Significance
DNA replication is error-prone. Mechanisms to recognize errors in DNA lead to arrest of cell proliferation at various checkpoints to allow for repair. Suppression of these mechanisms is necessary for recovery from these checkpoints and continuation of cell division. We show that signal transducer and activator of transcription 3 (STAT3), a protein overactive in many human cancers, can abnormally evade recognition of DNA errors and damage leading to bypass of a critical cell-cycle checkpoint and uncontrolled cell proliferation. While relevant to understanding cancer development and prevention, this will also bring fresh insights into the role of STAT3 in the central biology of cell proliferation, particularly since STAT3 is necessary for critical processes including embryonic development and immunity.
Keywords: autosomal dominant hyper-IgE syndrome, infectious mononucleosis, latent membrane protein 1, Epstein–Barr nuclear antigen
Abstract
DNA damage response (DDR) is a signaling network that senses DNA damage and activates response pathways to coordinate cell-cycle progression and DNA repair. Thus, DDR is critical for maintenance of genome stability, and presents a powerful defense against tumorigenesis. Therefore, to drive cell-proliferation and transformation, viral and cellular oncogenes need to circumvent DDR-induced cell-cycle checkpoints. Unlike in hereditary cancers, mechanisms that attenuate DDR and disrupt cell-cycle checkpoints in sporadic cancers are not well understood. Using Epstein–Barr virus (EBV) as a source of oncogenes, we have previously shown that EBV-driven cell proliferation requires the cellular transcription factor STAT3. EBV infection is rapidly followed by activation and increased expression of STAT3, which mediates relaxation of the intra-S phase cell-cycle checkpoint; this facilitates viral oncogene-driven cell proliferation. We now show that replication stress-associated DNA damage, which results from EBV infection, is detected by DDR. However, signaling downstream of ATR is impaired by STAT3, leading to relaxation of the intra-S phase checkpoint. We find that STAT3 interrupts ATR-to-Chk1 signaling by promoting loss of Claspin, a protein that assists ATR to phosphorylate Chk1. This loss of Claspin which ultimately facilitates cell proliferation is mediated by caspase 7, a protein that typically promotes cell death. Our findings demonstrate how STAT3, which is constitutively active in many human cancers, suppresses DDR, fundamental to tumorigenesis. This newly recognized role for STAT3 in attenuation of DDR, discovered in the context of EBV infection, is of broad interest as the biology of cell proliferation is central to both health and disease.
A central feature of cancer is suppression of the DNA damage response (DDR) to bypass cell-cycle checkpoints (1). Markers of DDR are prominent in precancerous lesions, but suppressed in cancer tissues (1, 2). Moreover, aberrant expression of oncogenes results in cell senescence or apoptosis, unless DDR is suppressed (3). However, the specific mechanisms that suppress DDR during development of oncogene-driven sporadic cancers are poorly understood. This is in contrast to hereditary forms of cancer whose development is generally aided by crippling mutations in genes central to the DDR (4). High-throughput sequencing of genomes from a large number of sporadic cancers failed to identify frequent driver mutations in DDR genes (5). Instead, a prominent finding was the presence of mutations in genes that activate cytokine- and growth factor-signaling pathways (6). We recognized that signaling through these pathways frequently activates STAT3, a well studied transcription activator.
STAT3 is a member of the signal transducer and activator of transcription (STAT) family. Ligation of various cytokine and growth factor receptors results in activation of STAT3, which primarily involves phosphorylation of a tyrosine residue (Y705) (7, 8). Phosphorylation can be mediated by receptor tyrosine kinases, such as the Janus-activated kinase (JAK) family kinases or less frequently by nonreceptor kinases such as Src (8, 9). STAT3 then activates transcription of a variety of genes; prominent among those are proproliferative and anti-apoptotic genes. STAT3 plays critical roles in embryogenesis and immunity. Deficiency of STAT3 results in death of mouse embryos by day 7 (10). Loss-of-function mutations in STAT3 in humans result in autosomal dominant hyper-IgE syndrome (AD-HIES or Job’s syndrome) (11). AD-HIES patients have a primary immunodeficiency disorder characterized by deficient TH17 cells, central memory T cells, and memory B cells (12–14). On the other hand, constitutive activation of STAT3, almost never associated with mutations in STAT3, is a feature of many human cancers (15). Such aberrant activation of STAT3 contributes to tumor initiation, progression, and metastasis (16, 17). Whether STAT3 can contribute to DDR attenuation during oncogene-driven cell proliferation has not been addressed. This question stemmed from the observations that sporadic cancers frequently exhibit mutations in growth factor- and cytokine-signaling genes, STAT3 is frequently activated in growth signaling pathways, and STAT3 is constitutively active in many human cancers. We used Epstein–Barr virus (EBV), classified by the WHO as a Group I carcinogen, to test the hypothesis that STAT3 attenuates DDR to facilitate oncogene-driven cell proliferation.
EBV infects most humans and persists for the life of the host in B lymphocytes. To establish such persistence, EBV-oncogenes must successfully drive cell proliferation, which can cause B-cell lymphoproliferative diseases or lymphomas particularly under conditions of immune suppression (18). Such EBV-oncogene driven lymphoproliferation can only be successful if cell-intrinsic barriers such as cell-cycle checkpoints imposed by DNA damage are overcome. We previously reported that an early event such as EBV-binding or internalization results in activation and increased expression of cellular STAT3 in primary B lymphocytes. STAT3 then critically contributes to cell proliferation and transformation by promoting cell survival and relaxing the intra-S phase cell-cycle checkpoint (19). We now find that STAT3 contributes to intra-S phase checkpoint relaxation by suppressing signaling downstream of the critical S phase kinase ATR despite detection of replication stress-associated DNA damage that results from EBV infection. Specifically, STAT3 interrupts ATR-to-Chk1 signaling by promoting loss of Claspin via caspase 7. These findings not only demonstrate how EBV exploits host STAT3 to interrupt DDR-signaling and drive cell proliferation past the S phase, but also reveal a function for STAT3 in regulation of DDR in response to replication stress.
Results
EBV Infection Leads to Replication Stress-Associated DNA Damage and Activation of ATR.
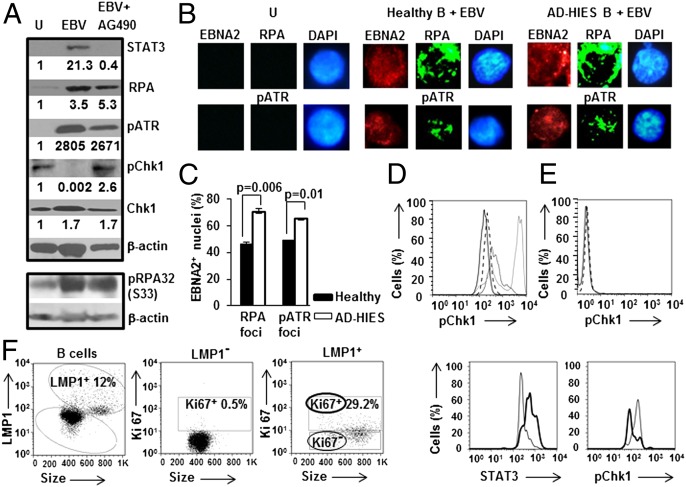
To address how EBV-mediated early activation and increase in STAT3 may contribute to relaxation of the intra-S phase checkpoint, we used three complementary approaches. First, AG490, a JAK inhibitor was included during EBV infection to impair STAT3 activation, and thereby secondarily suppress STAT3 mRNA and protein levels (19, 20). Second, to safeguard against potential off-target effects of AG490, primary B cells from patients with AD-HIES were infected with EBV. Patients with AD-HIES have a heterozygous dominant negative mutation in their STAT3 gene that renders the majority of cellular STAT3 nonfunctional despite normal levels of STAT3 protein (11). Third, in key experiments, we also used siRNA to STAT3 to control for potential STAT3-unrelated variations among patients. Because apoptosis and intra-S phase arrest of EBV-infected STAT3-deficient B cells (19) is consistent with EBV oncogene-driven replication stress (3, 21), we examined the effect of EBV infection on replication protein A (RPA) and ataxia telangiectasia and Rad3 related (ATR) proteins. Typically, RPA is recruited to single-stranded stretches of DNA in response to replication stress; this results in recruitment and activation of ATR (4). As shown in Fig. 1A, levels of RPA and phospho(p)ATR increased by day 4 after EBV infection, irrespective of STAT3 inhibition. Consistent with replication stress and detection of the associated DNA damage, we also observed RPA and pATR foci in 46–49% of EBV-infected [i.e., EB nuclear antigen (EBNA2)+] nuclei derived from healthy subjects (Fig. 1 B and C). Of AD-HIES-derived EBNA2+ nuclei, 65–70% stained for RPA and pATR foci (Fig. 1 B and C). This increase in infected, foci-positive AD-HIES nuclei relative to healthy subject-derived nuclei agreed with our earlier observation that EBV-infected AD-HIES-derived B cells accumulate in the S phase and likely do not undergo transformation (19). Of note, although an increase in RPA protein (Fig. 1A) was surprising, such increase following DNA damage has been observed by others (22). Furthermore, recent evidence (23) would suggest that increase in RPA levels may be a strategy, mediated by EBV, to stabilize replication forks during replication stress. To obtain additional confirmation of ATR activation besides its phosphorylation at S428 and recruitment to chromatin, we also examined phosphorylation of RPA32 at S33; this latter event has been used as a reliable marker for ATR activation (24). We found RPA32 phosphorylated at S33 following EBV infection, regardless of the presence of AG490 (Fig. 1A). Thus, EBV infection leads to replication stress and its detection as evidenced by recruitment of RPA to chromatin, and activation of ATR, whether or not STAT3 is functional.
Fig. 1.
EBV-infected cells demonstrate low levels of pChk1 despite ATR activation in response to replication stress-associated DNA damage. (A) Healthy subject-derived primary B lymphocytes exposed to EBV (with or without AG490) or uninfected (U) were subjected to immunoblotting after 4 d in culture using different antibodies. Numbers below blots indicate fold-change in quantity of protein compared with uninfected cells after normalization to β-actin; p: phospho. (B and C) Uninfected B cells (U) and healthy or AD-HIES B cells infected with EBV were harvested on day 4. Representative immunofluorescence images of nuclei stained with DAPI and for EBNA2 and costained for RPA or pATR are shown in B. Aggregate data from 100 EBNA2+ nuclei each from healthy and AD-HIES cells are shown in C; error bars: SEM. (D) B cells from healthy subjects and AD-HIES patients were uninfected or infected with EBV, harvested on day 4, evaluated by flow cytometry using antibodies to LMP1 and pChk1, and shown as histogram overlay of relative levels of pChk1 in uninfected healthy B cells (dashed line), LMP1+ healthy B cells (solid line), uninfected AD-HIES B cells (dotted line), and LMP1+ AD-HIES B cells (gray line). (E) Healthy subject-derived primary B cells were placed in culture in the presence (solid line) or absence (dashed line) of AG490, harvested on day 4, and evaluated by flow cytometry using anti-pChk1 antibody. (F) Peripheral B cells from a representative patient with infectious mononucleosis were stained with antibodies to LMP1, Ki67, STAT3, and pChk1. Ki67 is a cellular marker for proliferation. Gating strategy for LMP1and Ki67 expression is shown in the left hand panels. Right hand panels show histogram overlays of LMP1+Ki67+ proliferating cells (thick line) and LMP1+Ki67− nonproliferating cells (solid line) stained for STAT3 or pChk1. Positions of cells relative to each other on the X-axis within histograms indicate relative intracellular levels of STAT3 and pChk1. Data (except for panel F) are representative of at least 3 experiments.
EBV-Infected Cells with Functional STAT3 Demonstrate Low Levels of pChk1.
In response to replication stress, activated ATR phosphorylates the critical checkpoint kinase Chk1 which sets off a cascade of events culminating in activation of the intra-S phase checkpoint (4). As shown in Fig. 1A, an expected increase in phospho(p)Chk1 was observed when STAT3 function was impaired; in contrast, pChk1 was minimally detected when STAT3 was functional, despite unaffected levels of total Chk1. Similarly, when we used latent membrane protein (LMP) 1, a critical EBV oncoprotein, to mark infected cells, we found that cells derived from AD-HIES patients showed an increase in pChk1 compared with uninfected cells, whereas those derived from healthy subjects did not (Fig. 1D). Of note, treatment of uninfected cells with AG490 did not alter the level of pChk1 (Fig. 1E). To determine whether high levels of STAT3 but low levels of pChk1 also characterize EBV-infected proliferating B cells in vivo, we examined patients with primary EBV infection (infectious mononucleosis). Substantial numbers of EBV-infected B cells can be detected in the blood of such patients if they are identified very early after infection (25). We found 29.2% of LMP1+ peripheral B cells to be Ki67+ proliferating cells. These cells expressed higher levels of STAT3 but lower levels of pChk1 compared with LMP1+ Ki67− nonproliferating cells (Fig. 1F). Taken together, these results demonstrate that EBV-infected cells with functional STAT3, in which the intra-S phase checkpoint is relaxed (19), have low levels of pChk1. Furthermore, proliferating EBV-infected B cells in blood have high levels of STAT3 but low levels of pChk1.
STAT3 Suppresses pChk1 to Relax the Intra-S Phase Checkpoint.
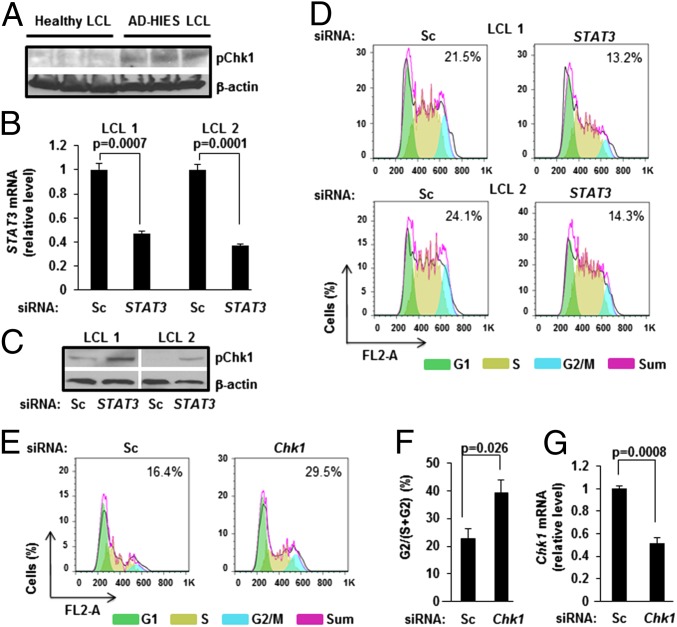
To further understand the relationship between STAT3 and pChk1, we examined EBV- transformed lymphoblastoid cell lines (LCL) derived from B cells of healthy subjects compared with those derived from AD-HIES patients. Consistent with observations in Fig. 1, AD-HIES LCL demonstrated higher levels of pChk1 compared with healthy LCL (Fig. 2A). When healthy LCL were transfected with siRNA to STAT3, we observed an increase in levels of pChk1 compared with cells transfected with scrambled siRNA (Fig. 2C). Moreover, when transfected with siRNA to STAT3, ∼40% fewer cells containing >2N DNA (S+G2) were in G2/M phase of the cell cycle, compared with scrambled siRNA-transfected cells (Fig. 2D). As expected, siRNA to STAT3 suppressed STAT3 mRNA levels (Fig. 2B). Thus, suppression of STAT3 causes increase in pChk1 and accumulation of cells in the S phase.
Fig. 2.
STAT3 suppresses pChk1 to promote progression of EBV-infected B cells past the S phase of the cell cycle. (A) Immunoblot comparing levels of pChk1 between three healthy subject-derived and three AD-HIES patient-derived EBV-lymphoblastoid cell lines (LCL). (B and C) Two healthy subject-derived LCL were transfected with siRNA to STAT3 or scrambled siRNA (Sc). Cells were harvested 36 h later and tested for STAT3 mRNA levels by qRT-PCR (B) and pChk1 levels by immunoblotting (C); error bars: SEM. (D) Two healthy subject-derived LCL were transfected with siRNA to STAT3 or scrambled siRNA in combination with FITC-conjugated scrambled siRNA to mark transfected cells. Cells were harvested 36 h later, and cell-cycle analysis was performed on live FITC-positive cells using flow cytometry. Numbers within boxes indicate percent G2/(S+G2) cells. (E and F) EBV-LCL from four AD-HIES patients were transfected with scrambled siRNA or siRNA to Chk1 in combination with FITC-conjugated scrambled siRNA to mark transfected cells. Cells were harvested 36 h later, and live FITC-positive cells were subjected to cell-cycle analysis by flow cytometry. Representative data with the percent G2/(S+G2) cells are shown in E, and aggregate data from four cell lines are shown in F. (G) EBV-LCL from four AD-HIES patients were transfected with siRNA to Chk1 or scrambled siRNA (Sc) and tested for Chk1 mRNA levels by qRT-PCR after 36 h; error bars: SEM. Transfection experiments were performed twice.
We reasoned that if STAT3 interferes with Chk1 function to relax the intra-S phase checkpoint, then experimental depletion of Chk1 in STAT3-deficient cells should allow more cells to progress from S to G2/M phase of the cell-cycle. When transfected with siRNA to Chk1, 1.7-fold more cells containing >2N DNA were in the G2/M phase, compared with scrambled siRNA-transfected cells (Fig. 2 E and F). As expected, siRNA to Chk1 suppressed Chk1 transcript levels (Fig. 2G). Thus, STAT3 impairs checkpoint-related functions of Chk1 to relax the intra-S phase checkpoint during EBV-driven cell proliferation. In total, these findings support a role for STAT3 in DDR-suppression resulting in bypass of intra-S phase checkpoint.
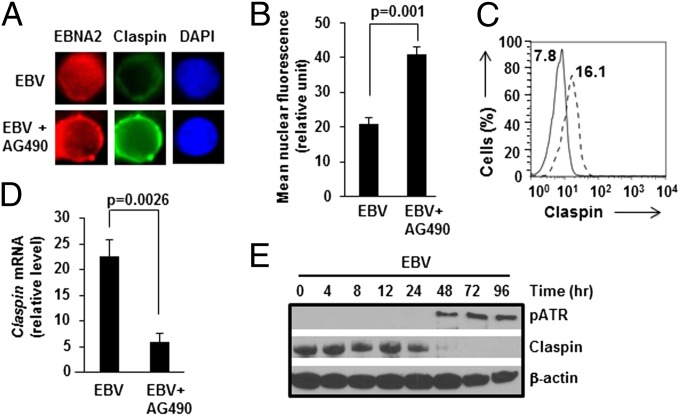
Cells with Functional STAT3 Demonstrate Early Loss of Claspin.
Suppression of pChk1 may involve regulation of total Chk1 levels or phosphoprotein levels. Similar Chk1 levels irrespective of STAT3 function (Fig. 1A) argued against STAT3-mediated regulation of Chk1 protein levels. With respect to the second possibility, the contribution of Claspin to ATR-mediated phosphorylation of Chk1 is well documented (26), prompting us to examine Claspin. We found Claspin levels in EBNA2+ nuclei (Fig. 3 A and B) and LMP1+ cells (Fig. 3C) to be lower when cells were infected with EBV in the absence of AG490 compared with when AG490 was added. Lower levels of Claspin were not explained by suppression of Claspin transcription, as Claspin mRNA was more abundant in EBV-infected cells that were untreated compared with AG490-treated cells (Fig. 3D). However, we observed a substantial loss of Claspin protein beyond 24 h following EBV infection (Fig. 3E). In addition to its checkpoint-related function, a prominent function of Claspin is to coordinate the functions of the helicase and polymerase complexes on DNA and ensure stability of the replisome (27). Therefore, although loss of Claspin in the setting of cell proliferation in our experiments was surprising, this observation was nevertheless consistent with those in metazoan cells in which depletion of Claspin did not impair DNA replication under conditions in which a large number of origins of replication were available (27–29); such conditions included those in which there was loss of checkpoint activation despite oncogene-driven replication stress (30), as we see here following EBV infection. Taken together, our findings suggest that STAT3 interferes with intra-S phase DDR-signaling via Claspin loss ahead of or coincident with expression of EBV oncoproteins by 24 h (19) and detection of DNA damage (Fig. 3E: appearance of pATR) by 48 h postinfection.
Fig. 3.
Cells with functional STAT3 demonstrate loss of Claspin after EBV infection. (A and B) Healthy subject-derived primary B cells were infected with EBV+/−AG490, harvested on day 4, and stained with DAPI and costained for EBNA2 and Claspin followed by imaging. Mean fluorescence intensities of Claspin in EBNA2+ nuclei were calculated; representative nuclei (A) and aggregate data from 30 nuclei each from EBV and EBV+AG490 cells (B) are shown; error bars: SEM. (C) Cells were infected and harvested as in A and B, and evaluated by flow cytometry using antibodies to LMP1 and Claspin. Histogram overlay of relative levels of Claspin in LMP1+ cells in the presence (dashed line) or absence (solid line) of AG490 is shown; numbers within the box indicate mean fluorescence intensities of histograms. (D) B cells infected with EBV in the presence or absence of AG490 were harvested on day 4 and examined for Claspin mRNA levels by qRT-PCR; error bars: SEM. (E) Extracts from cells harvested at indicated intervals after exposure of primary B cells to EBV were immunoblotted with antibodies to pATR and Claspin. Data are representative of three experiments.
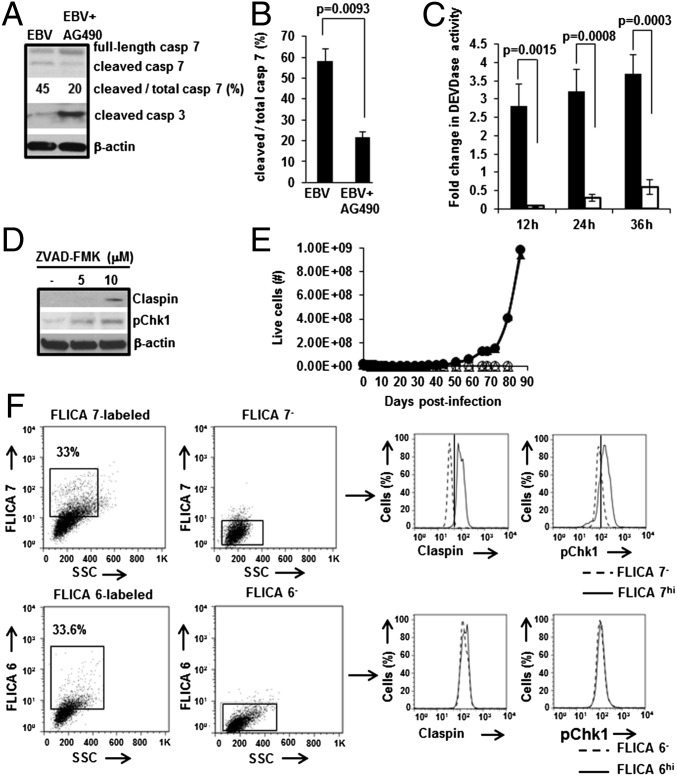
Caspase Function Is Necessary for Outgrowth of EBV-LCL, with Caspase 7 Mediating Loss of Claspin in EBV-Infected Cells.
Because caspase 7, an effector caspase, is known to degrade Claspin during apoptosis (31), we compared the ratio of cleaved to total caspase 7 between STAT3-intact (i.e., without AG490) and STAT3-impaired (i.e., with AG490) conditions. We found this ratio to be 2.7-fold higher in the absence of AG490 compared with when AG490 was present (Fig. 4 A and B). We also examined caspase 7 activity in vitro at different times after EBV-infection and observed a 2.8-fold increase in caspase 7 activity by 12 h postinfection only in the absence of AG490 (Fig. 4C). In contrast, the cleaved/active form of caspase 3, another effector caspase and a central mediator of apoptosis, was detected predominantly in the presence of AG490 (Fig. 4A). This observation is consistent with our earlier findings of apoptosis and suppression of Bcl-xL and Bcl-2 transcripts in cells infected in the presence of AG490 (19). To determine the contribution of caspase(s) to EBV-driven cell proliferation, we investigated the effects of ZVAD-FMK, a pan-caspase inhibitor, on levels of Claspin and pChk1 as well as outgrowth of LCL. We observed recovery of Claspin and pChk1 in the presence of ZVAD-FMK (Fig. 4D), linking caspase(s) to loss of Claspin and suppression of pChk1 levels during EBV infection. Furthermore, LCL failed to grow out in the presence of ZVAD-FMK (Fig. 4E), demonstrating the necessity of caspase(s) for EBV-driven cell proliferation.
Fig. 4.
Caspase 7 causes loss of Claspin in EBV-infected B cells. (A and B) Healthy subject-derived primary B cells were infected with EBV+/−AG490, harvested on day 4, and subjected to immunoblotting using antibodies to caspase 7 or cleaved caspase 3. The fraction of cleaved caspase 7 is shown as a percentage of total caspase 7. A representative immunoblot is shown in A, and densitometric quantitation of the fraction of cleaved to total caspase 7 from three experiments is shown in B; error bars, SEM. (C) Primary B cells were infected with EBV, placed in culture, harvested at indicated intervals of time, and extracts were assayed for DEVDase activity. Fold change in activity over uninfected cells was calculated for cells cultured without AG490 (solid bars) or with AG490 (open bars); error bars: SEM. (D) Primary B cells were infected with EBV in the presence of solvent control or ZVAD-FMK (5, 10 μM), harvested on day 4, and subjected to immunoblotting using anti-Claspin and anti-pChk1 antibodies. A representative immunoblot is shown. (E) Representative growth curves by Trypan blue staining of live cells harvested at periodic intervals following infection of primary B cells from two healthy subjects (+ ZVAD-FMK: open symbols; − ZVAD-FMK: closed symbols) are shown. (F) Healthy subject-derived LCL were treated with FLICA7 (Upper), FLICA6 (Lower), or mock-treated (FLICA7−, FLICA6−) and examined 6 h later for Claspin and pChk1 levels by flow cytometry. Histogram overlays on right compare levels of Claspin and pChk1 in FLICA7hi versus FLICA7− cells, and in FLICA6hi cells versus FLICA6− cells. Experiments were performed at least three times.
To test the contribution of caspase 7 toward loss of Claspin and suppression of pChk1, we used a Fluorescence Labeled Inhibitor of Caspase (FLICA) 7. When FLICA7 was added to LCL to simultaneously bind and inactivate active caspase 7, we observed increases in both Claspin and pChk1 levels (Fig. 4F). In contrast, FLICA6, specific for the third effector caspase, caspase 6, had no effect on Claspin and pChk1 levels. Although FLICA7 also inhibits active caspase 3, detection of very low levels of cleaved caspase 3 in cells with functional STAT3 (Fig. 4A) makes caspase 3 an unlikely target of FLICA7 in EBV-infected cells. In total, these results support a model in which EBV uses cellular STAT3 to promote caspase 7-mediated loss of Claspin, thereby suppressing DDR-signaling to facilitate EBV-oncogene driven cell proliferation (Fig. 5).
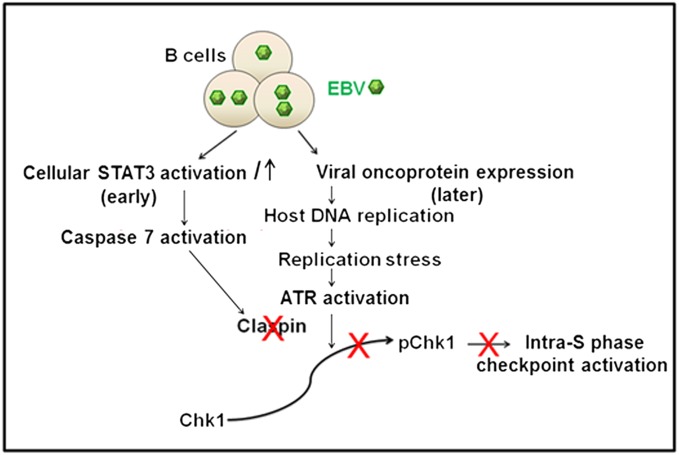
Fig. 5.
Model for STAT3-mediated attenuation of intra-S phase DDR signaling. EBV infection causes early activation and increase in STAT3, which, via caspase 7, causes loss of Claspin. As a result, Claspin is not available to assist ATR in phosphorylating Chk1 despite detection of DNA damage resulting from EBV-oncogene-induced replication stress. Because Chk1 is not phosphorylated, the intra-S phase checkpoint is not activated. As a result, cells advance beyond the S phase.
Discussion
Activation or overexpression of STAT3 is linked to many human cancers including EBV-related cancers (15, 32). Although the contribution of STAT3 to tumorigenesis has generally been attributed to its ability to transcriptionally activate prosurvival and proproliferative genes such as c-Myc and cyclins (33, 34), experiments presented here reveal another mechanism by which STAT3 exerts a proproliferative influence. Specifically, the findings reveal a mechanism that links STAT3 and regulation of DDR, a process fundamental to cell proliferation, and its suppression, a trait of all cancer (1). Indeed, accumulation of DNA damage is characteristic not only of hereditary cancers that have mutations in DDR genes (5) but of sporadic cancers where the mechanism of DDR-suppression is often unknown (4). Our findings also reveal that mutations in DDR genes (5) are not the only cause of suppression of DDR but that aberrant overexpression or activation of transcriptional regulatory proteins like STAT3 can influence molecular pathways to suppress DDR, thereby evading cell-cycle arrest by checkpoints. Furthermore, this STAT3-mediated mechanism of DDR-suppression provides mechanistic support for the oncogene-induced DNA replication stress model for cancer development (1) because many growth signaling pathways that are mutated in sporadic cancers (5) converge on STAT3.
DDR is fundamental to normal cell proliferation and STAT3 is known to regulate growth and differentiation of multiple cell lineages (35). This raises the possibility that STAT3 may assist in recovery from cell-cycle checkpoints by interrupting DDR-signaling during normal cell proliferation. Indeed proteasomal degradation of Claspin has been shown to be important for cells to enter mitosis (36) and we demonstrate that STAT3 mediates loss of Claspin to interrupt DDR-signaling. This supports the notion that STAT3, via Claspin loss, may also influence checkpoint recovery during normal cell proliferation with implications beyond cancer. Using primary human B cells and patients with a rare genetic disorder harboring STAT3 mutations adds biological relevance. For instance, recognition of the STAT3-mediated mechanism of DDR-suppression may help to better understand the basis for some of the immunologic deficits observed in AD-HIES patients, particularly those related to immunologic memory (12, 14).
Because STAT3 can transcriptionally activate thousands of genes (25), there may be differences in the manner in which STAT3 regulates the DDR in different experimental systems and in response to different types of DNA damage. For instance, an earlier study examined the role of STAT3 in activating the DDR in response to DNA strand breaks in already proliferating immortalized mouse embryonic fibroblasts (37). That study found that STAT3 was necessary for phosphorylation of ATM and ATR and their respective downstream targets Chk2 and Chk1, and therefore activation of the DDR; the effect on ATM activation was likely mediated by STAT3-driven transcription of MDC1. Our study addresses a fundamentally different question: Does STAT3 suppress the DDR to facilitate oncogene-driven cell proliferation during the initial stages of transformation of primary human cells? Contrary to the findings of STAT3-mediated increased pChk1 in an already immortalized murine cell line (37), our study demonstrates that STAT3 is necessary for suppressing phosphorylation of Chk1 via activation of caspase 7.
Although conventional thinking suggests that caspase-mediated apoptosis prevents cancer, our findings implicate caspases in a nonapoptotic role, i.e., cell proliferation. Indeed, in recent years, caspases have been implicated in nonapoptotic functions contributing to cell proliferation, migration, differentiation, and immunity (38). We now propose a mechanism which involves caspase 7-mediated loss of Claspin. The mechanism by which STAT3 activates caspase 7 in EBV-infected cells remains to be determined. Such cells, as we have demonstrated earlier, are almost uniformly nonapoptotic (19). Although we were able to detect caspase 7 function in vitro by 12 h, Claspin loss was observed only after 24 h post-EBV infection. This temporal lag may reflect issues of intracellular accessibility of Claspin to caspase 7 or the presence of a DEYD cleavage site in Claspin that deviates from the ideal caspase 7 cleavage site DEVD (31).
Using EBV as a tool to uncover DDR suppression by STAT3 provides insights into the biology underlying persistence of EBV in human B cells. Because ATR is critical to maintenance of genome integrity (39), interruption of its function by STAT3 also provides a likely explanation for the substantial genomic aberrations that have been observed early following EBV-mediated B-cell transformation (40). During EBV oncogene-driven cell proliferation, viral proteins EBNA3C and EBNA-LP intersect with DDR-signaling at several points to suppress it (41, 42). Compared with such EBV proteins, suppression of DDR-signaling by STAT3 begins earlier: STAT3-mediated molecular events that suppress DDR-signaling are set in motion before the oncoprotein EBNA2 and therefore before EBNA3C, EBNA-LP, or LMP1 are expressed. However, this does not exclude the possibility that at later times, these and other viral proteins may contribute toward or modulate STAT3-mediated DDR-suppression. Indeed, STAT3 can be transcriptionally induced by LMP1 in epithelial cells (43). Finally, our findings raise the possibility that similar host mechanisms may be exploited by other tumor viruses to promote virus persistence with the collateral effect of causing cancer.
With regard to anticancer therapeutics, STAT3 and Chk1 have independently been recognized as targets of anticancer drug development (4, 15). Our results provide a mechanistic link between the two, further lending support to these approaches. Chk1 inhibitors, several of which have been approved for clinical trials, aim to boost sensitization of cancer cells to genotoxic stress by imposing deficits in DNA replication and repair in addition to DDR signaling. Although this approach is very promising, partial suppression of Chk1, which may result in suppression of pChk1 levels as is caused by EBV, may instead promote inappropriate relaxation of cell-cycle checkpoints (4). On the other hand, cancers with constitutively active STAT3 and DDR suppression may already be primed for genotoxic therapy. Recognition of the STAT3-mediated mechanism of DDR-suppression now opens an area of investigation into therapeutic options for cancers with aberrantly active STAT3.
Materials and Methods
Study Subjects.
Blood was obtained from study subjects following informed consent. The study of human subjects was approved by the Institutional Review Boards at Stony Brook University, the National Institute of Allergy and Infectious Diseases, and the Garvan Institute of Medical Research. Healthy EBV-seronegative volunteers ranged from 18 to 28 y of age. Three infectious mononucleosis (IM) patients (ages 8, 14, and 14 y) had presented with 5–7 d of symptoms that included low grade fever, sore throat, malaise, and headache. Serologies of IM patients were consistent with primary EBV infection (presence of IgM and IgG to VCA but absence of IgG to EBNA). Peripheral blood B cells were obtained and EBV-LCL were derived from a total of six AD-HIES patients. Three of these had a mutation in the SH2 domain [J074 and J100 studied in Koganti et al. (19); patient 7 described in Avery et al. (12)] and three in the DNA-binding domain [patient 4 described in Ma et al. (13) and patients 6 and 8 described in Avery et al. (12)].
Isolation of Primary B Lymphocytes and Infection with EBV.
Peripheral blood B cells were isolated by negative selection and infections with EBV were performed as described (19).
Culture Conditions.
Newly infected B cells and previously established EBV-LCL were grown in culture using conditions described (19). For experiments using AG490 (25 μM) or ZVAD-FMK (5, 10 μM), chemicals were added at time 0 to cultures. For experiments examining long-term outgrowth, chemicals were supplemented at the initial concentration every fourth day. We had experimentally determined 25 μM AG490 to be minimally toxic to EBV-infected B-cell lines.
Antibodies.
The following primary antibodies were used for immunologic applications: mouse anti-human RPA, mouse anti-human phospho-RPA32 (S33), goat anti-human Claspin, rabbit anti-human STAT3, mouse anti-human β-actin, mouse anti-LMP1, rabbit anti-human pATR (S428), rabbit anti-human pChk1 (S345), rabbit anti-human Caspase 7, rabbit anti-human cleaved Caspase 3, mouse anti-human total Chk1, PE-conjugated anti-human Ki67, and rat anti-EBNA2 (clone R3) (44). Secondary antibodies included HRP-anti-mouse Ab, HRP-anti-rabbit Ab, HRP-anti-goat Ab, HRP-anti-rat Ab, PE-anti-mouse IgG1, PE-anti-mouse IgG, PE-anti-rat IgG, FITC-anti-mouse IgG, FITC-anti-rabbit IgG, Alexa 647-anti-rabbit IgG, biotin-anti-goat IgG detected by HRP-Avidin, FITC-Avidin, or PECy7-Avidin. Isotype-matched control antibodies including PE-mouse IgG1, mouse IgG1, mouse IgG2b, rat IgG2a, normal rabbit sera, and goat sera were used as negative controls for FACS staining.
Flow Cytometry.
Cells were stained with antibodies as described (25), data were acquired using a FACS Calibur, and analyzed using FlowJo software. For assessment of cell-cycle distribution, cells were stained with 50 μg/mL propidium iodide supplemented with 1 μg/mL RNase A.
Immunofluorescence Microscopy.
Cells were stained as for flow cytometry, washed, cytospun onto glass slides, air dried, and mounted with DAPI Prolong Gold Anti-fade (Life Technologies). Images were acquired at 40× magnification on an AxioScope A1 microscope (Zeiss) with SPOT v4.0 software. Quantitation of nuclear Claspin was performed using Axiovision software (4.8.2). Intensity of FITC fluorescence was calculated for each EBNA2+ nucleus and average values for 30 nuclei were plotted. When counting cells with nuclear foci, images were blinded and counted by two individuals; only nuclei with ≥5 foci were considered positive.
Immunoblotting.
Total extracts from 1 × 106 per mL cells were analyzed by immunoblotting as described (25).
Transfections.
EBV-LCL were transfected with 100 μM siRNA [targeting STAT3 (sc-29493), Chk1 (sc-29269), scrambled (sc-37007), or FITC-scrambled (sc-36869); Santa Cruz Biotechnology] as described (25). In experiments requiring cell-cycle analyses, combinations of siRNA (targeted or scrambled) and FITC-scrambled siRNA were transfected at 3:1 ratio to identify transfected cells by flow cytometry.
Caspase-Related Assays.
DEVDase activity in extracts of uninfected and EBV-infected (with or without AG490) cells was measured following immunoprecipitation of caspase 7 using the CaspSELECT caspase 7 immunoassay kit (MBL International) using manufacturer’s instructions. Caspase 7- and 6-specific inhibitors FAM FLICA caspase 7 and the Green FLICA caspase 6 (Immunochemistry Technologies) were used according to manufacturer’s instructions.
Quantitative RT-PCR.
RNA was isolated and relative transcript levels were determined using the ΔΔCt method with gene-specific primers (25). Individual samples were assayed in triplicate. Sequences of primers for 18S rRNA and STAT3 are as described (25). Sequences for Claspin and Chk1 primers are as follows: Claspin (Forward: 5′TTAGCATGCTTCCAGAAACG3′; Reverse: 5′TCCATTAAACCACGGCTAGG3′), Chk1 (Forward: 5′ TGGGCTATCAATGGAAGAAAA3′; Reverse: 5′ TCATCCATTTCTAACAAATTCACTT3′).
Statistical Analyses.
P values were calculated by comparing the means of two groups of interest using unpaired Student t test.
Acknowledgments
We are grateful to Dr. Patrick Hearing for his valuable comments. We thank Dr. Steven Holland at the National Institute for Allergy and Infectious Diseases for providing access to AD-HIES patient samples, and AD-HIES patients as well as healthy blood donors at the National Institutes of Health Primary Immune Deficiency Clinic for their participation. This study was supported by funds from the Research Foundation for the State University of New York (to S.B.-M.), by a Resident Research Grant from the American Academy of Pediatrics (to J.H.-Y.), and from the National Health and Medical Research Council of Australia and New South Wales Cancer Council (to S.G.T.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Halazonetis TD, Gorgoulis VG, Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319(5868):1352–1355. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- 2.Bartkova J, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434(7035):864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 3.Bartkova J, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444(7119):633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 4.Smith J, Tho LM, Xu N, Gillespie DA. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res. 2010;108:73–112. doi: 10.1016/B978-0-12-380888-2.00003-0. [DOI] [PubMed] [Google Scholar]
- 5.Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability—an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11(3):220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 6.Greenman C, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446(7132):153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raz R, Durbin JE, Levy DE. Acute phase response factor and additional members of the interferon-stimulated gene factor 3 family integrate diverse signals from cytokines, interferons, and growth factors. J Biol Chem. 1994;269(39):24391–24395. [PubMed] [Google Scholar]
- 8.Schindler C, Darnell JE., Jr Transcriptional responses to polypeptide ligands: The JAK-STAT pathway. Annu Rev Biochem. 1995;64:621–651. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 9.Silva CM. Role of STATs as downstream signal transducers in Src family kinase-mediated tumorigenesis. Oncogene. 2004;23(48):8017–8023. doi: 10.1038/sj.onc.1208159. [DOI] [PubMed] [Google Scholar]
- 10.Takeda K, et al. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA. 1997;94(8):3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland SM, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357(16):1608–1619. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 12.Avery DT, et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med. 2010;207(1):155–171. doi: 10.1084/jem.20091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma CS, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205(7):1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegel AM, et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;35(5):806–818. doi: 10.1016/j.immuni.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu H, Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev Cancer. 2004;4(2):97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 16.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8(4):945–954. [PubMed] [Google Scholar]
- 17.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat Rev Cancer. 2009;9(11):798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350(13):1328–1337. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- 19.Koganti S, de la Paz A, Freeman AF, Bhaduri-McIntosh S. B lymphocytes from patients with hypomorphic mutation in STAT3 resist Epstein-Barr virus-driven cell proliferation. J Virol. 2013;88(1):516–524. doi: 10.1128/JVI.02601-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J, et al. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res. 2005;65(3):939–947. [PubMed] [Google Scholar]
- 21.Di Micco R, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444(7119):638–642. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
- 22.Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300(5625):1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 23.Toledo LI, et al. ATR prohibits replication catastrophe by preventing global exhaustion of RPA. Cell. 2013;155(5):1088–1103. doi: 10.1016/j.cell.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 24.Anantha RW, Vassin VM, Borowiec JA. Sequential and synergistic modification of human RPA stimulates chromosomal DNA repair. J Biol Chem. 2007;282(49):35910–35923. doi: 10.1074/jbc.M704645200. [DOI] [PubMed] [Google Scholar]
- 25.Hill ER, et al. Signal transducer and activator of transcription 3 limits Epstein-Barr virus lytic activation in B lymphocytes. J Virol. 2013;87(21):11438–11446. doi: 10.1128/JVI.01762-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeong SY, Kumagai A, Lee J, Dunphy WG. Phosphorylated claspin interacts with a phosphate-binding site in the kinase domain of Chk1 during ATR-mediated activation. J Biol Chem. 2003;278(47):46782–46788. doi: 10.1074/jbc.M304551200. [DOI] [PubMed] [Google Scholar]
- 27.Errico A, Costanzo V. Differences in the DNA replication of unicellular eukaryotes and metazoans: Known unknowns. EMBO Rep. 2010;11(4):270–278. doi: 10.1038/embor.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Errico A, et al. Tipin/Tim1/And1 protein complex promotes Pol alpha chromatin binding and sister chromatid cohesion. EMBO J. 2009;28(23):3681–3692. doi: 10.1038/emboj.2009.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Errico A, Costanzo V, Hunt T. Tipin is required for stalled replication forks to resume DNA replication after removal of aphidicolin in Xenopus egg extracts. Proc Natl Acad Sci USA. 2007;104(38):14929–14934. doi: 10.1073/pnas.0706347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodward AM, et al. Excess Mcm2-7 license dormant origins of replication that can be used under conditions of replicative stress. J Cell Biol. 2006;173(5):673–683. doi: 10.1083/jcb.200602108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke CA, Bennett LN, Clarke PR. Cleavage of claspin by caspase-7 during apoptosis inhibits the Chk1 pathway. J Biol Chem. 2005;280(42):35337–35345. doi: 10.1074/jbc.M506460200. [DOI] [PubMed] [Google Scholar]
- 32.Nepomuceno RR, Snow AL, Robert Beatty P, Krams SM, Martinez OM. Constitutive activation of Jak/STAT proteins in Epstein-Barr virus-infected B-cell lines from patients with posttransplant lymphoproliferative disorder. Transplantation. 2002;74(3):396–402. doi: 10.1097/00007890-200208150-00017. [DOI] [PubMed] [Google Scholar]
- 33.Shields BJ, Hauser C, Bukczynska PE, Court NW, Tiganis T. DNA replication stalling attenuates tyrosine kinase signaling to suppress S phase progression. Cancer Cell. 2008;14(2):166–179. doi: 10.1016/j.ccr.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: Role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7(1):41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 35.Tangye SG, Cook MC, Fulcher DA. Insights into the role of STAT3 in human lymphocyte differentiation as revealed by the hyper-IgE syndrome. J Immunol. 2009;182(1):21–28. doi: 10.4049/jimmunol.182.1.21. [DOI] [PubMed] [Google Scholar]
- 36.Peschiaroli A, et al. SCFbetaTrCP-mediated degradation of Claspin regulates recovery from the DNA replication checkpoint response. Mol Cell. 2006;23(3):319–329. doi: 10.1016/j.molcel.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Barry SP, et al. STAT3 modulates the DNA damage response pathway. Int J Exp Pathol. 2010;91(6):506–514. doi: 10.1111/j.1365-2613.2010.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miura M. Apoptotic and nonapoptotic caspase functions in animal development. Cold Spring Harb Perspect Biol. 2012;4:a008664. doi: 10.1101/cshperspect.a008664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cimprich KA, Cortez D. ATR: An essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9(8):616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lacoste S, et al. Chromosomal rearrangements after ex vivo Epstein-Barr virus (EBV) infection of human B cells. Oncogene. 2010;29(4):503–515. doi: 10.1038/onc.2009.359. [DOI] [PubMed] [Google Scholar]
- 41.Nikitin PA, et al. An ATM/Chk2-mediated DNA damage-responsive signaling pathway suppresses Epstein-Barr virus transformation of primary human B cells. Cell Host Microbe. 2010;8(6):510–522. doi: 10.1016/j.chom.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saha A, Kaul R, Murakami M, Robertson ES. Tumor viruses and cancer biology: Modulating signaling pathways for therapeutic intervention. Cancer Biol Ther. 2010;10(10):961–978. doi: 10.4161/cbt.10.10.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kung CP, Meckes DG, Jr, Raab-Traub N. Epstein-Barr virus LMP1 activates EGFR, STAT3, and ERK through effects on PKCdelta. J Virol. 2011;85(9):4399–4408. doi: 10.1128/JVI.01703-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kremmer E, et al. Rat monoclonal antibodies differentiating between the Epstein-Barr virus nuclear antigens 2A (EBNA2A) and 2B (EBNA2B) Virology. 1995;208(1):336–342. doi: 10.1006/viro.1995.1157. [DOI] [PubMed] [Google Scholar]