Significance
Suppression of NAD+-dependent sirtuin 1 (SIRT1) is linked to dementia or Alzheimer’s disease (AD) and the metabolic syndrome (MS). Because advanced glycation end products (AGEs) promote MS and neurotoxicity, we conducted studies of C57BL6 mice fed isocaloric diets containing defined AGEs [methyl-glyoxal derivatives (MG)] to determine whether food AGEs promote AD and MS. MG+-fed, but not MG−-fed, mice developed brain SIRT1 deficiency, amyloid-β deposits, cognitive and motor deficits, and MS. These findings were validated in older healthy humans with high baseline circulating MG levels by a time-dependent decline in cognition and insulin sensitivity. The data suggest that food-derived AGEs, an environmental factor, contribute to both AD and MS by causing chronic SIRT1 suppression. Importantly, reduction of food-derived AGEs is feasible and may provide an effective treatment strategy for both these epidemics.
Keywords: neural, insulin resistance, obesity, nutrition, caloric restriction
Abstract
Age-associated dementia and Alzheimer’s disease (AD) are currently epidemic. Neither their cause nor connection to the metabolic syndrome (MS) is clear. Suppression of deacetylase survival factor sirtuin 1 (SIRT1), a key host defense, is a central feature of AD. Age-related MS and diabetes are also causally associated with suppressed SIRT1 partly due to oxidant glycotoxins [advanced glycation end products (AGEs)]. Changes in the modern diet include excessive nutrient-bound AGEs, such as neurotoxic methyl-glyoxal derivatives (MG). To determine whether dietary AGEs promote AD, we evaluated WT mice pair-fed three diets throughout life: low-AGE (MG−), MG-supplemented low-AGE (MG+), and regular (Reg) chow. Older MG+-fed mice, similar to old Reg controls, developed MS, increased brain amyloid-β42, deposits of AGEs, gliosis, and cognitive deficits, accompanied by suppressed SIRT1, nicotinamide phosphoribosyltransferase, AGE receptor 1, and PPARγ. These changes were not due to aging or caloric intake, as neither these changes nor the MS were present in age-matched, pair-fed MG− mice. The mouse data were enhanced by significant temporal correlations between high circulating AGEs and impaired cognition, as well as insulin sensitivity in older humans, in whom dietary and serum MG levels strongly and inversely associated with SIRT1 gene expression. The data identify a specific AGE (MG) as a modifiable risk factor for AD and MS, possibly acting via suppressed SIRT1 and other host defenses, to promote chronic oxidant stress and inflammation. Because SIRT1 deficiency in humans is both preventable and reversible by AGE reduction, a therapeutic strategy that includes AGE reduction may offer a new strategy to combat the epidemics of AD and MS.
Cognitive dysfunction is currently one of the most prevalent and important polygenic age-related diseases (1–3). A link has been identified between dementia, the most frequent form of which is Alzheimer’s disease (AD), and the metabolic syndrome (MS) or diabetes type 2 (T2D) (1, 3), conditions largely related to environmental factors (4, 5). An emerging view suggests that there is a compromise in innate defense mechanisms preceding these conditions that is due to sustained elevation of oxidant stress (OS) (6).
Modulation of the environment, i.e., caloric excess or caloric restriction (CR), can influence cognitive function (7, 8); however, the calorie-sensitive pathway(s) involved are unknown. NAD+-dependent sirtuin 1 (SIRT1), an NAD+-dependent deacetylase that positively regulates neuronal, immune, and endocrine responses, is down-regulated in aging-related diseases and is thought to contribute to cognitive decline (1, 9–11). Restoration of brain SIRT1 is widely implicated in the benefits of CR on the aging brain (7–9, 11).
Glycotoxins or advanced glycation end products (AGEs) are a class of OS-promoting agents implicated in diabetes and aging, including brain injury due to AD and stroke (6, 12–14). Certain AGEs, such as the derivatives of methyl-glyoxal-imidazolone-H1 (MG-H1) amplify the proinflammatory properties of amyloid β1–42 (Aβ) or tau protein (15–17). High MG levels in brain or the circulation are linked to cognitive decline in elderly subjects (15, 17, 18).
Food-derived AGEs have emerged as contributors to chronic diseases due to their abundance in thermally altered nutrients (19, 20). AGE restriction in nutritionally and nutritionally balanced diets delayed metabolic and vascular diseases and extended lifespan in mice (21, 22). The role of diet-derived AGEs in systemic AGE toxicity was confirmed in studies using a defined MG-supplemented low-AGE diet (MG+). Old MG+ mice, but not MG− mice, developed age-related MS and kidney and cardiac fibrosis, associated with inflammation and SIRT1 depletion in insulin-sensitive tissues (22). AGE restriction also improved insulin resistance and inflammation in humans (23, 24).
We therefore postulated that oral AGEs, in addition to causing MS, might also predispose to dementia, and these both might be prevented by AGE restriction (22). The MG+/MG− mouse model provides an opportunity to explore the link of oral AGEs to these chronic conditions, free of either genetic or caloric manipulations.
Herein, we show that cognitive dysfunction develops in parallel with metabolic changes in old mice fed defined AGEs (MG+) but not in AGE-restricted (MG−) mice. These findings were supported by clinical findings, introducing previously unidentified evidence of AGEs as a modifiable risk factor for both AD and MS.
Results
Chronic Oral MG+ Promotes Systemic and Brain Changes in Old WT Mice.
MG+ (18 mo) mice fed an MG-supplemented diet had higher body weight than pair-fed age-matched MG− mice fed a low-AGE diet (Table 1), a finding attributed to the higher amount of AGE-modified visceral fat found only in MG+ (and Reg 24–26 mo) mice (22). Higher serum AGEs [serum εN-carboxymethyl-lysine (sCML) and sMG], plasma 8-isoprostanes, and lower adiponectin levels were noted in MG+ and Reg mice (Table 1 and Fig. 1A), suggesting elevated OS in these two groups, but not in MG− mice (22). MG+ and Reg mice were also insulin resistant, based on higher fasting insulin and leptin levels (Table 1) and on an abnormal i.p. glucose tolerance test (IGTT) (SI Materials and Methods) (22).
Table 1.
Mice characteristics
| Groups | MG− | MG+ | Reg |
| Number/group | 12 (6 F/6 M) | 12 (6 F/6 M) | 8 (4 F/4 M) |
| Body weight (g) | 30.8 ± 0.52 | 35.1 ± 1.8* | 33.5 ± 1.5 |
| Brain weight (g) | 0.48 ± 0.1 | 0.46 ± 0.05 | 0.48 ± 0.1 |
| Brain/body weight ratio | 0.017 ± 0.004† | 0.013 ± 0.001* | 0.014 ± 0.003 |
| Food intake (g/d) | 4.8 ± 0.8 | 4.9 ± 0.05 | 5 ± 0.7 |
| Food MG intake (nmol/d) | 0.67 × 104† | 1.9 × 104‡ | 1.5 × 104 |
| Serum CML (sCML, U/mL) | 21.9 ± 1.2§ | 49.9 ± 1.1‡ | 42.8 ± 1.5 |
| Serum MG (sMG, nmol/mL) | 0.83 ± 0.2† | 2.08 ± 0.29‡,¶ | 1.59 ± 0.2 |
| Fasting blood glucose (mg/dL) | 82.6 ± 2.8 | 81.4 ± 4 | 83.1 ± 2.4 |
| Fasting insulin (nmol/L) | 0.24 ± 0.02§ | 0.41 ± 0.07* | 0.45 ± 0.02 |
| Adiponectin (μg/mL) | 13.7 ± 1.2† | 7.8 ± 1.0‡ | 8.2 ± 0.4 |
| Leptin (ng/mL) | 10.3 ± 0.9† | 22.7 ± 0.9‡ | 18.0 ± 0.8 |
| 8-Isoprostane (pg/mL) | 88 ± 5.5† | 267 ± 32.4‡,¶ | 174 ± 22.3 |
| Brain protein CML (U/g brain) | 100.3 ± 12.2 | 166.3 ± 19.4* | 142.6 ± 10 |
| Brain protein MG (nmol/g brain) | 63.4 ± 6.3 | 90.1 ± 11.05* | 78.4 ± 6.5 |
| Brain lipid CML (U/g brain) | 229.5 ± 15.5 | 352.5 ± 39.2* | 290.4 ± 14.3 |
| Brain lipid MG (nmol/g brain) | 6.3 ± 1.3 | 10.0 ± 0.6* | 7.5 ± 0.5 |
Mice were WT C57BL6. MG− denotes 18-mo mice on low-AGE diet. MG+ denotes 18-mo mice on a MG-supplemented low-AGE diet. Reg denotes 24- to 26-mo control mice on standard NIH-31 open formula diet. Data are means ± SEM. *P < 0.05 and ‡P < 0.01 between MG+ and MG− mice; ¶P < 0.05 between MG+ and Reg mice; †P < 0.05 and §P < 0.01 between MG− and Reg mice.
Fig. 1.
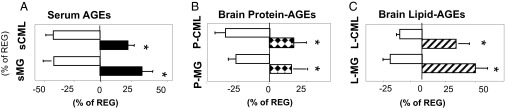
Oral MG+ leads to increased systemic and brain protein and lipid AGEs. Data are from 18-mo WT C57BL6 mice pair-fed MG+ or MG− diet and control (Reg) mice (24–26 mo, n = 8/group). (A) Serum CML and MG levels. (B) Brain protein CML and protein MG. (C) Brain lipid CML and lipid MG. Data are percent (mean ± SEM) above or below Reg controls (as shown in Table 1). *P < 0.05, MG+ vs. MG− mice.
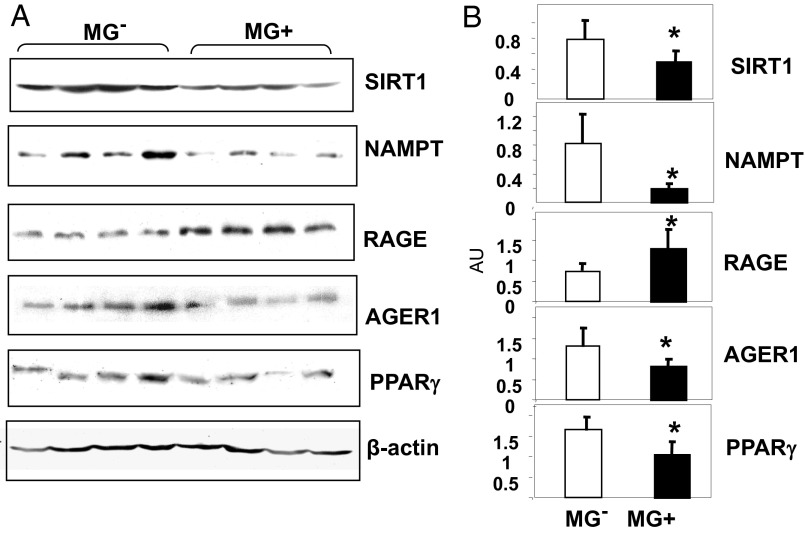
Both brain protein- and lipid-associated AGE levels in Reg and MG+ mice were higher than in MG− mice (Table 1 and Fig. 1 B and C). Brain tissue from MG+ mice had reduced protein levels of SIRT1 and of the [NAD+/NADH]-regulating nicotinamide phosphoribosyltransferase (NAMPT), relative to MG− mice (Fig. 2 A and B), suggesting that exogenous AGEs induce parallel changes in brain and in the periphery (22). Brain AGE receptor 1 (AGER1) and PPARγ levels were reduced, and receptor for AGEs (RAGE) levels were enhanced in MG+, compared with MG−, brain tissue (Fig. 2 A and B) (22).
Fig. 2.
Oral MG+ alters brain SIRT1, NAMPT, AGER1, RAGE, and PPARγ protein expression in MG+-fed mice brains. (A) Representative Western blots from brain extracts of 18-mo C57BL6 mice fed an MG+ or MG− diet (n = 3–5) and (B) densitometry of A, shown as ratio (mean ± SEM) of target protein to β-actin. *P < 0.05 vs. MG− mice.
Oral MG+ Reduces ADAM10 Transcriptional Activity and Promotes Aβ Accumulation.
A disintegrin and metalloproteinase binding protein 10 (ADAM10) modulates amyloid precursor protein (APP) and soluble APP-beta (APP, etc.) (sAPP-β) levels, limiting the accumulation of Aβ1–42, and is regulated by SIRT1 (25). In this context, ADAM10 mRNA and protein levels in MG+ and Reg brain were significantly lower than in MG− brain (Fig. 3 A and B, i and ii). Levels of total APP and sAPP-β, the product cleaved by β-secretase, were similar in MG+ and Reg brains. In contrast, sAPP-β levels and the sAPP-β:APP ratio were lower in MG− brains than in MG+ or Reg brains (Fig. 3C). Furthermore, the levels of Aβ in the MG+ and Reg brains were significantly higher than in the MG− brain (Fig. 3D).
Fig. 3.
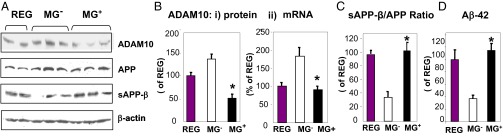
Oral MG+ suppresses ADAM10 expression and increases Aβ in brain. Data are from 18-mo C57BL6 mice fed an MG+ or MG− diet and Reg mice (24–26 mo, n = 8/group). (A) Western blots of brain extracts for ADAM10, APP, and soluble or sAPPβ (n = 3–5). β-actin is used as control. (B, i) Densitometry of ADAM10 protein data shown in A and (B, ii) of ADAM10 mRNA levels, by RT-PCR, shown as AU (n = 5/group). (C) Densitometry of sAPPβ and APP shown in A and expressed as sAPPβ/APP ratio. (D) Aβ1–42 levels. Data in B–D are shown as percent (mean ± SEM) of Reg. *P < 0.05 MG+ vs. MG− mice.
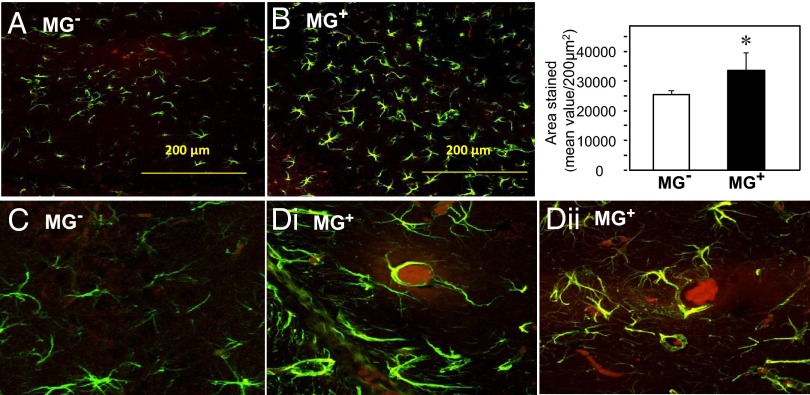
Morphometric analysis of hippocampal (HC) areas for anti–GFAP-positive glia indicated significantly more cells and levels of activation in MG+ than in MG− HC (Fig. 4 A, B, and Inset). MG+ HC had prominent AGE-positive aggregates colocalizing with GFAP-positive cells in areas of dense glial populations (Fig. 4 C and D, i and ii). In contrast, MG− HC sections displayed fewer cells and no AGE-positive clusters (Fig. 4C). No cortical differences were noted by specific nuclear protein staining.
Fig. 4.
Oral MG+ promotes brain gliosis and AGE deposits. Confocal microscopy of coronal hippocampal sections from (A) MG+ and (B) MG− mice (n = 8/group) immunostained for glia cells (Magnification, 20×), and (C) MG− and (D, i and ii) MG+ for both glia and AGEs, using anti-GFAP and anti-AGE, as primary antibodies and Alexa Fluor-488 (green) and Fluor-594 (red), respectively, as secondary antibodies (magnification, 200×). Bar graph shows the quantification of GFAP staining. (Scale bar, 200 µm.) *P < 0.05, MG+ vs. MG− mice.
Neocortical SIRT1 Expression Is Suppressed by Chronic MG+ Excess.
Chronically elevated MG levels could directly or indirectly predispose fetal neurons to injury. SIRT1 and NAMPT were suppressed in MG+ neuronal cells compared with cells from MG− cells (Fig. S1 A–C). Reduced AGER1 levels (Fig. S1C) were consistent with higher intraneuronal RAGE, AGEs, and reactive oxygen species (ROS) levels in Reg and MG+ neurons than in MG− neurons (Fig. S1 D and G). Moreover, ADAM10 was markedly suppressed in MG+ neurons but not in MG− neurons (Fig. S1E).
Prolonged ex vivo stimulation of Reg neurons with MG-BSA (>72 h) resulted in a dose-dependent suppression of SIRT1, AGER1, and ADAM10 (Fig. S2 A and B), changes that were associated with increased ROS (Fig. S2C).
Chronic MG+ Impairs Learning and Memory.
Basic motor coordination and balance learning skills were first evaluated with the rotarod test. MG− mice performed for a longer distance and at a higher speed before falling from the rod compared with MG+ mice (Fig. 5 A and B). MG+ mice showed a lower latency than MG− mice (Fig. 5C). On testing object recognition, MG+ fed mice showed poor exploratory behavior with a lower discriminatory capacity between a familiar and a novel object than MG− mice (Fig. 5D), which spent ∼70% of the time exploring the new object. On testing object replacement, MG+-fed mice performed better, but this was not significant (Fig. 5E).
Fig. 5.
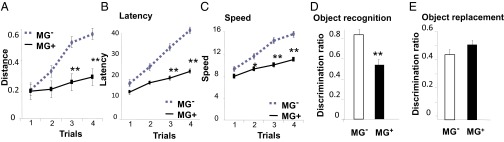
Oral MG+ impairs learning and memory capacities. (A–C) Accelerating rotarod test indicating that MG+ mice (straight line) can travel a shorter distance (A), have reduced latency until fall (B), and a slower speed than MG− mice (broken line) (C). Data (mean ± SEM) were analyzed by two-way repeated-measures ANOVA. n = 10/group. *P < 0.05, **P < 0.01 vs. MG− mice. (D) Object recognition and (E) object replacement tests show that MG+ mice have a deficient memory compared with MG− mice. Data are shown as mean ± SEM (n = 10/group, **P < 0.01 vs. MG− mice).
High MG Correlates with Dietary AGE Intake and SIRT1 Suppression in Older Humans.
At baseline, the cohort’s body mass index (BMI) and metabolic and biochemical parameters (n = 93, ≥60 y old, educated, 68% female) were within the range expected for their age, as were calorie and dietary AGE intake (dAGE) (24, 26). Baseline cognitive function [by Mini Mental State Examination (MMSE)] was also normal (Table S1).
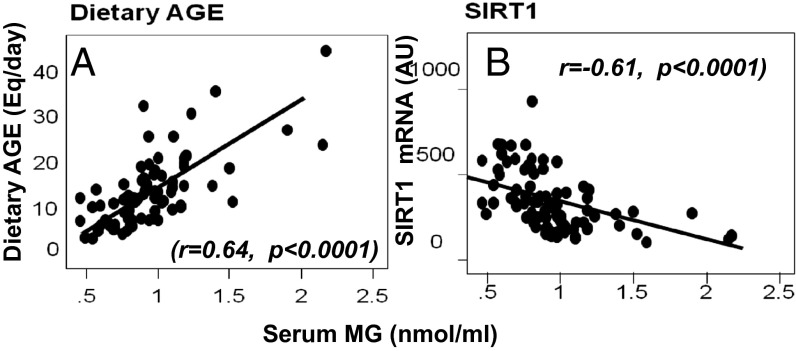
Baseline sMG levels correlated positively with dAGE intake (Fig. 6A) and inversely with mononuclear cell (MNC) SIRT1 mRNA levels (Fig. 6B and Table S2). In addition, baseline dAGE and sMG levels both correlated with sCML, plasma 8-isoprostanes, leptin, MNC TNFα protein, and RAGE mRNA, but inversely with SIRT1 mRNA and adiponectin levels (Table S2 and Fig. S3).
Fig. 6.
Serum MG levels correlate directly with dietary AGE intake (A) and inversely with MNC SIRT1 mRNA (B). Baseline fasting sMG levels, shown as mean ± SEM (nmol/mL), are plotted against daily dietary AGE intake, shown as Eq/d (A) or against MNC SIRT1 mRNA of healthy older adults (B). Fitted regression lines are as shown.
High MG Levels in Older Humans Correlate with Temporal Changes in Cognition and Insulin Sensitivity.
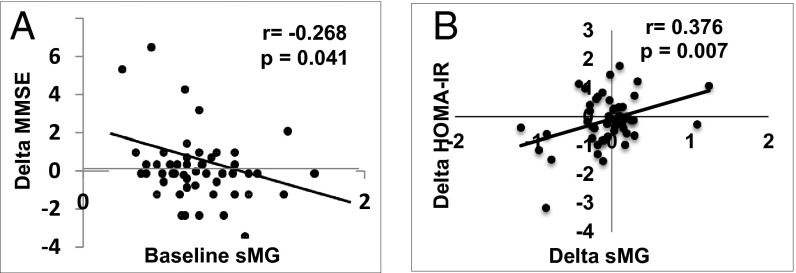
High baseline sMG levels predicted a cognitive decline over time (9 mo, P = 0.041; Fig. 7A), which remained significant after adjusting for age, sex, education, and baseline MMSE. Temporal changes in homeostasis model assessment (HOMA)-IR, a marker of insulin resistance, also correlated with changes in sMG (Fig. 7B and Fig. S4A), as well as with sCML (Fig. S4B). No other metabolic changes were noted.
Fig. 7.
(A) Human serum MG (sMG) levels predict changes in cognition over time. Levels of fasting sMG at baseline are plotted against changes (Δ) in MMSE of healthy older adults, over 9 mo. (B) Changes (Δ) in sMG levels over time correlate with changes (Δ) in HOMA-IR over the same period. All tests were performed in fasting sera; fitted regression lines are as shown.
Discussion
We report that age-related dementia (AD) and MS may be causally linked to high levels of food AGEs, specifically MG. The data extend our previous findings on AGEs promoting the MS in older animals and humans (22–24). The mouse study further reproduces the cognitive and metabolic conditions recently found to be linked in humans (1, 4, 5). The clinical study validates the mouse model and demonstrates that high sMG, a marker of dietary AGE intake and IR (23), may also be a determinant of dementia in older adults (17). It further validates the relevance of dietary AGEs to MS and AD in humans. Because AGEs can be modified in humans, recognition that this underappreciated risk factor plays a role in AD and MS may open unique therapeutic avenues.
Brain deposits of AGEs and Aβ are thought to be age related (13–16, 27–31). The current study shows that both of these elements were increased in brains of old MG+ mice to levels similar to those in old Reg controls. An important insight provided herein is that these changes cannot be attributed to aging or caloric intake alone, because the levels of AGEs and Aβ were significantly lower in strain- and age-matched pair-fed MG− mice.
Brain dysfunction has also been associated with the MS and T2D, conditions linked to nutrient intake (8, 9, 11). We previously showed that MG+ mice had features of the MS, including AGE-modified white adipose tissue (WAT) accumulation and SIRT1 suppression (22). The effects of calories and CR on cognition were previously thought to be directly related to brain SIRT1 expression (10, 11). We found that modern diets are also replete with prooxidant AGEs, including MG (19–21). These data, coupled with the fact that the MG+ diet induces systemic inflammation and SIRT1 suppression in this study independently of caloric intake (21, 22), provides a clear link between SIRT1 deficiency of the aging brain and glycotoxins. Mouse models of CR, AD, or SIRT1 expression in mice reveal that SIRT1 plays a central role in brain function (2, 8, 25). However, the fact that SIRT1 and NAMPT, AGER1, and PPARγ were suppressed in MG+ but not in MG− brains indicates that loss of multiple defense mechanisms in aging may reflect the impact of sustained high OS and that AGEs could play a seminal role (22). The MG− mouse data further demonstrate that SIRT1 deficiency may be preventable in mice regardless of caloric intake.
Changes in the SIRT1 pathway have been linked to AGE receptor levels (22–24). AGE receptors are expressed in brain neurons, microglia, and endothelium. AGER1, an anti-AGE receptor, was up-regulated in the brain of MG− mice, whereas RAGE, a signaling receptor linked to oxidative stress and neurotoxicity, was decreased (30, 31). Because systemic AGER1 also inhibits SIRT1 suppression (22), it could have a similar effect on SIRT1 in the brain. In the current study, we found that neurons from MG− mice had higher AGER1 levels, which were associated with higher levels of SIRT1 and lower levels of intracellular AGE and ROS. In contrast, reduced AGER1 in Reg and MG+ neurons, by delaying the clearance of AGE-modified proteins such as AGE-Aβ (13), could account for the increased amounts of AGE deposits, suppression of SIRT1, and glial activation seen in the brains of MG+ mice but not in MG− mice.
The data from neocortical neurons of MG+ mice further suggest a placental mode of transfer of excessive AGEs to the fetal brain, which might render the brain more susceptible to OS injury. The findings might be of relevance to the increasing incidence of dementia in younger adults with the MS or diabetes. Importantly, this injurious process appears to be preventable in brains of MG− mice, a finding of significant therapeutic import. Whether this involves epigenetic changes or an altered gut microbiome is a critical subject for further inquiry (32, 33).
SIRT1 deficiency leads to impaired insulin receptor signaling in adipose tissue from MG+ mice (22). It is not known if this pathway is altered in the brains of MG+ mice, although this might provide a mechanistic link to the insulin-resistant state shown to be associated with cognitive decline (1, 3, 34). Nonetheless, the prominent gliosis noted in the hippocampus of the MG+ mice, coupled with suppressed SIRT1 and AGER1, is consistent with an MG-mediated inflammatory response. The AGE aggregates observed in these mice could have elicited inflammatory responses (27–30), partly via RAGE activation (14, 35). Whether the effects in MG+ mice are a reflection of altered blood-brain barrier, high intracerebral OS, or both, remains to be established. However, the fact that lower MG levels in MG− brains were associated with lower OS and RAGE suggests that lowering external AGEs could exert significant benefits.
In this context, SIRT1 also regulates liver X receptor, forkhead box subgroup O, and PPARγ, important factors in brain plasticity (2, 11, 36). Additionally, PPARγ, which promotes amyloid clearance and suppresses glial activation (37), was decreased in MG+ and Reg compared with MG− brains. Thus, low PPARγ may delay Aβ clearance, a hypothesis supported by higher levels of Aβ levels and gliosis in brains of MG+ and Reg mice compared with MG− mice.
SIRT1 also limits Aβ accumulation by directing APP processing via ADAM10 and α-secretase transcription (25). Because SIRT1 deficiency in MG+ mice was associated with reduced ADAM10 levels, this may partly account for the increased APPβ/total APP ratio and the higher Aβ generation in MG+ and Reg mice. The absence of these changes in the MG− mice further supports the view that altered brain homeostasis may stem from sustained exposure to neurotoxic AGEs.
Importantly, impaired spatial learning and recognition memory in MG+ mice mirrored cognitive changes in older humans (11, 38, 39). Significantly, these were absent in MG− mice, offering previously unidentified direct in vivo evidence that oral AGEs can impair cognition.
Because the MG+ mice also manifested in parallel metabolic (22) and cognitive changes, the data may identify MG as a causal link between AD and MS (3, 24). Herein, we found a significant temporal decline in cognition in subjects with high baseline sMG level, together with a strong inverse correlation between baseline levels of dietary or serum AGEs and MNC SIRT1 gene levels (24). Furthermore, changes in insulin resistance temporally correlated with changes in serum AGE levels. Together with the animal data, these clinical findings reinforce the fact that chronic exposure to exogenous AGEs can weaken host defenses well in advance of cognitive or metabolic disturbances. A critical finding afforded by the animal studies is that AGE restriction prevented the loss of both conditions, highlighting glycotoxins as a modifiable risk for AD and MS in humans. Because aspects of the MS in humans may improve after AGE restriction (23, 24), it is possible that cognition can also improve in humans. Given the major public health potential of these findings, larger clinical trials are warranted.
Materials and Methods
Animal Studies.
We used WT C57BL/6 mice [derived from National Institutes of Aging, CR colony, bred for >10 generations and fed an MG-supplemented diet (MG+)], because older MG+ mice, similar to older WT Reg mice, develop MS (SI Materials and Methods) (22). The diets were identical in caloric content, but MG+ and Reg diets contained ∼2- to 2.3-fold more MG-H1 and CML than the MG− diet (Table 1) (21). C57BL/6 mice, fed the NIH-31 open formula (Reg), were used as controls (SI Materials and Methods) (21). Based on an abnormal IGTT, which appeared in MG+ mice (at 18 mo) and in Reg mice (at 24–26 mo), all mice, including MG− mice (18 mo) were killed; blood and brain tissues were processed as needed.
AGE Determinations.
AGEs in sera (mouse and human), mouse brain tissue, and cultured neuronal cells were determined by ELISAs for MG-H1 (3D11 mab) and CML (4G9 mab) for protein and lipid AGEs (SI Materials and Methods) (21–23, 40–42).
Cell Culture and Treatments.
Embryonic E14 primary brain neuronal cells from MG+, MG−, and Reg mice were derived and cultured as described with minor modifications (SI Materials and Methods) (8, 11). Cell extracts were obtained immediately or after 24–72 h, as needed. Neuronal cells from Reg brain were chronically exposed to different doses of MG-BSA or BSA (72 h).
Immunohistochemistry.
Anesthetized mice were transcardially perfused with 4% (wt/vol) paraformaldehyde (PF) and the brain was removed, processed for immunohistochemistry, and viewed by a confocal microscope (Zeiss LSM 510 Meta) (SI Materials and Methods) (8, 36).
Brain Functional Testing.
Motor coordination, balance, and motor learning were tested on the accelerating rotarod device (Series 8; IITC Life Science) (SI Materials and Methods) (38). Object recognition and placement memory tests were conducted, and data were video recorded and analyzed (SI Materials and Methods) (39).
Human Studies.
This study was an observational study in healthy adult (n = 93), 60-y-old, New York City residents who provided informed consent. Participants were evaluated at baseline and 9 mo later for time-dependent changes in serum AGEs, markers of OS, and inflammation, insulin resistance, and cognition. Information was collected on medical history, medications, and caloric and AGE intake. Exclusion criteria included evidence of diabetes, cardiovascular or kidney disease, neuropsychiatric disease, and cancer. Cognition was assessed by a Clinical Dementia Rating (CDR) scale and by a MMSE score, with normality defined as a CDR score of 0 (nondemented) and an MMSE score above the 10th percentile of age and education norms (17). All cognition tests were performed by trained research coordinators at the Icahn School of Medicine Alzheimer’s Disease Research Center. After an initial evaluation, participants underwent MMSE and provided a fasting blood sample (at 0 and 9 mo). Plasma or sera was used for routine blood tests, and AGEs (sCML and sMG), adiponectin, leptin, insulin levels, and HOMA index and MNCs were used for gene assessment by RT-PCR (SI Materials and Methods) (23, 24).
60-y-old, New York City residents who provided informed consent. Participants were evaluated at baseline and 9 mo later for time-dependent changes in serum AGEs, markers of OS, and inflammation, insulin resistance, and cognition. Information was collected on medical history, medications, and caloric and AGE intake. Exclusion criteria included evidence of diabetes, cardiovascular or kidney disease, neuropsychiatric disease, and cancer. Cognition was assessed by a Clinical Dementia Rating (CDR) scale and by a MMSE score, with normality defined as a CDR score of 0 (nondemented) and an MMSE score above the 10th percentile of age and education norms (17). All cognition tests were performed by trained research coordinators at the Icahn School of Medicine Alzheimer’s Disease Research Center. After an initial evaluation, participants underwent MMSE and provided a fasting blood sample (at 0 and 9 mo). Plasma or sera was used for routine blood tests, and AGEs (sCML and sMG), adiponectin, leptin, insulin levels, and HOMA index and MNCs were used for gene assessment by RT-PCR (SI Materials and Methods) (23, 24).
Statistics.
Animal data were expressed as means ± SEM, and differences were determined by Student t test. For comparisons among the three groups, one-way ANOVA with Bonferroni correction analysis was performed. The behavioral data were analyzed using repeated-measures ANOVA with multiple comparison tests performed with Bonferroni’s adjustment. Significance was set at P < 0.05.
Human data were analyzed for relationships between variables at baseline using mean ± SD and quartiles, based on regression models and partial Spearman correlation coefficients and adjusting for age and sex. The temporal relationship between baseline sMG and Δ MMSE was explored using general linear regression models adjusting for baseline MMSE and years of education, age, and sex. Also assessed were the correlations of Δ sMG, Δ sCML, and Δ HOMA-IR, using Spearman correlation coefficients, by Stata, version 11. Data with two-sided P < 0.05 were considered significant.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants AG23188 (to H.V.) and M01-RR-00071 (to the GCRC of Icahn School of Medicine at Mount Sinai).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Commentary on page 4743.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316013111/-/DCSupplemental.
References
- 1.Janson J, et al. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53(2):474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 2.Guarente L, Franklin H. Franklin H. Epstein Lecture: Sirtuins, aging, and medicine. N Engl J Med. 2011;364(23):2235–2244. doi: 10.1056/NEJMra1100831. [DOI] [PubMed] [Google Scholar]
- 3.Talbot K, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy VP, Zhu X, Perry G, Smith MA. Oxidative stress in diabetes and Alzheimer’s disease. J Alzheimers Dis. 2009;16(4):763–774. doi: 10.3233/JAD-2009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loy CT, Twigg SM. Growth factors, AGEing, and the diabetes link in Alzheimer’s disease. J Alzheimers Dis. 2009;16(4):823–831. doi: 10.3233/JAD-2009-0997. [DOI] [PubMed] [Google Scholar]
- 6.Vlassara H, Striker GE. AGE restriction in diabetes mellitus: A paradigm shift. Nat Rev Endocrinol. 2011;7(9):526–539. doi: 10.1038/nrendo.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srivastava S, Haigis MC. Role of sirtuins and calorie restriction in neuroprotection: Implications in Alzheimer’s and Parkinson’s diseases. Curr Pharm Des. 2011;17(31):3418–3433. doi: 10.2174/138161211798072526. [DOI] [PubMed] [Google Scholar]
- 8.Qin W, et al. Regulation of forkhead transcription factor FoxO3a contributes to calorie restriction-induced prevention of Alzheimer’s disease-type amyloid neuropathology and spatial memory deterioration. Ann N Y Acad Sci. 2008;1147:335–347. doi: 10.1196/annals.1427.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126(2):257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Bonda DJ, et al. The sirtuin pathway in ageing and Alzheimer disease: Mechanistic and therapeutic considerations. Lancet Neurol. 2011;10(3):275–279. doi: 10.1016/S1474-4422(11)70013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fusco S, et al. A role for neuronal cAMP responsive-element binding (CREB)-1 in brain responses to calorie restriction. Proc Natl Acad Sci USA. 2012;109(2):621–626. doi: 10.1073/pnas.1109237109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huebschmann AG, Regensteiner JG, Vlassara H, Reusch JE. Diabetes and advanced glycoxidation end products. Diabetes Care. 2006;29(6):1420–1432. doi: 10.2337/dc05-2096. [DOI] [PubMed] [Google Scholar]
- 13.Vitek MP, et al. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc Natl Acad Sci USA. 1994;91(11):4766–4770. doi: 10.1073/pnas.91.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmerman GA, et al. Neurotoxicity of advanced glycation endproducts during focal stroke and neuroprotective effects of aminoguanidine. Proc Natl Acad Sci USA. 1995;92(9):3744–3748. doi: 10.1073/pnas.92.9.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srikanth V, et al. Methylglyoxal, cognitive function and cerebral atrophy in older people. J Gerontol A Biol Sci Med Sci. 2013;68(1):68–73. doi: 10.1093/gerona/gls100. [DOI] [PubMed] [Google Scholar]
- 16.Li XH, et al. Methylglyoxal induces tau hyperphosphorylation via promoting AGEs formation. Neuromolecular Med. 2012;14(4):338–348. doi: 10.1007/s12017-012-8191-0. [DOI] [PubMed] [Google Scholar]
- 17.Beeri MS, et al. Serum concentration of an inflammatory glycotoxin, methylglyoxal, is associated with increased cognitive decline in elderly individuals. Mech Ageing Dev. 2011;132(11-12):583–587. doi: 10.1016/j.mad.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed N, et al. Protein glycation, oxidation and nitration adduct residues and free adducts of cerebrospinal fluid in Alzheimer’s disease and link to cognitive impairment. J Neurochem. 2005;92(2):255–263. doi: 10.1111/j.1471-4159.2004.02864.x. [DOI] [PubMed] [Google Scholar]
- 19.Koschinsky T, et al. Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci USA. 1997;94(12):6474–6479. doi: 10.1073/pnas.94.12.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birlouez-Aragon I, et al. A diet based on high-heat-treated foods promotes risk factors for diabetes mellitus and cardiovascular diseases. Am J Clin Nutr. 2010;91(5):1220–1226. doi: 10.3945/ajcn.2009.28737. [DOI] [PubMed] [Google Scholar]
- 21.Cai W, et al. Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan. Am J Pathol. 2008;173(2):327–336. doi: 10.2353/ajpath.2008.080152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai W, et al. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc Natl Acad Sci USA. 2012;109(39):15888–15893. doi: 10.1073/pnas.1205847109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uribarri J, et al. Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: Potential role of AGER1 and SIRT1. Diabetes Care. 2011;34(7):1610–1616. doi: 10.2337/dc11-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uribarri J, et al. Suppression of native defense mechanisms, SIRT1 and PPARγ, by dietary glycoxidants precedes disease in adult humans; relevance to lifestyle-engendered chronic diseases. Amino Acids. 2013;46(2):301–309. doi: 10.1007/s00726-013-1502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses β-amyloid production by activating the α-secretase gene ADAM10. Cell. 2010;142(2):320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Vlassara H, et al. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: Role of the antiinflammatory AGE receptor-1. J Clin Endocrinol Metab. 2009;94(11):4483–4491. doi: 10.1210/jc.2009-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickson DW, et al. Glycation and microglial reaction in lesions of Alzheimer’s disease. Neurobiol Aging. 1996;17(5):733–743. doi: 10.1016/0197-4580(96)00116-9. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt AM, et al. The role of RAGE in amyloid-beta peptide-mediated pathology in Alzheimer’s disease. Curr Opin Investig Drugs. 2009;10(7):672–680. [PubMed] [Google Scholar]
- 29.Ko SY, Lin YP, Lin YS, Chang SS. Advanced glycation end products enhance amyloid precursor protein expression by inducing reactive oxygen species. Free Radic Biol Med. 2010;49(3):474–480. doi: 10.1016/j.freeradbiomed.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Li JJ, Dickson D, Hof PR, Vlassara H. Receptors for advanced glycosylation endproducts in human brain: Role in brain homeostasis. Mol Med. 1998;4(1):46–60. [PMC free article] [PubMed] [Google Scholar]
- 31.Takuma K, et al. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc Natl Acad Sci USA. 2009;106(47):20021–20026. doi: 10.1073/pnas.0905686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford D, Ions LJ, Alatawi F, Wakeling LA. The potential role of epigenetic responses to diet in ageing. Proc Nutr Soc. 2011;70(3):374–384. doi: 10.1017/S0029665111000851. [DOI] [PubMed] [Google Scholar]
- 33.Marques SC, et al. Epigenetic regulation of BACE1 in Alzheimer’s disease patients and in transgenic mice. Neuroscience. 2012;220:256–266. doi: 10.1016/j.neuroscience.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 34.Correia SC, et al. Insulin-resistant brain state: The culprit in sporadic Alzheimer’s disease? Ageing Res Rev. 2011;10(2):264–273. doi: 10.1016/j.arr.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deane R, et al. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122(4):1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michán S, et al. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30(29):9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandrekar-Colucci S, Karlo JC, Landreth GE. Mechanisms underlying the rapid peroxisome proliferator-activated receptor-γ-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer’s disease. J Neurosci. 2012;32(30):10117–10128. doi: 10.1523/JNEUROSCI.5268-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monville C, Torres EM, Dunnett SB. Comparison of incremental and accelerating protocols of the rotarod test for the assessment of motor deficits in the 6-OHDA model. J Neurosci Methods. 2006;158(2):219–223. doi: 10.1016/j.jneumeth.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Barker GR, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27(11):2948–2957. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uribarri J, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110(6):911–916, e12. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bucala R, et al. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc Natl Acad Sci USA. 1994;91(20):9441–9445. doi: 10.1073/pnas.91.20.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu MX, et al. The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996;271(17):9982–9986. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.