mice. PLoS ONE. 2013;8(3):e60493. doi: 10.1371/journal.pone.0060493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waget A, et al. Physiological and pharmacological mechanisms through which the DPP-4 inhibitor sitagliptin regulates glycemia in mice. Endocrinology. 2011;152(8):3018–3029. doi: 10.1210/en.2011-0286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.