Significance
Here, we show how, mechanistically, inflammation-recruited macrophages may stimulate beta-cell proliferation in the pancreas, and specifically identify that TGFβ1 and EGF, which are secreted by M2 macrophages, induce SMAD7 expression in beta cells. SMAD7 not only activates cell cycle activators but also induces the nuclear exclusion of cell cycle inhibitors to promote beta-cell replication. Our study thus reveals a molecular pathway to induce beta-cell proliferation through enhanced SMAD7 activity specifically in beta cells.
Keywords: TGFβ superfamily signaling pathway, epidermal growth factor receptor signaling pathway
Abstract
Determination of signaling pathways that regulate beta-cell replication is critical for beta-cell therapy. Here, we show that blocking pancreatic macrophage infiltration after pancreatic duct ligation (PDL) completely inhibits beta-cell proliferation. The TGFβ superfamily signaling inhibitor SMAD7 was significantly up-regulated in beta cells after PDL. Beta cells failed to proliferate in response to PDL in beta-cell–specific SMAD7 mutant mice. Forced expression of SMAD7 in beta cells by itself was sufficient to promote beta-cell proliferation in vivo. M2, rather than M1 macrophages, seem to be the inducers of SMAD7-mediated beta-cell proliferation. M2 macrophages not only release TGFβ1 to directly induce up-regulation of SMAD7 in beta cells but also release EGF to activate EGF receptor signaling that inhibits TGFβ1-activated SMAD2 nuclear translocation, resulting in TGFβ signaling inhibition. SMAD7 promotes beta-cell proliferation by increasing CyclinD1 and CyclinD2, and by inducing nuclear exclusion of p27. Our study thus reveals a molecular pathway to potentially increase beta-cell mass through enhanced SMAD7 activity induced by extracellular stimuli.
Because exogenous insulin administration does not adequately replace the functional deficit of pancreatic beta cells in diabetes, the cure for diabetes would ideally entail either replacement or regeneration of insulin-producing beta cells (1, 2). In rodents, accumulating data suggest that beta-cell replication, rather than differentiation from progenitor cells, is the main contributor to any beta-cell mass increase, both in normal physiologic situations and under particular pathological conditions (3–7). However, the factors that lead to this enhanced beta-cell mass in these settings have not been well delineated (8).
Transforming growth factor β (TGFβ) superfamily signaling pathways are essential for proper pancreas development (9–12). Moreover, modulation of TGFβ superfamily signaling pathways can affect proper islet development, function, and beta-cell replication (13–16). TGFβ superfamily signaling entails phosphorylation of intracellular R-SMAD proteins, such as SMAD2 and SMAD3, which form heteromeric complexes with SMAD4. The activated SMAD complexes translocate to the nucleus, where they regulate the transcription of target genes (17). SMAD7 is a general antagonist against all superfamily signaling. SMAD7 can be induced at the transcriptional level (18), predominantly after binding of superfamily ligands to a receptor (17, 19). SMAD7 can block R-SMAD phosphorylation (20), degrade type I receptors (21), and even exert an inhibitory effect in the nucleus (22). Moreover, SMAD7 expression can be induced by nonsuperfamily signals such as IFNγ/STAT (17, 23–26) or TNFα/NF-κB (27). SMAD2 nuclear translocation can similarly be affected by other nonsuperfamily pathways (26). This cross talk between the TGFβ superfamily and other signaling pathways, mediated by SMAD2 and SMAD7, adds another layer of complexity and may explain why regulation of superfamily signaling differs between cell types and among different physiologic conditions (17, 23–27). SMAD7 appears to play an important role during pancreas development and function (12, 14) and in some disease processes (28). Nevertheless, its possible involvement in adult beta-cell replication has only recently been reported (16) and not yet well characterized.
We have previously compared two experimental models of beta-cell proliferation, partial pancreatectomy (PPX) and partial duct ligation (PDL) (7, 15, 16). Beta-cell proliferation after PPX is robust, presumably resulting from an increased workload demand on the residual beta cells after surgical removal of a significant amount of the functional beta-cell mass. However, inflammation may be the major trigger for beta-cell proliferation after PDL, because beta cells should not seemingly be directly affected by ligation of the major pancreatic duct (15). In line with this view, tissue injury in the PDL pancreas is accompanied by a substantial infiltration of inflammatory cells, which here we hypothesized may potentially promote beta-cell proliferation via secretion of growth factors. Indeed, inflammatory cells, and macrophages in particular (marked by F4/80 expression), can regulate reparative processes (29). Besides the classically activated macrophages (also called M1 macrophages), which respond to inflammatory stimuli and appear early on, there are also the alternatively activated macrophages (M2 macrophages), which appear later to mediate humoral immunity and tissue repair (30–32). M2 macrophages are known to secrete a wide range of chemokines, enzymes, and growth factors to promote neovascularization, fibrosis, and tissue repair (30–33). However, their effects on pancreatic beta-cell proliferation have not been investigated.
Here, we found that blocking pancreatic macrophage infiltration after PDL completely inhibited PDL-triggered beta-cell proliferation. SMAD7 was significantly up-regulated in beta cells after PDL. With a series of in vivo and in vitro gain-of-function and loss-of-function experiments, we show that SMAD7 is not only necessary but also sufficient to mediate M2-macrophage–triggered beta-cell proliferation. M2 macrophages release high levels of TGFβ1 and epidermal growth factor (EGF), leading to up-regulation of SMAD7 in beta cells. Further effects of TGFβ1 and EGF on SMAD2 and other possible SMAD7-related intracellular factors seem to lead to beta-cell proliferation (18).
Results
PDL Is an Inflammation Model with an Increase in Beta-Cell Proliferation.
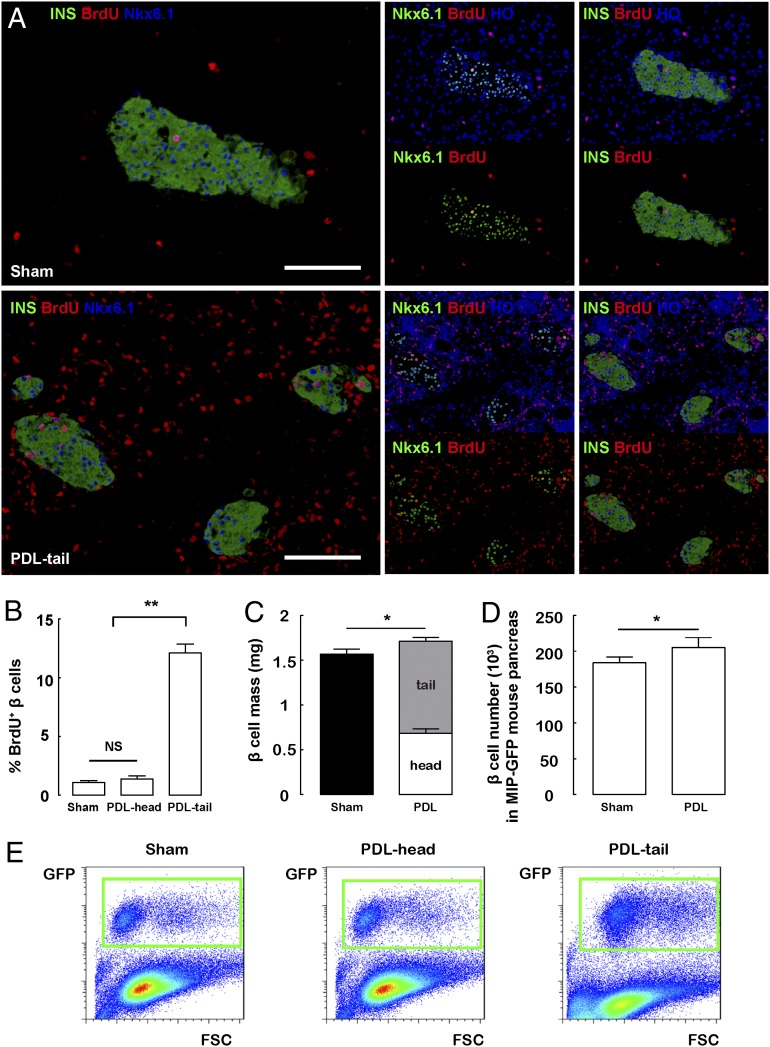
PDL is a model of complete destruction of exocrine acinar cells in the ligated portion of the pancreas, along with a severe local inflammation, as evident from the significant local infiltration by CD45+ (panleukocyte marker) cells, and accumulation of inflammatory factors IL-6, IFN-γ, and tumor necrosis factor (TNF) (15). We and others have previously shown that, in C57BL/6 mice, there is no substantial beta-cell neogenesis (7, 34–41), but an increase in beta-cell proliferation in the ligated pancreas early after PDL (15, 36, 38, 42). Indeed, a 7-d continuous labeling with BrdU (15, 43, 44) immediately after PDL showed a significant increase in beta-cell proliferation. Both insulin and Nkx6.1 were used as beta-cell markers to precisely define beta cells (Fig. 1 A and B) (45, 46). Moreover, the increase in beta-cell proliferation after PDL resulted in a slight but significant increase in beta-cell mass, or beta-cell number (Fig. 1 C–E and Table S1). Because no beta cells are surgically removed after PDL, there should not be a substantial increase in the “per-cell” beta-cell workload, and therefore the increase in beta-cell proliferation after PDL may result specifically from local inflammation (15).
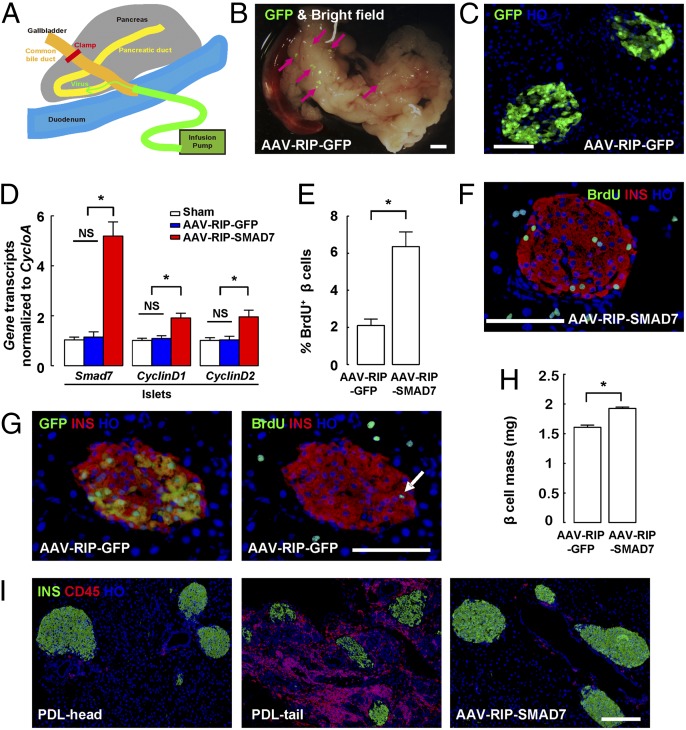
Fig. 1.
PDL is an inflammation model with an increase in beta-cell proliferation. (A and B) A 7-d continuous labeling with BrdU immediately after PDL was performed. (A) Representative images of BrdU, insulin (INS), and Nkx6.1 triple immunostaining in sham-treated pancreas (Sham) and the ligated tail part of the pancreas after PDL (PDL-tail) are shown. (B) Both insulin and Nkx6.1 were used as beta-cell markers to precisely define proliferating beta cells. Quantification showed a nearly 10-fold increase in beta-cell proliferation in the PDL-tail, compared with Sham or the unligated head part of the pancreas after PDL (PDL-head). (C and D) Quantification of beta-cell mass (C) and beta-cell number (D) after PDL. (E) Representative FACS scatter plots for quantification of total pancreatic beta-cell number 7 d after PDL in MIP-GFP mice, based on GFP expression in beta cells. GFP+ beta cells are in green boxes. *P < 0.05. (Scale bars: 50 μm.)
Recruited Macrophages in the PDL Pancreas Trigger Beta-Cell Proliferation.
Because inflammatory macrophages have been reported to play an essential role during inflammatory neovascularization, fibrosis, and tissue remodeling (30–32), we hypothesized that the recruited macrophages in the ligated pancreas after PDL may also stimulate beta-cell proliferation. First, we performed immunostaining for F4/80, a specific marker for macrophages, on tissue sections from control sham-operated pancreas (sham), from the unligated head part of the pancreas (PDL-head), and from the ligated tail part of the pancreas (PDL-tail) 1 wk after PDL. We found very few F4/80+ cells in either sham or PDL-head pancreas (no difference), but we found a robust and impressive increase in F4/80+ cells in the PDL-tail pancreas (Fig. 2 A and B). Thus, PDL in C57BL/6 mice is indeed an inflammation model with robust macrophage infiltration and concomitant beta-cell proliferation.
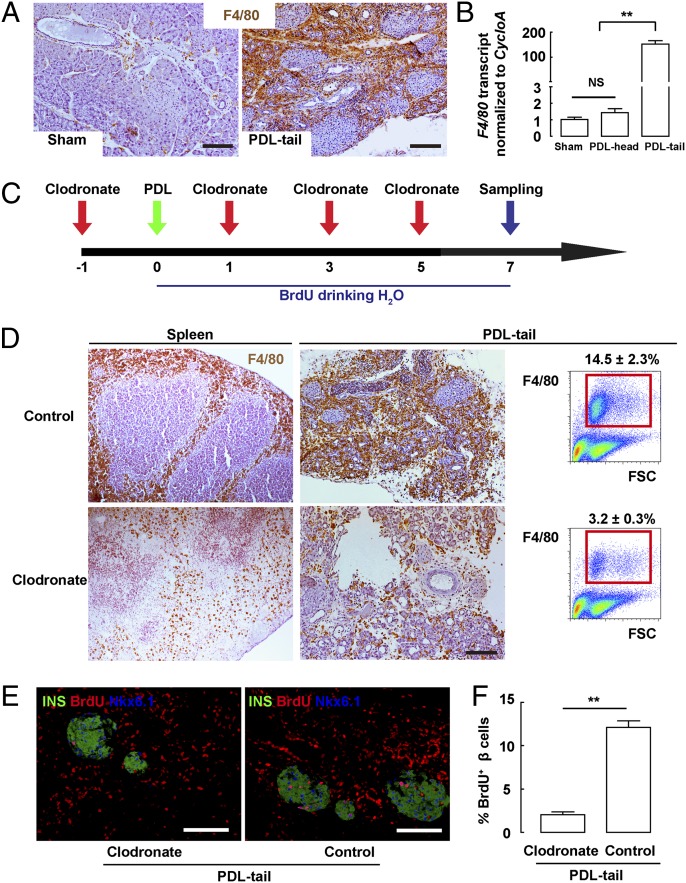
Fig. 2.
Inhibition of macrophage infiltration prevents beta-cell proliferation after PDL. (A) Representative images of F4/80 immunostaining in tissue sections from Sham and PDL-tail pancreas 7 d after PDL. (B) F4/80 transcripts were quantified by RT-qPCR, showing a roughly 150-fold increase in the PDL-tail, compared with Sham or the PDL-head pancreas. (C) A myeloid-specific ablating liposome, clodronate, was i.v. injected every other day starting 1 d before PDL. Control mice received injections of a control liposome. BrdU was continuously provided in the drinking water for 7 d after PDL, at which point the mice were harvested. (D) Representative images of F4/80 immunostaining of the spleen and PDL-tail pancreas and the quantification by FACS. The percentage of F4/80+ cells decreased from 14.5 ± 2.3% of the total cells in control pancreas (control), to 3.2 ± 0.3% in clodronate-treated pancreas (Clodronate). (E) Representative images of BrdU, insulin, and Nkx6.1 triple immunostaining in control and clodronate-treated pancreas. (F) Quantification showed that BrdU+ beta cells significantly decreased in the clodronate-treated pancreas, compared with controls. **P < 0.01. (Scale bars: 50 μm.)
To explore whether the recruited macrophages may affect beta-cell proliferation after PDL, we i.v. injected clodronate (47, 48), a myeloid-ablating liposome that induces apoptosis of macrophages, every other day starting from 1 d before PDL (Fig. 2C). Control mice received injections of control liposome. Immediately after PDL, BrdU was provided in the drinking water for 7 d, at which point the mice were then harvested. All mice remained euglycemic and had normal glucose tolerance (Fig. S1).
Our data show a significant reduction in F4/80+ cells in the spleen in the clodronate-treated mice, compared with controls. Similarly, the number of F4/80+ cells in PDL-tail dramatically decreased. We quantified the percentage of F4/80+ cells in pancreatic digests by FACS and found that it decreased from 14.5 ± 2.3% of the total cells in the control PDL-tail pancreas (injected with control liposome) to 3.2 ± 0.3% of the total cells in the clodronate-treated PDL-tail pancreas (Fig. 2D). Hence, clodronate efficiently reduced the recruitment and/or retention of inflammatory monocytes/macrophages in the PDL-tail.
Next, we wanted to determine whether this reduction in inflammatory macrophages may affect the increase in beta-cell proliferation after PDL. We found that the percentage of BrdU-labeled beta cells significantly decreased in the clodronate-treated PDL-tail (2.4 ± 0.3%) compared with control PDL-tail pancreas (12.3 ± 1.1%), demonstrating that the decrease in the local inflammatory macrophages indeed correlated with a decrease in beta-cell proliferation (Fig. 2 E and F) (Fig. S2 and Table S2). These data suggest that infiltrating macrophages are necessary for the increase in beta-cell proliferation after PDL.
SMAD7 Is Up-Regulated in Beta Cells After PDL.
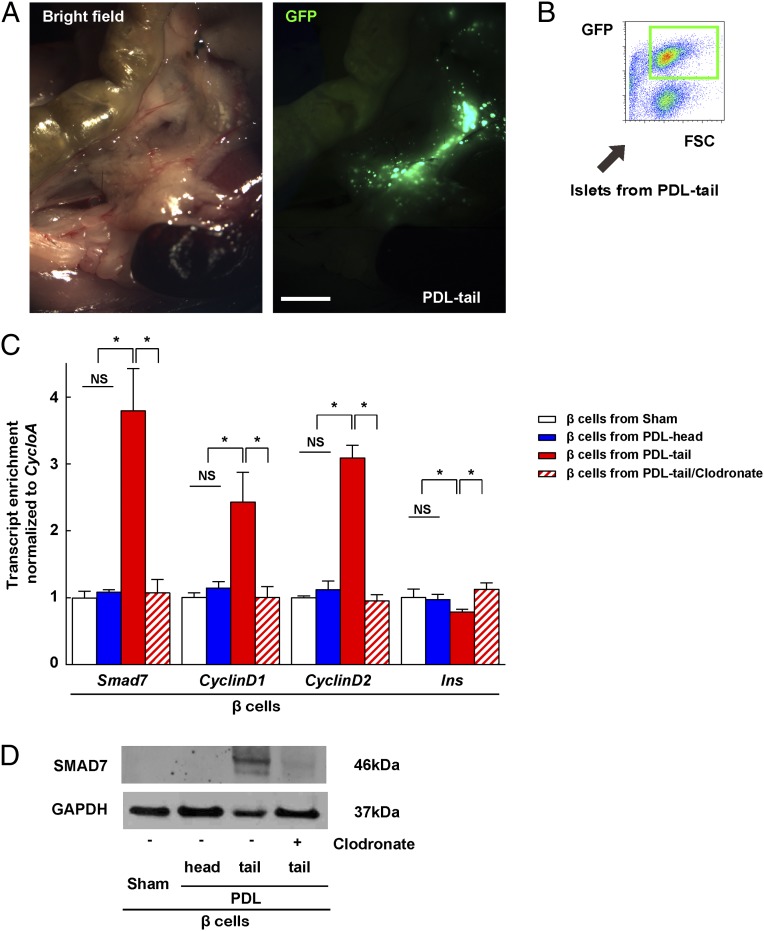
We next tried to determine the mechanism by which macrophages promote beta-cell proliferation in the PDL-tail. Therefore, beta cells were isolated from the PDL-tail, PDL-head, or the sham-operated pancreas of MIP-GFP mice by FACS (Fig. 3 A and B). The purity of beta cells was assured by examining markers of various pancreatic cell types as described before (7, 49). We found a significant increase in the mRNA for Smad7, and two cell cycle activators CyclinD1 and CyclinD2, in beta cells from the PDL-tail pancreas (50–52). Moreover, the increases in Smad7, CyclinD1, and CyclinD2 were completely inhibited in beta cells isolated from the clodronate-treated PDL-tail, suggesting that the recruited macrophages are responsible for the increase in Smad7, CyclinD1, and CyclinD2 in beta cells (Fig. 3C). The changes in SMAD7 protein were confirmed by Western blot on purified beta cells (Fig. 3D). Moreover, a modest but significant down-regulation of insulin, Pdx1, NeuroD1, MafA, and Nkx6.1 gene transcripts was also detected in the beta cells in the PDL-tail (Fig. 3C and Fig. S3), consistent with our previous findings that some beta cells may undergo a certain degree of dedifferentiation after PDL (7).
Fig. 3.
SMAD7 is up-regulated in beta cells after PDL. (A) Gross image of PDL in MIP-GFP mice. (B) Beta cells were FAC sorted from the islets of PDL-tail, PDL-head, or clodronate-treated PDL-tail pancreas of MIP-GFP mice. A representative flow cytometry image is shown. (C) RT-qPCR showed a significant increase in Smad7, CyclinD1, and CyclinD2 transcripts and a modest but significant decrease in insulin in beta cells from PDL-tail, all of which were inhibited by clodronate treatment. (D) SMAD7 protein was analyzed by Western blot on purified beta cells. GAPDH was used as a protein loading control. *P < 0.05. NS, no significance. (Scale bar: 1 mm.)
SMAD7 Is Necessary for Macrophage-Induced Beta-Cell Proliferation.
To determine whether macrophages promote beta-cell proliferation through up-regulation of SMAD7, we generated beta-cell–specific SMAD7 mutant mice (INS-Cre; Tomato; SMAD7fx/fx) by crossing SMAD7fx/fx (12); Rosa26CAGTomato and INS-Cre (7) mice. These mice are euglycemic and have a normal glucose tolerance (Fig. S1), and the beta cells in these mice are lineage-tagged with Tomato to allow isolation of beta cells based on red fluorescence by FACS. Our data showed a roughly 98% labeling efficiency of beta cells in these mice. INS-Cre; Tomato mice (without SMAD7fx/fx) were used as a control.
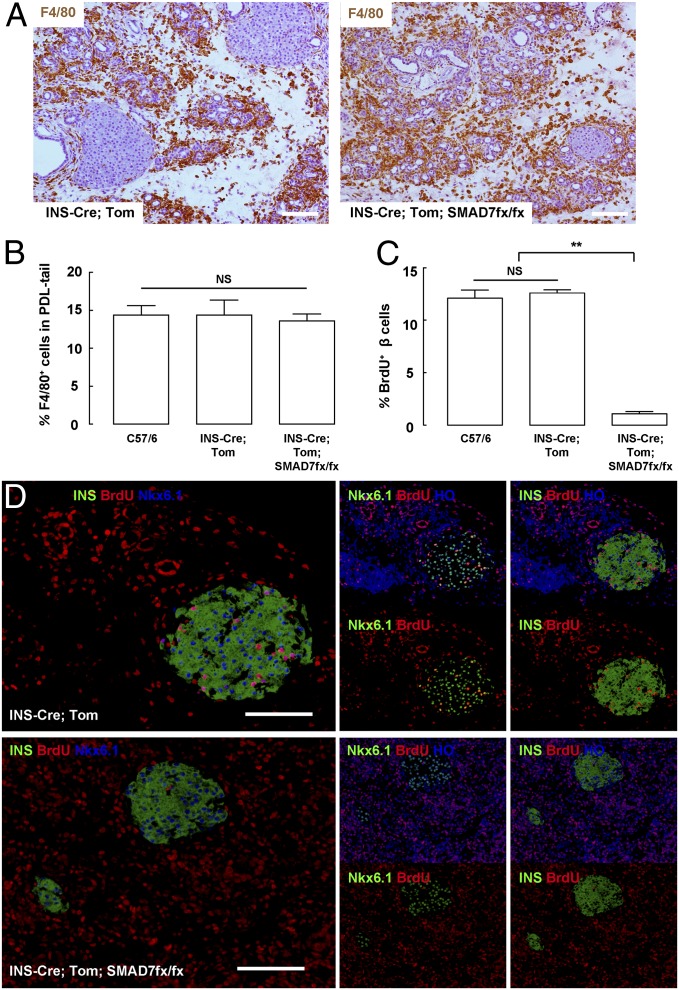
Macrophage infiltration after PDL was unaltered in beta-cell–specific SMAD7 mutant mice, by F4/80 immunohistochemistry (Fig. 4A), and by FACS (Fig. 4B). Beta-cell proliferation in beta-cell-specific SMAD7 mutant mice after PDL was significantly lower (1.1 ± 0.2%) than control INS-Cre; Tomato mice (11.9 ± 1.6%) (Fig. 4 C and D, and Table S3). Tomato+ beta cells were isolated from the pancreas by FACS, confirming the complete loss of Smad7, and a marked decrease of CyclinD1 and CyclinD2 in the beta cells from beta-cell–specific SMAD7 mutant mice after PDL (Fig. S4). These data suggest that macrophages promote beta-cell proliferation through up-regulation of SMAD7 in beta cells.
Fig. 4.
SMAD7 is necessary for macrophage-induced beta-cell proliferation after PDL. (A and B) Macrophage infiltration after PDL was unchanged in beta-cell–specific SMAD7 mutant mice by representative F4/80 immunostaining (A), and by FACS (B). (C and D) Beta-cell proliferation after PDL significantly decreased in beta-cell–specific SMAD7 mutant mice, shown by quantification (C) and by representative images (D). **P < 0.01. NS, no significance. (Scale bars: 50 μm.)
SMAD7 Is Sufficient to Promote Beta-Cell Proliferation.
Next, we tested whether up-regulation of SMAD7 in beta cells alone, without PDL and macrophage infiltration, is sufficient to promote beta-cell proliferation. For this purpose, we generated an adenoassociated virus (AAV) to express SMAD7 under the control of the rat insulin promoter (RIP), to specifically express SMAD7 in beta cells (AAV-RIP-SMAD7) and thus avoid potential off-target effects of SMAD7 overexpression in nonbeta pancreatic cells (53, 54). AAV-RIP-GFP virus was also generated to be used as a control.
We then used our recently developed intraductal virus delivery system (34, 55) to efficiently express SMAD7 in beta cells in vivo (Fig. 5A). C57BL/6 mice that received either AAV-RIP-SMAD7, or an identical titer of AAV-RIP-GFP as a control, also received BrdU in the drinking water for a period of 7 d, starting immediately after viral infusion. GFP+ islets were readily visualized grossly (Fig. 5B), and in pancreatic sections (Fig. 5C) 7 d after AAV-RIP-GFP infusion. Quantification showed a labeling efficiency of 73.4 ± 6.5% for beta cells, with an extremely high specificity of beta-cell labeling (less than 0.2% of nonbeta cells expressing GFP). SMAD7 transcripts were further quantified from isolated whole islets from the AAV-RIP-SMAD7–infused mice, showing a significant increase compared with islets from the mice infused with control virus. Moreover, increases in both CyclinD1 and CyclinD2 transcripts were also detected in the islets from AAV-RIP-SMAD7–infused mice, suggesting forced expression of SMAD7 in beta cells induced up-regulation of CyclinD1 and CyclinD2 expression (Fig. 5D). Further analysis showed a significant increase in beta-cell proliferation in the mice that received AAV-RIP-SMAD7 (6.4 ± 0.8%) compared with control mice that received AAV-RIP-GFP (2.1 ± 0.4%) (Fig. 5 E–G, Fig. S5, and Table S4). One month after AAV infusion, beta-cell mass in mice that received AAV-RIP-SMAD7 increased by 19.7 ± 1.4% compared with mice that received AAV-RIP-GFP (Fig. 5H). Because AAV induced little increase in local inflammation, as evident from immunostaining for a panleukocyte marker, CD45 (Fig. 5I), our data thus suggest that AAV-RIP-SMAD7 infusion leads to increased beta-cell proliferation directly due to expressing SMAD7 in beta cells, rather than indirectly by increasing local inflammation. This conclusion is further supported by the fact that there was little increase in beta-cell proliferation after infusion with the control AAV-RIP-GFP (Fig. 5 E–G, Fig. S5, and Table S4). These data suggest that SMAD7 is not only necessary but also sufficient to mediate the macrophage-triggered beta-cell proliferation.
Fig. 5.
SMAD7 is sufficient to promote beta-cell proliferation. (A) An intraductal virus delivery system is shown. (B and C) Virus-transduced GFP+ islets were readily visualized grossly (B), and in pancreatic sections (C) 7 d after AAV-RIP-GFP virus infusion. The arrows point to GFP+ islets. (D) Smad7, CyclinD1, and CyclinD2 transcripts significantly increased in the islets isolated from mice that received AAV-RIP-SMAD7 viral infusion, compared with islets isolated from mice that received control virus infusion. (E) Quantification of BrdU+ beta cells. (F) Representative images for GFP (direct fluorescence), insulin, and BrdU staining in mice that received AAV-RIP-GFP virus infusion. The arrow points to a BrdU+INS+ cell. (G) Representative images for insulin and BrdU double immunostaining in mice that received AAV-RIP-SMAD7 virus infusion. (H) Quantification of beta-cell mass. (I) Representative images for insulin and CD45 double immunostaining in mice that received AAV-RIP-SMAD7 virus infusion. PDL-head and PDL-tail pancreas were used as controls. *P < 0.05. NS, no significance. (Scale bars: 50 μm.)
Recruited Macrophages in the PDL Pancreas Are Mainly M2 Macrophages.
We have shown that PDL-recruited macrophages are associated with up-regulated SMAD7 in beta cells, which in turn activates the cell cycle activators CyclinD1 and CyclinD2, to promote beta-cell proliferation. Next, we wanted to determine which subtype(s) of macrophages may be necessary for beta-cell proliferation after PDL. Therefore, M2 and M1 macrophages were separated by using FACS for two different M2 macrophages markers, CD163 and CD206 (30–32) in the F4/80+ cell fraction from the PDL-tail pancreas. Our data showed a similar percentage of CD206+ (75.2 ± 8.3%) and CD163+ (72.5 ± 5.3%) macrophages (F4/80+) in the PDL-tail (Fig. 6A). We then isolated CD206+F4/80+ cells (representing M2 macrophages) and CD206−F4/80+ cells (representing M1 macrophages) from the PDL-tail, and analyzed their gene expression profiles. The highly enriched iNOS (M1 macrophage marker) in the M1 macrophage fraction and the highly enriched Arginase (M2 macrophage marker) (30–32) in the M2 macrophage fraction confirmed the quality of FACS and the purity of the macrophage subtype fractions (Fig. 6B). These data suggest that the majority of the recruited macrophages in PDL-tail are M2 macrophages.
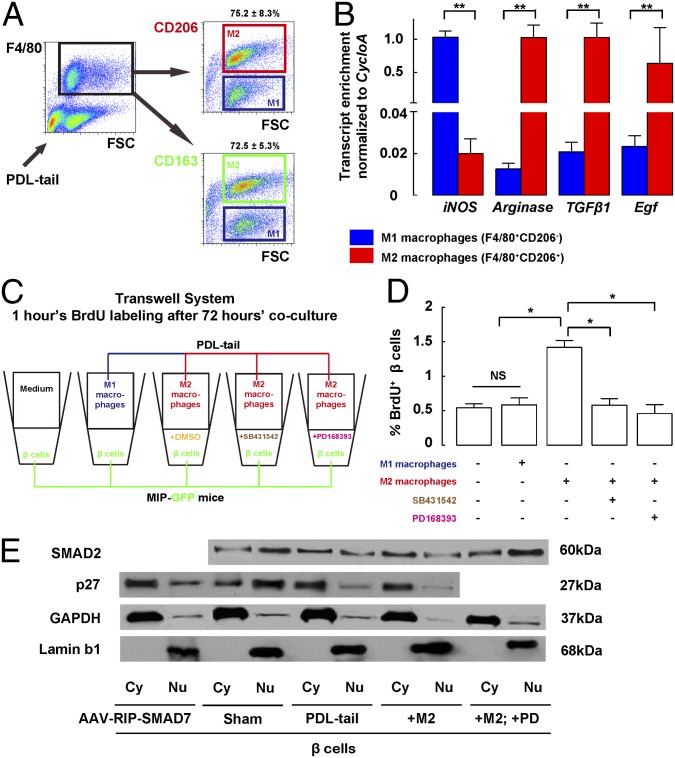
Fig. 6.
M2 macrophages promote beta-cell proliferation via interplay of TGFβ and EGF receptor signaling pathways. (A and B) Recruited macrophages in PDL pancreas are mainly M2 macrophages. (A) M1 and M2 macrophages were separated by using FACS for two different M2 macrophages markers, CD163 or CD206 in the F4/80+ cell fraction from PDL-tail pancreas. Our data showed a similar percentage of CD206+ (75.2 ± 8.3%) and CD163+ (72.5 ± 5.3%) macrophages in the PDL-tail. (B) Gene expression profiles of CD206+F4/80+ cells (representing M2 macrophages) and CD206− F4/80+ cells (representing M1 macrophages) from PDL-tail. The highly enriched iNOS (M1 macrophage marker) in the M1 macrophage fraction and the highly enriched Arginase (M2 macrophage marker) in the M2 macrophage fraction confirmed the purity of the macrophage subtype fractions by FACS. M2 macrophages expressed much higher mRNA levels of TGFβ1 and Egf, compared with M1 macrophages. (C) Purified primary beta cells isolated from untreated MIP-GFP mice were cocultured with M1 macrophages (CD206−F480+) or M2 macrophages (CD206+F480+) isolated from the PDL-tail, or with control medium, in a Transwell system. Ten micromolar SB431542 (a specific TGFβ receptor I inhibitor), or 0.1 μM PD168393 (a specific EGFR inhibitor), with DMSO alone as a control, was added to the medium of the cocultured beta cells and M2 macrophages to block TGFβ signaling or EGFR signaling, respectively. After 72-h culture, BrdU was given for 1 h before beta cells were sampled and analyzed for their proliferation. (D) Whereas incubation with M1 macrophages did not affect beta-cell proliferation, compared with control medium, incubation with M2 macrophages significantly increased the percentages of beta cells that incorporated BrdU. Notably, the increase in beta-cell proliferation by M2 macrophages was completely blocked by either SB431542 or PD168393. (E) Cytoplasmic and nuclear proteins were separated for analysis. Nuclear SMAD2 did not increase in beta cells from either the PDL-tail pancreas, or when cocultured with M2 macrophages, but significantly increased in beta cells cocultured with M2 macrophages when the EGFR inhibitor PD168393 (PD) was applied. However, nuclear p27 was significantly decreased in beta cells from AAV-RIP-SMAD7–infused pancreas, or the PDL-tail pancreas, or when cocultured with M2 macrophages. GAPDH and Lamin b1 were used as loading controls for cytoplasmic and nuclear proteins, respectively, and also used as a control for the purity of the proteins. *P < 0.05. **P < 0.01. NS, no significance.
M2 Rather than M1 Macrophages Promote Beta-Cell Proliferation.
To find out which type(s) of macrophages may induce beta-cell proliferation, we cocultured M1 macrophages (CD206−F480+) or M2 macrophages (CD206+F480+) that were isolated from the PDL-tail, with purified primary beta cells isolated from untreated MIP-GFP mice (7, 49) in a Transwell system (Fig. 6C). After 72-h culture, BrdU was given for 1 h before beta cells were sampled and analyzed for their proliferation. Although incubation with M1 macrophages did not affect beta-cell proliferation (0.57 ± 0.08%) compared with control medium (0.54 ± 0.03%), incubation with M2 macrophages significantly increased the percentage of beta cells that incorporated BrdU (1.43 ± 0.21%) (Fig. 6D), suggesting that M2 macrophages, rather than M1 macrophages, are able to induce beta-cell proliferation in the PDL pancreas. The increases in mRNA for Smad7, CyclinD1, and CyclinD2 were also detected in the beta cells that were cocultured with M2 macrophages, consistent with the in vivo findings in PDL (Fig. S6). However, when beta cells from beta-cell–specific SMAD7KO mice were instead used in the coculture with M2 macrophages, the absence of SMAD7 in beta cells resulted in loss of the increase in beta-cell proliferation and failure to induce CyclinD1 and CyclinD2 in beta cells, suggesting that M2-induced beta-cell replication is SMAD7 dependent (Fig. S7).
M2 Macrophages Increase Beta-Cell Proliferation Through Interplay Between TGFβ and EGF Receptor Signaling Pathways.
We previously showed that specific knockout of TGFβ receptor I and II in beta cells substantially inhibited beta-cell proliferation after PDL (15), but here we also find that inhibition of the pan-TGFβ superfamily signaling inhibitor, SMAD7, also inhibited beta-cell proliferation after PDL. These seemingly paradoxical data suggest that signaling pathways other than specifically TGFβ receptor I and II may also be involved here. Likely candidates would include BMPs, activins, and non-TGFβ superfamily signaling pathways, which have all been reported to regulate both SMAD2 and SMAD7 (17, 23–27).
We thus screened for the candidate factors that may be released from M2 macrophages to affect beta-cell proliferation. Among the numerous factors tested, we detected a significant increase in the mRNA levels of TGFβ1 and Egf in the PDL-tail pancreas (selected genes shown in Fig. S8) and specifically in M2 macrophages (Fig. 6B). Modest increases in TGFβ2, TGFβ3, Bmp4, and Bmp7 were also detected in the PDL-tail pancreas, but the recruited macrophages did not seem to be the predominant source of them (Fig. S8). Notably, activins and other BMPs did not up-regulate. These data suggest that both TGFβ and EGF receptor (EGFR) signaling pathways in beta cells may be directly affected by M2 macrophages in the PDL-pancreas, because EGF signals through EGFR, and because both TGFβ receptor I and II, and EGFR have been reported to be active in pancreatic beta cells (9–11, 56–58).
Binding of TGFβ1 to type II receptor not only leads to phosphorylation of the type I receptor, which subsequently phosphorylates SMAD2 with translocation to the nucleus but also increases SMAD7 levels as a potential negative feedback to attenuate SMAD2 signaling (17, 23–26, 59). Thus, we added a specific TGFβ receptor I inhibitor, SB431542 (60–62), to the medium of the cocultured beta cells with M2 macrophages, at a concentration of 10 μM to block the effect from TGFβ1 (Fig. 6C). DMSO, the solvent for SB431542, was added to the control wells with identical volume, with no effect on beta-cell proliferation. Notably, the increase in beta-cell proliferation induced by M2 macrophages was completely blocked by SB431542 (Fig. 6D). Here, the increases in SMAD7, CyclinD1, and CyclinD2 mRNA in beta cells cocultured with M2 macrophages were completely blocked (Fig. S6), suggesting that beta cells may activate SMAD7 via TGFβ1/receptors signaling. However, we failed to detect a significant increase in the nuclear SMAD2 (phosphorylated form) either in the isolated beta cells from the PDL-tail pancreas, or in the beta cells that were cocultured with M2 macrophages (Fig. 6E). Because EGF has been reported to inhibit SMAD2 nuclear translocation (17, 23, 24, 26), we hypothesized that activation of beta-cell EGFR signaling by M2-macrophage–derived EGF may prevent SMAD2 nuclear translocation and thus prevent the known cell cycle inhibitory effect of SMAD2 on beta cells (26). Thus, we added an EGFR inhibitor, PD168393 (dissolved in DMSO) (63), to the medium of the cocultured beta cells with M2 macrophages, at a concentration of 0.1 μM (Fig. 6C). Notably, the increase in beta-cell proliferation induced by M2 macrophages was completely blocked by PD168393 (Fig. 6D). Here, a significant increase in nuclear SMAD2 was detected in PD168393-treated, M2-macrophage–cocultured beta cells (Fig. 6E). These data suggest that inhibition of EGFR signaling was permissive for active TGFβ superfamily-mediated SMAD2 activity in beta cells, perhaps due to TGFβ1 release from M2 macrophages, with reestablishment of nuclear translocation of SMAD2. Our data also suggest that, in the PDL-tail pancreas, a TGFβ1-induced inhibitory effect on beta-cell proliferation (through SMAD2) is blocked by a concomitant EGF-induced inhibition of SMAD2 nuclear translocation, but still allowing SMAD7 up-regulation, which may lead to beta-cell proliferation through mechanisms other than SMAD2 inhibition (Fig. 7). These results may explain the seemingly paradoxical finding that knockout of either TGFβ receptor I and II, or of the pan-TGFβ superfamily inhibitor SMAD7, can both inhibit beta-cell proliferation in the PDL-tail pancreas.
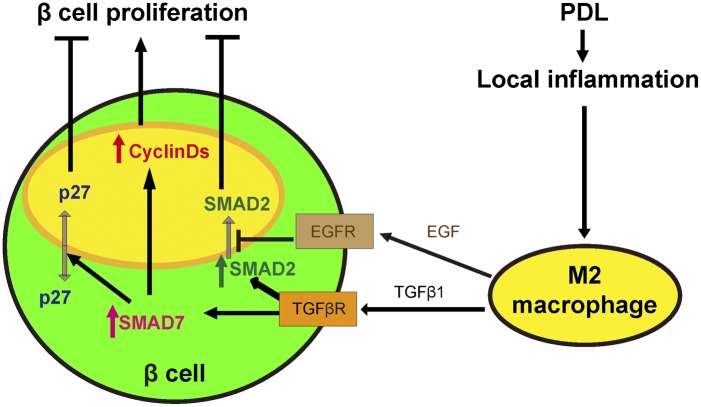
Fig. 7.
Schematic for hypothetical SMAD7-dependent, M2 macrophage-induced beta-cell proliferation. PDL induces severe local inflammation. The recruited M2 macrophages release high levels of TGFβ1 and EGF. TGFβ1 induces SMAD7 up-regulation and SMAD2 phosphorylation. The nuclear translocation of phosphorylated SMAD2 is efficiently inhibited, possibly through activation of EGFR signaling pathways in response to EGF. Thus, without the SMAD2-induced inhibitory effect, the increased SMAD7 induces beta-cell proliferation, possibly by increasing the cell cycle activators CyclinD1 and CyclinD2, and by nuclear exclusion of the cell cycle inhibitor p27.
SMAD7 Also Increases Beta-Cell Proliferation Through Nuclear Exclusion of p27.
We have shown that SMAD7 expression led to up-regulated CyclinD1 and CyclinD2 to promote beta-cell proliferation. Because it has been reported that inhibition of TGFβ signaling reduces the nuclear accumulation of the Cyclin-dependent kinase inhibitor 1β (p27) to promote beta-cell proliferation (64), we examined whether p27 may play a role in PDL-macrophage–induced, SMAD7-dependent beta-cell proliferation. We indeed detected a decrease in nuclear p27 in the beta cells from the PDL-tail pancreas, beta cells from AAV-RIP-SMAD7–infused pancreas, and beta cells cocultured with M2 macrophages (Fig. 6E). These results suggest that, besides inducing CyclinD1 and CyclinD2, SMAD7 may also promote beta-cell proliferation through nuclear exclusion of the cell cycle inhibitor, p27 (Fig. 7).
Discussion
Enhancing beta-cell mass could be an ideal cure for diabetes. Because accumulating data suggest that beta-cell replication is the main source of increased beta-cell mass in adults (3–7), determination of signaling pathways that regulate beta-cell replication are critical. In the current study, we examined the mechanism underlying PDL-induced beta-cell replication in C57BL/6 mice (15, 36, 38, 42), which appears to be triggered by local inflammation (7, 15). Although differences in mouse strain or surgical technique could affect the outcome of PDL, most studies do not support beta-cell neogenesis after PDL (7, 15, 34–41, 65), unless the PDL is combined with a chemical beta-cell toxin treatment (66, 67). Of note here, the residual beta cells that survived chemical beta-cell toxin treatment may lose replication potential (65–67). This toxicity may also explain why beta-cell regeneration has not been seen after chemical beta-cell toxin treatment. We only detected a very modest increase (∼10%) in beta-cell mass 1 wk after PDL, consistent with several previous reports (35, 37, 38), but in contrast with one report that has shown a doubling of beta-cell mass within this period (42). The reason for this discrepancy may result from tissue remodeling and edema after PDL that have led to an overestimation of beta-cell mass in some situations, which has been well discussed previously (35, 37, 38).
Here, we found a substantial infiltration of inflammatory macrophages into the ligated pancreas after PDL. In a loss-of-function experiment using clodronate to deplete macrophages, we showed that macrophage infiltration is necessary for the increase in beta-cell replication after PDL. Further support for the strength of the link between macrophages and beta-cell proliferation includes the fact that the literature is rife with evidence of the specificity of clodronate for macrophage depletion, rather than depletion of other cells. Also, we used liposome vehicle as a control to further exclude the possibility of nonspecific effects. Clodronate treatment is essentially a loss-of-function experiment for macrophages, and thus our in vivo clodronate data strongly suggest a causative link between macrophages and SMAD7 activation in beta cells/beta-cell proliferation. To exclude the possible effect of an intermediate cell type, the in vitro coculture system involved only M2 macrophages and purified beta cells. Therefore, the SMAD7 up-regulation in beta cells in the presence of only macrophages is likely to result from a direct communication between these two cell types, although the possible involvement of some other intermediate factors or cofactors cannot be completely excluded.
Next, we examined the possible signaling pathways in beta cells that may be targeted by macrophage-induced beta-cell proliferation. We found a significant increase in expression of the TGFβ superfamily signaling inhibitor SMAD7 in beta cells after PDL, as well as a significant increase in expression of the inflammatory cytokines TGFβ1 and EGF, in either the PDL-tail pancreas, or in isolated macrophages from the PDL-tail pancreas. These results are consistent with previous reports that SMAD7 can be induced by TGFβs, NF-κB, BMPs, and other inflammatory cytokines (68–72).
Then, we tried to determine whether macrophages promote beta-cell proliferation through up-regulation of SMAD7. In a loss-of-function experiment, beta cells failed to proliferate in response to PDL in beta-cell–specific SMAD7 mutant mice, confirming a necessary role for SMAD7. Thus, we believe that SMAD7 is necessary for macrophage-induced beta-cell proliferation. Furthermore, in a gain-of-function experiment, otherwise unperturbed beta cells significantly increased their proliferation after forced SMAD7 expression in beta cells in vivo, despite the absence of an influx of macrophages and despite the absence of PDL. Of note, the degree of inflammation caused by our intraductal infusion system with AAV seems minimal compared with PDL. Moreover, the increase in beta-cell proliferation after control viral infusion was not significant in our study. Taken together, these data suggest that SMAD7 is necessary and sufficient to mediate the macrophage-induced beta-cell proliferation after PDL.
To study this process more closely, we used an in vitro Transwell coculture system of macrophages from the PDL-tail along with beta cells. M2 rather than M1 macrophages were shown to induce SMAD7 up-regulation and to have a proliferative effect on beta cells. A less potent effect of M2 macrophages on beta-cell replication in vitro compared with in vivo may have resulted from a suboptimal environment for macrophages in the culture system, which may then dampen the release of growth factors by macrophages, compared with in vivo in the PDL pancreas. Although M2 macrophages have been extensively studied for their central role in neovascularization, fibrosis, and cell growth during tissue repair (30–32), our study provides strong evidence, which was previously unidentified, that they may also have a proliferative effect on pancreatic beta cells.
We previously found that the specific deletion of TGFβ receptor I and II in beta cells substantially inhibited beta-cell proliferation after PDL (15). Although TGFβ1 binding can activate both SMAD2 and SMAD7 (17, 23–26), we failed to detect an increase in SMAD2 in the nuclei of beta cells after PDL, suggesting that nuclear translocation of SMAD2 in beta cells after PDL has actually been inhibited. Although SMAD7 is considered canonically as a major inhibitor for all TGFβ superfamily pathways, it is also capable of affecting many other pathways. In turn, both SMAD2 and SMAD7 can be regulated by other signaling pathways (17, 23–27, 69, 72, 73). This pathway cross talk mediated by SMAD2 and SMAD7 substantially complicates the interpretation of results in various experimental models (17, 23–27). EGF has been reported to inhibit SMAD2 nuclear translocation and thus to attenuate some TGFβ signaling (17, 23, 24, 26). M2 macrophages release high levels of EGF in addition to TGFβ1. Thus, we found that a potential TGFβ1-induced inhibitory effect on beta-cell proliferation (through SMAD2) appeared to be blocked by a concomitant EGF-induced inhibition of SMAD2 nuclear translocation. The macrophage-induced SMAD7 up-regulation appeared to then be able to lead to beta-cell proliferation through mechanisms other than SMAD2 inhibition. These results may explain the seemingly paradoxical result that knockout of either TGFβ receptor I and II, or of the major TGFβ inhibitor, SMAD7, can both lead to inhibition of beta-cell proliferation in the PDL-tail pancreas. However, PPX, in which the TGFβ signaling pathway is significantly inhibited, may induce beta-cell proliferation through interplay of other pathways (15, 16).
Here, we showed that SMAD7 directly up-regulated the cell-cycle activators CyclinD1 and CyclinD2, and induced nuclear exclusion of the cell cycle inhibitor p27, to promote beta-cell proliferation, consistent with a previous report (64). Interestingly, insulin transcripts in beta cells showed a modest but significant decrease in the PDL pancreas. These data are consistent with previous findings that beta cells may undergo a certain degree of dedifferentiation during aging (74), under stress (75), after PPX (16), or specifically after PDL (7).
We have recently reported that up-regulation of SMAD7 in beta cells after PPX was accompanied by an increase in SMAD7+PP+ cells that might had derived from transiently dedifferentiated beta cells (16). However, no combined lineage tracing and loss-of-function experiments were done to define a causal link between SMAD7 activation and these double-positive cells (16). Therefore, SMAD7 activation in beta cells does not necessarily lead to formation of these SMAD7+PP+ cells. Here, we studied beta-cell replication in a different model—PDL. We detected SMAD7 up-regulation in beta cells after PDL but did not find an increase in the number of SMAD7+PP+ cells. These data suggest that SMAD7 up-regulation may not be the direct trigger for formation of SMAD7+PP+ cells.
A previous study by Smart et al. (14) showed that overexpression of SMAD7 in beta cells led to a loss of beta-cell identity and function. Compared with this report, in our study, the degree of increase in SMAD7 expression in beta cells after PDL, or after AAV-RIP-SMAD7 infection, appears much more modest. Because SMAD7-induced effects seem to be dose dependent (17), we believe that the modest increase in SMAD7 in our study may down-regulate TGFβ signaling in beta cells, resulting in a decrease in hormone production and an increase in cell cycle activators to allow beta cells to divide. However, the presumably much higher levels of SMAD7 in beta cells seen in the study by Smart et al. may cause beta cells to lose their identity, fully dedifferentiate, resulting in the development of overt diabetes, as reported (14, 75). If SMAD7 up-regulation does not persist, beta cells may be able to then redifferentiate, and diabetes may be reversed (14).
Here, we present a model of SMAD7-dependent M2 macrophage-mediated beta-cell proliferation during PDL-induced pancreatic inflammation (Fig. 7). PDL induces severe local inflammation. The recruited M2 macrophages release high levels of TGFβ1 and EGF. Although TGFβ1 can directly lead to up-regulation of SMAD7 mRNA and SMAD2 phosphorylation, nuclear translocation of phosphorylated SMAD2 does not occur, perhaps due to EGFR signaling activity. Thus, without the inhibitory effects of TGFβ-induced SMAD2 activity, increased SMAD7 may be able to induce beta-cell proliferation, possibly by increasing the cell cycle activators CyclinD1 and CyclinD2, and by nuclear exclusion of the cell cycle inhibitor p27.
Our study not only reveals a pathway to regulate beta-cell proliferation after PDL but also suggests the possibility of using the SMAD7 pathway to induce endogenous beta-cell proliferation (through extracellular manipulations) for translation to clinical diabetic therapy. Future studies may focus on dissection of the further downstream pathways in beta cells in response to M2 macrophages and definition of the exact extracellular signals that lead to the SMAD7-induced beta-cell trophic effects.
Materials and Methods
Mouse Manipulation.
All mouse experiments were approved by the Animal Research and Care Committee at the Children's Hospital of Pittsburgh and the University of Pittsburgh Institutional Animal Care and Use Committee. BAC transgenic insulin promoter Cre reporter (INS-Cre) mice and MIP-GFP mice have been described before (7). C57BL/6 and Rosa26CAGTomato (Tomato) mice were purchased from The Jackson Laboratory. SMAD7fx/fx was generated before (12). In total, 98.2 ± 7.3% beta cells from INS-Cre; Tom; SMAD7fx/fx mouse pancreas were labeled with Tomato. Nonspecific labeling of other cell types was not detected. All mice used in current experiments are 10-wk-old males and have a C57BL/6 background. PDL was performed and quality controlled as described by us previously (7, 15). Two hundred microliters of clodronate or control liposome (Clodronateliposomes) was injected in the tail vein of mice every other day, starting 1 d before PDL, until the end of the experiment. Pancreatic intraductal virus infusion was performed as described before (34). Briefly, after anesthetizing the animals, the duodenum was isolated to expose the common bile duct, after which a microclamp (Roboz; RS-7439) was placed on the common bile duct above the branching of the pancreatic duct. A 31-gauge blunt-ended catheter (World Precision Instruments) was then put into the common bile duct through the sphincter of Oddi in the duodenum, which was then clamped with another microclamp to prevent backflow. The other end of the catheter is connected to a microinfusion apparatus, which delivers 150 μL of AAV8 virus (titration of 108) via the catheter at a rate of 10 μL/min. After viral infusion, the hole created by the catheter in the duodenum was closed with 6-0 suture. Blood glucose levels were measured using Accu-119 Chek glucose meter (Roche) as described before (15). For i.p. glucose tolerance test, mice were fasted for 16 h, and then were injected with glucose (2 g/kg), as described before (15).
Pancreatic Digestion, Islet Isolation, and FACS.
Digestion of the pancreas and islet and beta-cell isolation from MIP-GFP mice have been described previously (15, 49). For examining nuclear and cytoplasmic forms of the proteins, mice were perfused with 4% (vol/vol) PFA for 20 min before pancreas digestion. Isolation of APC-conjugated F4/80 (eBioscience), FITC-conjugated CD206, and Brilliant Violet 421-conjugated CD163 (BD Bioscience) are used for analysis and isolation of macrophages or their subtypes. The purity of sorted cells (beta cells or macrophages or macrophage subtypes) was evaluated by analysis of cell type-specific markers by reverse transcription–quantitative PCR (RT-qPCR) as described before (7, 49).
Virus Production.
AAV serotype 8 vectors were generated by transfection of human embryonic kidney 293 cells as described before (53, 54). ORFs of mouse SMAD7 gene was amplified from cDNA of E17 pancreas. RIP was amplified from rat genomic DNA. GFP was amplified from pLVX-IRES-ZsGreen (Clontech). Purified AAV vectors were filtered and stored at −80 °C. Titration of viral vectors was determined by Viral P24 ELISA kit (Clontech).
Cell Culture.
Primary beta cells were cultured as described previously (7, 49). Macrophages were cultured in the same medium as the beta cells for the Transwell coculture system. After 72-h coculture, 1 mM BrdU was added for 1 h before beta cells were harvested and analyzed for their proliferation. SB431542 is a specific TGFβR1 inhibitor (60–62). PD168393 (Millipore) is a specific EGFR inhibitor (63). SB431542 or PD168393 was added to the medium of the cocultured beta cells and M2 macrophages at a concentration of 10 and 0.1 μM in DMSO, respectively, at the beginning of coculture. DMSO was added in control.
Western Blot.
Total protein was extracted with radioimmunoprecipitation assay buffer. Nuclear and cytoplasmic proteins were isolated with Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific). Primary antibodies for Western blot are rabbit polyclonal anti-GAPDH (loading control for total protein, or cytoplasmic protein; Cell Signaling), anti-Lamin b1 (load control for nuclear protein; Cell Signaling), anti-SMAD7 (Santa Cruz), and anti-SMAD2 and anti-p27 (Cell Signaling). Secondary antibody is HRP-conjugated anti-rabbit (Jackson Labs).
Isolation of Genomic DNA and PCR for SMAD7 Mutant.
Genomic DNA extraction and conventional PCR have been described previously (15, 49). Primers for examining SMAD7 mutant are 5′-GGAGCAGGGCATCGATGTGG-3′ (sense) and 5′-GACAGACTCGACAATGGCAGCG-3′ (antisense), which amplifies a genomic DNA PCR product of 1.7 kb from wild-type mice, 2.2 kb from SMAD7floxed knock-in mice, and 0.7 kb from SMAD7floxed knock-in mice after Cre recombination.
Isolation of RNA and RT-qPCR.
RNA extraction and RT-qPCR have been described previously (7, 15, 49). Primers were all purchased from Qiagen. They are CycloA (QT00247709), F4/80 (QT00099617), Smad7 (QT00124607), CyclinD1 (QT00154595), CyclinD2 (QT00170618), Ins (QT00114289), iNOS (QT00100275), Arginase (QT00134288), TGFβ1 (QT00145250), TGFβ2 (QT00106806), TGFβ3 (QT00166838), Egf (QT00151018), Bmp4 (QT00111174), Bmp7 (QT00096026), and P27 (QT01058708). RT-qPCR values were normalized against CycloA, which proved to be stable across the samples. Fold changes to control are shown in the figures.
Immunohistochemistry.
All pancreas samples were fixed and cryoprotected in 30% sucrose overnight before freezing, as described before (7, 15, 49). GFP and Tomato were detected by direct fluorescence. Primary antibodies for immunostaining are as follows: guinea pig polyclonal anti-insulin (Dako), rabbit polyclonal anti-F4/80 (Invitrogen) and anti-Nkx6.1 (a kind gift from Maike Sander, University of California, San Diego, La Jolla, CA), and rat polyclonal anti-BrdU (Abcam) and anti-CD45 (BD). Secondary antibodies for indirect fluorescent staining are Cy2-, Cy3-, or Cy5-conjugated rat, rabbit, and guinea pig specific (Jackson ImmunoResearch Labs). Nuclear staining is performed with Hoechst solution (BD). Staining and imaging of sections were performed as described previously (15). For avidin–biotin complex staining, incubation with HRP-conjugated secondary antibodies was followed by DAB development (Dako).
Quantifications.
Quantification of total pancreatic beta-cell number was performed by first using flow cytometry on 2% of the complete pancreatic digests from MIP-GFP mice, and then multiplying that beta-cell number by 50. Total beta-cell number in the PDL-pancreas was determined by adding the head and tail pancreas beta-cell numbers, which were analyzed independently. Quantification by immunohistochemistry was performed on the basis of at least five sections that were 100 µm apart for each mouse. Here, quantification of beta-cell mass was performed as has been described previously (49). The pancreata were trimmed of all nonpancreatic tissue, weighed, fixed, and cryoprotected in 30% sucrose overnight before freezing in a way to allow longitudinal sections from tail to head of the pancreas to be obtained. Sections at 100-µm intervals from whole pancreas were immunostained for insulin and analyzed using ImageJ software. The relative cross-sectional area of beta cells was determined by quantification of the cross-sectional area occupied by beta cells divided by the cross-sectional area of total tissue. Each section was analyzed to estimate beta-cell and total tissue area. The beta-cell mass per pancreas was estimated as the product of the relative cross-sectional area of beta cells per total tissue and the weight of the pancreas. The beta-cell mass was calculated by examining pancreata from five animals for each group. Quantification of beta-cell proliferation was based on BrdU+ beta cells. Beta cells were determined by insulin, or both insulin and Nkx6.1 staining. At least 2,000 cells were counted for each mouse. If the percentage of positive cells was low, counting continued beyond 2,000 cells until at least 50 positive cells were counted. Five mice were analyzed in each experimental condition. All RT-qPCR data were from five samples for each condition.
Data Analysis.
All values are depicted as mean ± SEM. All data were statistically analyzed by two-tailed Student t test. Significance was considered when P < 0.05.
Supplementary Material
Acknowledgments
Special thanks to Alexis J. Styche, Robert J. Lakomy, Maria Branca, and Lauren Brink for technical assistance in flow cytometry, laser capture microdissection, confocal microscopy, and mouse genotyping. Thanks to Dr. Marilyn Diaz from National Institutes of Health (NIH) to generate and kindly provide SMAD7fx mice. This work was supported, in whole or in part, by Cochrane–Weber Endowed Fund in Diabetes Research Grant NO19831 (to X.X.), NIH Grants R01 DK083541 and R01 DK098196 (to G.K.G.), and the Children's Hospital of Pittsburgh Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.C.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321347111/-/DCSupplemental.
References
- 1.Pipeleers D, Ling Z. Pancreatic beta cells in insulin-dependent diabetes. Diabetes Metab Rev. 1992;8(3):209–227. doi: 10.1002/dmr.5610080303. [DOI] [PubMed] [Google Scholar]
- 2.Gaglia JL, Shapiro AM, Weir GC. Islet transplantation: Progress and challenge. Arch Med Res. 2005;36(3):273–280. doi: 10.1016/j.arcmed.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 4.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12(5):817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Meier JJ, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57(6):1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest. 2004;114(7):963–968. doi: 10.1172/JCI22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao X, et al. No evidence for β cell neogenesis in murine adult pancreas. J Clin Invest. 2013;123(5):2207–2217. doi: 10.1172/JCI66323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cozar-Castellano I, et al. Molecular control of cell cycle progression in the pancreatic beta-cell. Endocr Rev. 2006;27(4):356–370. doi: 10.1210/er.2006-0004. [DOI] [PubMed] [Google Scholar]
- 9.Sanvito F, et al. TGF-beta 1 influences the relative development of the exocrine and endocrine pancreas in vitro. Development. 1994;120(12):3451–3462. doi: 10.1242/dev.120.12.3451. [DOI] [PubMed] [Google Scholar]
- 10.Kim SK, et al. Activin receptor patterning of foregut organogenesis. Genes Dev. 2000;14(15):1866–1871. [PMC free article] [PubMed] [Google Scholar]
- 11.Miralles F, Battelino T, Czernichow P, Scharfmann R. TGF-beta plays a key role in morphogenesis of the pancreatic islets of Langerhans by controlling the activity of the matrix metalloproteinase MMP-2. J Cell Biol. 1998;143(3):827–836. doi: 10.1083/jcb.143.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Gohary Y, et al. Smad signaling pathways regulate pancreatic endocrine development. Dev Biol. 2013;378(2):83–93. doi: 10.1016/j.ydbio.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szabat M, Johnson JD. Modulation of β-cell fate and function by TGFβ ligands: A superfamily with many powers. Endocrinology. 2013;154(11):3965–3969. doi: 10.1210/en.2013-1880. [DOI] [PubMed] [Google Scholar]
- 14.Smart NG, et al. Conditional expression of Smad7 in pancreatic beta cells disrupts TGF-beta signaling and induces reversible diabetes mellitus. PLoS Biol. 2006;4(2):e39. doi: 10.1371/journal.pbio.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao X, et al. TGFβ receptor signaling is essential for inflammation-induced but not β-cell workload-induced β-cell proliferation. Diabetes. 2013;62(4):1217–1226. doi: 10.2337/db12-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Gohary Y, et al. A smad signaling network regulates islet cell proliferation. Diabetes. 2014;63(1):224–236. doi: 10.2337/db13-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan X, Chen YG. Smad7: Not only a regulator, but also a cross-talk mediator of TGF-β signalling. Biochem J. 2011;434(1):1–10. doi: 10.1042/BJ20101827. [DOI] [PubMed] [Google Scholar]
- 18.Afrakhte M, et al. Induction of inhibitory Smad6 and Smad7 mRNA by TGF-beta family members. Biochem Biophys Res Commun. 1998;249(2):505–511. doi: 10.1006/bbrc.1998.9170. [DOI] [PubMed] [Google Scholar]
- 19.Itoh S, ten Dijke P. Negative regulation of TGF-beta receptor/Smad signal transduction. Curr Opin Cell Biol. 2007;19(2):176–184. doi: 10.1016/j.ceb.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi H, et al. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell. 1997;89(7):1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S, et al. Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol Cell Biol. 2007;27(12):4488–4499. doi: 10.1128/MCB.01636-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kretzschmar M, Doody J, Massagué J. Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature. 1997;389(6651):618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- 24.Kretzschmar M, Doody J, Timokhina I, Massagué J. A mechanism of repression of TGFbeta/Smad signaling by oncogenic Ras. Genes Dev. 1999;13(7):804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulloa L, Doody J, Massagué J. Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway. Nature. 1999;397(6721):710–713. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- 26.Dai F, Lin X, Chang C, Feng XH. Nuclear export of Smad2 and Smad3 by RanBP3 facilitates termination of TGF-beta signaling. Dev Cell. 2009;16(3):345–357. doi: 10.1016/j.devcel.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bitzer M, et al. A mechanism of suppression of TGF-beta/SMAD signaling by NF-kappa B/RelA. Genes Dev. 2000;14(2):187–197. [PMC free article] [PubMed] [Google Scholar]
- 28.Kuang C, et al. In vivo disruption of TGF-beta signaling by Smad7 leads to premalignant ductal lesions in the pancreas. Proc Natl Acad Sci USA. 2006;103(6):1858–1863. doi: 10.1073/pnas.0508977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11(11):762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10(8):554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 32.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9(4):259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grunewald M, et al. VEGF-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell. 2006;124(1):175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 34.Xiao X, et al. Neurogenin3 activation is not sufficient to direct duct-to-beta cell transdifferentiation in the adult pancreas. J Biol Chem. 2013;288(35):25297–25308. doi: 10.1074/jbc.M113.484022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rankin MM, et al. β-Cells are not generated in pancreatic duct ligation-induced injury in adult mice. Diabetes. 2013;62(5):1634–1645. doi: 10.2337/db12-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solar M, et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. 2009;17(6):849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Kopp JL, et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138(4):653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chintinne M, et al. Beta cell count instead of beta cell mass to assess and localize growth in beta cell population following pancreatic duct ligation in mice. PLoS One. 2012;7(8):e43959. doi: 10.1371/journal.pone.0043959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kopinke D, et al. Lineage tracing reveals the dynamic contribution of Hes1+ cells to the developing and adult pancreas. Development. 2011;138(3):431–441. doi: 10.1242/dev.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desai BM, et al. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest. 2007;117(4):971–977. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan FC, et al. Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development. 2013;140(4):751–764. doi: 10.1242/dev.090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132(2):197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 43.Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes. 2009;58(6):1365–1372. doi: 10.2337/db08-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54(9):2557–2567. doi: 10.2337/diabetes.54.9.2557. [DOI] [PubMed] [Google Scholar]
- 45.Salpeter SJ, et al. Systemic regulation of the age-related decline of pancreatic β-cell replication. Diabetes. 2013;62(8):2843–2848. doi: 10.2337/db13-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou JX, et al. Combined modulation of polycomb and trithorax genes rejuvenates β cell replication. J Clin Invest. 2013;123(11):4849–4858. doi: 10.1172/JCI69468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Rooijen N, van Nieuwmegen R. Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. An enzyme-histochemical study. Cell Tissue Res. 1984;238(2):355–358. doi: 10.1007/BF00217308. [DOI] [PubMed] [Google Scholar]
- 48.van Rooijen N, van Kesteren-Hendrikx E. Clodronate liposomes: Perspectives in research and therapeutics. J Liposome Res. 2002;12(1-2):81–94. doi: 10.1081/lpr-120004780. [DOI] [PubMed] [Google Scholar]
- 49.Xiao X, et al. Hypoglycemia reduces vascular endothelial growth factor A production by pancreatic beta cells as a regulator of beta cell mass. J Biol Chem. 2013;288(12):8636–8646. doi: 10.1074/jbc.M112.422949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kushner JA, et al. Cyclins D2 and D1 are essential for postnatal pancreatic beta-cell growth. Mol Cell Biol. 2005;25(9):3752–3762. doi: 10.1128/MCB.25.9.3752-3762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He LM, et al. Cyclin D2 protein stability is regulated in pancreatic beta-cells. Mol Endocrinol. 2009;23(11):1865–1875. doi: 10.1210/me.2009-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kushner JA. Beta-cell growth: An unusual paradigm of organogenesis that is cyclin D2/Cdk4 dependent. Cell Cycle. 2006;5(3):234–237. doi: 10.4161/cc.5.3.2399. [DOI] [PubMed] [Google Scholar]
- 53.Guo P, et al. Rapid and simplified purification of recombinant adeno-associated virus. J Virol Methods. 2012;183(2):139–146. doi: 10.1016/j.jviromet.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo P, et al. A simplified purification method for AAV variant by polyethylene glycol aqueous two-phase partitioning. Bioengineered. 2013;4(2):103–106. doi: 10.4161/bioe.22293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo P, et al. Specific transduction and labeling of pancreatic ducts by targeted recombinant viral infusion into mouse pancreatic ducts. Lab Invest. 2013;93(11):1241–1253. doi: 10.1038/labinvest.2013.113. [DOI] [PubMed] [Google Scholar]
- 56.Miettinen PJ, et al. Impaired migration and delayed differentiation of pancreatic islet cells in mice lacking EGF-receptors. Development. 2000;127(12):2617–2627. doi: 10.1242/dev.127.12.2617. [DOI] [PubMed] [Google Scholar]
- 57.Brown ML, Schneyer AL. Emerging roles for the TGFbeta family in pancreatic beta-cell homeostasis. Trends Endocrinol Metab. 2010;21(7):441–448. doi: 10.1016/j.tem.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miettinen PJ, et al. Downregulation of EGF receptor signaling in pancreatic islets causes diabetes due to impaired postnatal beta-cell growth. Diabetes. 2006;55(12):3299–3308. doi: 10.2337/db06-0413. [DOI] [PubMed] [Google Scholar]
- 59.Nakao A, et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389(6651):631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 60.Callahan JF, et al. Identification of novel inhibitors of the transforming growth factor beta1 (TGF-beta1) type 1 receptor (ALK5) J Med Chem. 2002;45(5):999–1001. doi: 10.1021/jm010493y. [DOI] [PubMed] [Google Scholar]
- 61.Inman GJ, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62(1):65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 62.Laping NJ, et al. Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novel inhibitor of the TGF-beta type I receptor kinase activity: SB-431542. Mol Pharmacol. 2002;62(1):58–64. doi: 10.1124/mol.62.1.58. [DOI] [PubMed] [Google Scholar]
- 63.Pu YS, et al. Epidermal growth factor receptor inhibitor (PD168393) potentiates cytotoxic effects of paclitaxel against androgen-independent prostate cancer cells. Biochem Pharmacol. 2006;71(6):751–760. doi: 10.1016/j.bcp.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki T, et al. TGF-β signaling regulates pancreatic β-cell proliferation through control of cell cycle regulator p27 expression. Acta Histochem Cytochem. 2013;46(2):51–58. doi: 10.1267/ahc.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cavelti-Weder C, et al. Pancreatic duct ligation after almost complete β-cell loss: Exocrine regeneration but no evidence of β-cell regeneration. Endocrinology. 2013;154(12):4493–4502. doi: 10.1210/en.2013-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chung CH, Hao E, Piran R, Keinan E, Levine F. Pancreatic β-cell neogenesis by direct conversion from mature α-cells. Stem Cells. 2010;28(9):1630–1638. doi: 10.1002/stem.482. [DOI] [PubMed] [Google Scholar]
- 67.Hao E, Lee SH, Levine F. Efficient β-cell regeneration by a combination of neogenesis and replication following β-cell ablation and reversal of pancreatic duct ligation. Stem Cells. 2013;31(11):2388–2395. doi: 10.1002/stem.1492. [DOI] [PubMed] [Google Scholar]
- 68.Stopa M, et al. Participation of Smad2, Smad3, and Smad4 in transforming growth factor beta (TGF-beta)-induced activation of Smad7. The TGF-beta response element of the promoter requires functional Smad binding element and E-box sequences for transcriptional regulation. J Biol Chem. 2000;275(38):29308–29317. doi: 10.1074/jbc.M003282200. [DOI] [PubMed] [Google Scholar]
- 69.Brodin G, Ahgren A, ten Dijke P, Heldin CH, Heuchel R. Efficient TGF-beta induction of the Smad7 gene requires cooperation between AP-1, Sp1, and Smad proteins on the mouse Smad7 promoter. J Biol Chem. 2000;275(37):29023–29030. doi: 10.1074/jbc.M002815200. [DOI] [PubMed] [Google Scholar]
- 70.Park SH. Fine tuning and cross-talking of TGF-beta signal by inhibitory Smads. J Biochem Mol Biol. 2005;38(1):9–16. doi: 10.5483/bmbrep.2005.38.1.009. [DOI] [PubMed] [Google Scholar]
- 71.Hong S, et al. Smad7 binds to the adaptors TAB2 and TAB3 to block recruitment of the kinase TAK1 to the adaptor TRAF2. Nat Immunol. 2007;8(5):504–513. doi: 10.1038/ni1451. [DOI] [PubMed] [Google Scholar]
- 72.Wang C, et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412(6844):346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 73.Mazars A, et al. Evidence for a role of the JNK cascade in Smad7-mediated apoptosis. J Biol Chem. 2001;276(39):36797–36803. doi: 10.1074/jbc.M101672200. [DOI] [PubMed] [Google Scholar]
- 74.Kushner JA. The role of aging upon β cell turnover. J Clin Invest. 2013;123(3):990–995. doi: 10.1172/JCI64095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell. 2012;150(6):1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.