Breast cancer gene implicated in brain size control
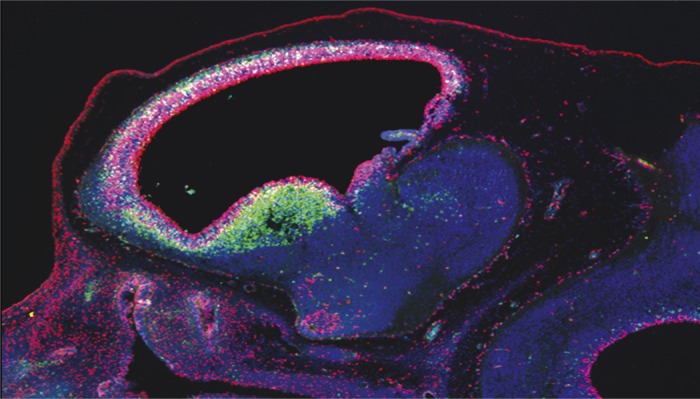
Deletion of BRCA1 within the neural stemcell/progenitor compartment (Nestin-CRE) in an E12.5 mouse embryo displays abnormally high levels of apoptosis as defined by activated Caspase 3 signal (green).
The breast cancer susceptibility gene 1 (BRCA1) is a tumor suppressor gene known to influence breast and ovarian cancer risk, but the BRCA1 protein, which helps repair DNA damage, is highly expressed in the frequently dividing neural epithelial and stem cells of embryos. To determine the functional significance of BRCA1 in embryonic neural progenitors, Gerald Pao et al. (pp. E1240–E1248) created genetically modified mice lacking BRCA1 in the embryonic brain, and found that the mutant mice displayed severe defects, including cell death and tissue volume loss, in several of the brain’s laminated structures implicated in cognition, memory, motor control, and sensation, namely the neocortex, cerebellum, hippocampus, and olfactory bulb. The authors found that experimental deletion of the tumor suppressor gene p53 partially restored the normal lamination of the affected brain structures in the BRCA1-deficient mice, likely because BRCA1 loss leads to neural progenitor cell death mediated by p53. However, defects in cell polarity and centrosomes—organelles involved in cell division—of certain neuronal types in BRCA1 mutant mice were rectified only when another signaling protein called ATM was deleted. The findings suggest a model wherein BRCA1 lies at the confluence of DNA repair and centrosomal functions mediated by a signaling cascade—involving p53 and ATM—that likely controls brain volume. According to the authors, the BRCA1 gene might have evolved to control brain size and architecture in primates. — P.N.
Bacteria programmed to monitor gut environment
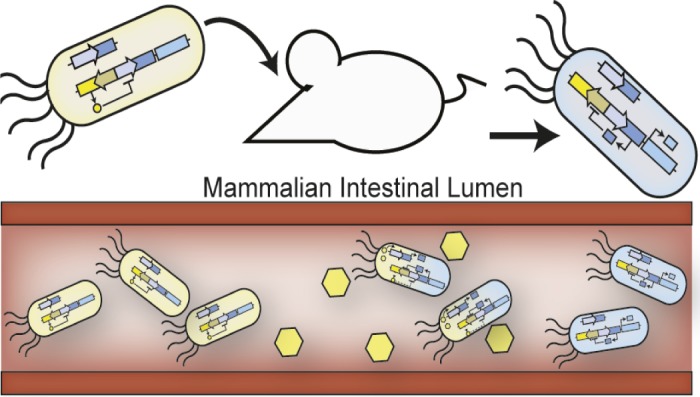
Engineered E. coli produce a detectable signal upon exposure to an environmental stimulus inside a mouse intestine.
Researchers have demonstrated that genetic systems can be engineered to detect, record, and report signals from an environment in vitro. Jonathan Kotula et al. (pp. 4838–4843) attempted to engineer a genetic system in Escherichia coli gut bacteria to enable environmental monitoring inside a living system. The authors first introduced genes from the cI/Cro genetic switch of phage lambda, a bacterial virus that commonly infects E. coli, into the E. coli genome. When the engineered E. coli were cultured in vitro and exposed to anhydrotetracycline, an inactive antibiotic, the chemical triggered the phage genes to begin producing Cro proteins. In the absence of anhydrotetracycline, the bacteria’s phage genes produced cI proteins. The authors next transferred the genetic switch system to an E. coli strain isolated from and adapted to the mouse gut, and fed the engineered E. coli to mice. Analysis of mouse fecal samples confirmed that the engineered bacteria had become established in the guts of the mice and were producing cI proteins. After feeding the mice anhydrotetracycline, the E. coli collected from fecal samples predominantly produced Cro proteins, suggesting that the genetic switch had been triggered by exposure to anhydrotetracycline, similar to the in vitro observation. According to the authors, the results demonstrate that a genetic reporting system can stably operate inside a living organism, enabling noninvasive probing of a complex internal environment. — J.J.
Study reveals multiple cellular origins of lung cancer
Adenocarcinomas are a common type of lung cancer often found in the outer area of the lung. Researchers have identified mutations in the K-RAS gene as a common cause, but the specific type of lung cell that gives rise to these malignant tumors has been a subject of intense debate. Kate Sutherland et al. (pp. 4952–4957) infected mice with viral vectors that activated a mutant form of the K-RAS gene, specifically in either alveolar type 2 (AT2) cells or Clara cells—two cell types thought to produce lung adenocarcinomas. Using histological and microscopy techniques, the authors found that the K-RAS mutation caused both of these cell types to form adenocarcinomas, but these tumors primarily originated from AT2 cells rather than from Clara cells. Moreover, the cell type of origin determined the specific location and molecular and cellular properties of the lung tumors, especially during the early stages of tumor development. The findings show that multiple cellular routes can lead to the formation of lung adenocarcinomas with distinct properties. According to the authors, the cell type of origin may also determine responses to treatment, suggesting that personalized treatments targeting the relevant cellular pathway might improve clinical outcomes for patients. — J.W.
Diet, diabetes, and dementia
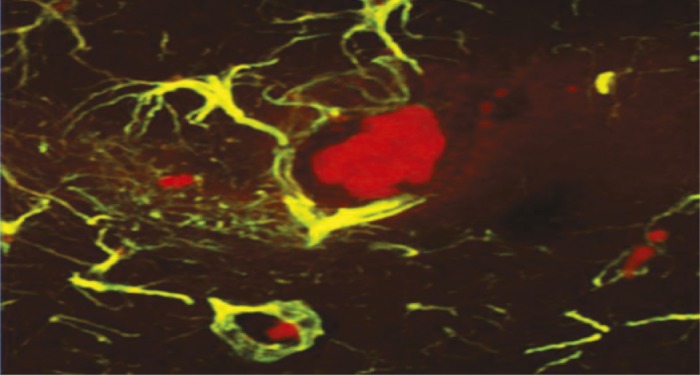
Mouse brain hippocampus showing advanced glycation endproducts (red clusters) within microglia, the brain’s immune cells (green).
Advanced glycation endproducts (AGEs), compounds present in many heat-processed foods, have been linked to diabetes and brain injury when present in the bloodstream and brain at high levels, but the mechanism linking the compounds to those conditions is largely unknown. Weijing Cai et al. (pp. 4940–4945) tracked cognitive health in mice and humans that ingested AGEs at the high levels typical of a Western diet to determine whether AGEs cause suppression of SIRT1, a deacetylase enzyme that regulates neuronal, immune, and endocrine function, and is suppressed in individuals with neurodegenerative and metabolic diseases. Mice chronically fed a diet high in certain reactive AGEs had high levels of AGEs in their brains, and SIRT1 levels in their blood and brain tissue were suppressed, compared with mice that were fed a diet low in AGEs. The authors report that the mice fed the high AGE diet developed metabolic syndrome, declines in cognitive and motor abilities, and amyloid-β42 deposits, a component of the plaques characteristic of Alzheimer’s disease. In contrast, the mice fed low AGEs did not display similar changes. In a clinical study, healthy humans over the age of 60 with high AGE-blood levels experienced cognitive declines over a 9-month period and signs of insulin resistance that correlated with high AGE levels and SIRT1 suppression. The results suggest that dietary AGEs suppress production of SIRT1, likely contributing over time to metabolic syndrome and symptoms of dementia, according to the authors. — J.J.
Modeling technique predicts untested protein kinase inhibitor efficacy
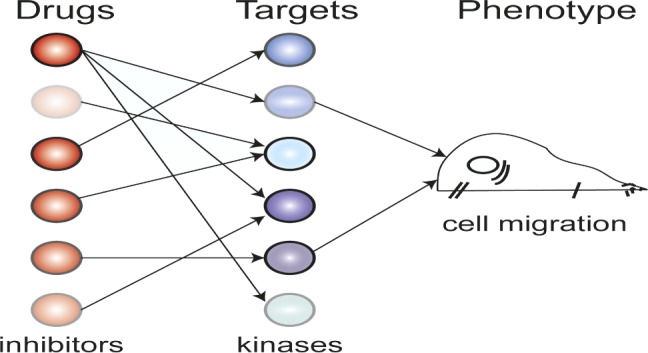
Schematic illustrating polypharmacology in cell migration.
Protein kinase inhibitors (PKIs), a promising class of anticancer therapeutics, have notoriously nonspecific targets. Recent advances in high-throughput screening have allowed researchers to profile the drug target specificities for hundreds of PKIs, revealing that even well characterized inhibitors thought to be specific can interact with off-target kinases. Seeking a different approach, Taranjit Gujral et al. (pp. 5048–5053) selected 32 PKIs with different effects on the activity of 300 kinases and tested how each inhibitor affects cell migration. With mathematical modeling and a well-established variable selection method known as elastic net regularization, the authors identified candidate kinases that were most likely to be involved in epithelial and mesenchymal cell migration in a specific cellular phenotype, based on the differences between the inhibitor profiles. The authors then performed gene knockdown experiments and verified that the majority of the candidates were in fact important kinases for cancer cell migration in the phenotype. The findings offer a means to predict cell-type specific responses to previously untested PKIs and could contribute to the identification of new cancer drugs, according to the authors. — T.J.
A model liver cell line for hepatitis
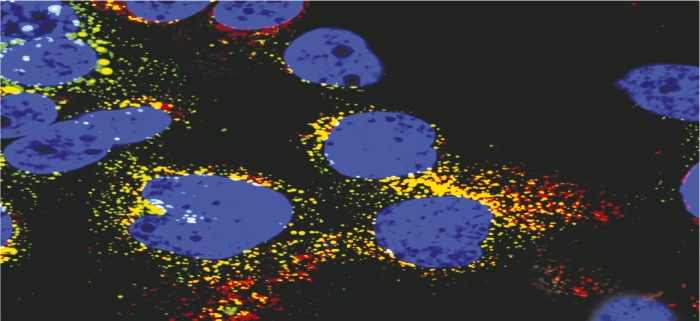
Coinfection of experimental cell line HLCZ01 by hepatitis B and C.
Experimental cell lines that support the full lifecycle of human hepatitis B virus (HBV) and hepatitis C virus (HCV) infections have not been developed, leaving gaps in understanding virus entry, replication, and assembly, and HBV/HCV coinfection. Darong Yang et al. (pp. E1264–E1273) isolated hepatoma cells from a human male with chronic HCV and screened for cell lines that would support the full lifecycle of HBV and HCV. The authors report that one cell line, HLCZ01, could be successfully infected with both laboratory and clinical strains of HBV and HCV. The infected HLCZ01 cultures produced viral proteins and full HBV and HCV particles that could then infect naive HLCZ01 cultures, suggesting that the HLCZ01 cell line can support the full lifecycle of both viruses. HBV and HCV infection of HLCZ01 cell cultures was also blocked by the antibodies that can protect healthy liver cells from infection, suggesting a similar mode of infection. Upon coinfection of HLCZ01 with both HBV and HCV, the authors observed no inhibition of infection of either virus due to the presence of the other, suggesting that the cell line could be used to study the pathogenesis of hepatitis coinfections. The similarity of HLCZ01 infection to that of normal liver cells, and the susceptibility of HLCZ01 to infection by a variety of clinical HBV and HCV isolates, makes HLCZ01 a viable tool to develop vaccines and antiviral therapies, according to the authors. — J.J.


