Significance
Carbapenem-resistant Klebsiella pneumoniae has emerged globally as a multidrug-resistant hospital pathogen for which there are few treatment options. Clinical isolates classified by multilocus sequence typing (ST) as ST258 are the most widespread. The basis for the success of ST258 organisms above and beyond antibiotic resistance is not known, nor is it clear whether infections are caused by a single clone. We used genome sequencing to reveal unexpected genetic diversity among ST258 organisms (thus disproving the single-clone hypothesis) and identified a recombination hotspot that accounts for the majority of divergence—and presumably for serologic variation—among ST258 clinical isolates. Our findings will facilitate the development of new clinical strategies designed to prevent or treat infections caused by multidrug-resistant K. pneumoniae.
Keywords: antibiotic resistance, carbapenemase, Enterobacteriaceae, plasmid
Abstract
Infections caused by drug-resistant bacteria are a major problem worldwide. Carbapenem-resistant Klebsiella pneumoniae, most notably isolates classified as multilocus sequence type (ST) 258, have emerged as an important cause of hospital deaths. ST258 isolates are predominantly multidrug resistant, and therefore infections caused by them are difficult to treat. It is not known why the ST258 lineage is the most prevalent cause of multidrug-resistant K. pneumoniae infections in the United States and other countries. Here we tested the hypothesis that carbapenem-resistant ST258 K. pneumoniae is a single genetic clone that has disseminated worldwide. We sequenced to closure the genomes of two ST258 clinical isolates and used these genomes as references for comparative genome sequencing of 83 additional clinical isolates recovered from patients at diverse geographic locations worldwide. Phylogenetic analysis of the SNPs in the core genome of these isolates revealed that ST258 K. pneumoniae organisms are two distinct genetic clades. This unexpected finding disproves the single-clone hypothesis. Notably, genetic differentiation between the two clades results from an ∼215-kb region of divergence that includes genes involved in capsule polysaccharide biosynthesis. The region of divergence appears to be a hotspot for DNA recombination events, and we suggest that this region has contributed to the success of ST258 K. pneumoniae. Our findings will accelerate research on novel diagnostic, therapeutic, and vaccine strategies designed to prevent and/or treat infections caused by multidrug resistant K. pneumoniae.
Antimicrobial resistance is a significant problem for treatment of infectious diseases worldwide. Carbapenem-resistant Enterobacteriaceae, especially Klebsiella pneumoniae, have emerged as important causes of morbidity and mortality among hospital-acquired and long-term care–associated infections (1, 2). Notably, there is very high mortality (∼30–70%) among patients with bacteremia or pulmonary infections caused by carbapenemase-producing K. pneumoniae (1, 3–7). The large majority of these isolates are characterized genetically as multilocus sequence type 258 (ST258) and are presumed to be clonally related by descent (1, 8, 9). Strains of ST258 are resistant to all β-lactam antibiotics and typically contain plasmid-borne genes that encode aminoglycoside-modifying enzymes and chromosomal mutations that confer fluoroquinolone resistance (2). Carbapenem resistance in ST258 strains is predominantly encoded by K. pneumoniae carbapenemase (KPC), and the gene, blaKPC, is located on a transposable element (Tn4401) that is integrated into many different plasmids (10, 11). Thus, blaKPC (along with other plasmid-associated resistance elements) is exchanged readily among K. pneumoniae and other Enterobacteriaceae, a feature key to the spread and high prevalence of multidrug-resistant strains (12).
Infections caused by carbapenem-resistant K. pneumoniae occur primarily in individuals who have significant clinical risk factors for acquiring these pathogens. These attributes suggest that disease is caused by poor/defective host defense rather than by enhanced bacterial virulence or transmissibility. However, there are no data that bear on these issues, nor is there a clear explanation for the predominance of ST258 globally. Therefore, it is possible that there has been clonal dissemination of an ST258 strain or clone that has high transmissibility and/or enhanced capacity to circumvent killing by the innate immune system and/or to escape the humoral immune response by antigenic variation. To test the clonal-dissemination hypothesis, we sequenced to closure the genomes of two KPC-producing ST258 K. pneumoniae clinical isolates and then performed comparative whole-genome sequencing of 83 additional K. pneumoniae clinical isolates obtained from human infections at diverse geographic locations worldwide. Our data provide information that is important for our understanding of the success of ST258 as a human pathogen, extending beyond antibiotic resistance.
Results and Discussion
Genome Sequence of Carbapenem-Resistant ST258 K. pneumoniae Clinical Isolates.
The two reference ST258 K. pneumoniae clinical isolates, one with mucoid and the other with nonmucoid colony morphology, were obtained in 2010 from patients with urinary tract infections at two separate healthcare institutions in New Jersey (Dataset S1). The genomes of these two KPC-producing ST258 isolates were 5,266,518 bp for the isolate named NJST258_1 and 5,293,301 bp for the isolate named NJST258_2 (Fig. 1), similar in length to other K. pneumoniae genomes (range, 5.3–5.6 Mbp) (13–15).
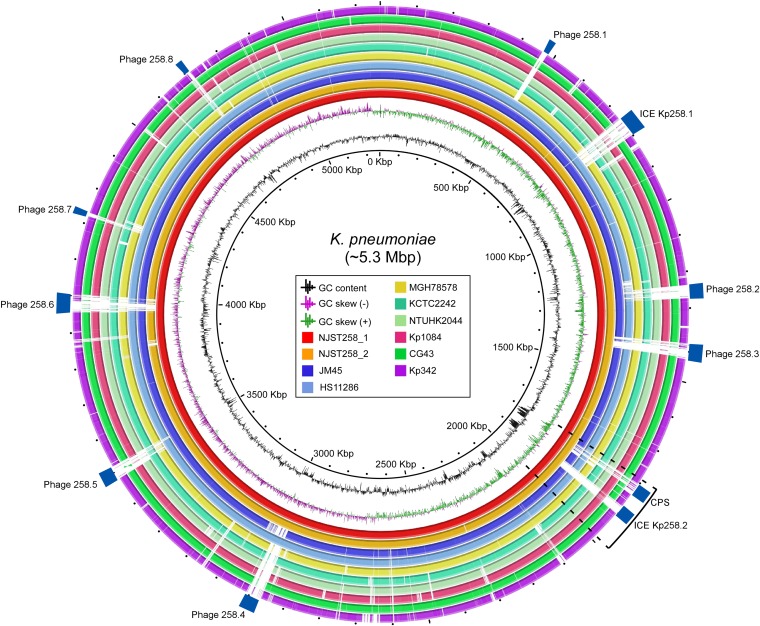
Fig. 1.
K. pneumoniae genomes. Selected K. pneumoniae genomes were compared with the two reference ST258 genomes, NJST258_1 and NJST258_2 (red and orange inner circles). Notable features, such as prophages (Phage) and other MGEs or variable regions, are indicated by rectangles. An RD is indicated by the bracket and dotted lines.
NJST258_1 has eight putative prophages (phages 1–8, designated 258.1–8), 22 insertion sequences (IS), and two integrated conjugative elements (ICE) (Fig. 1 and Table 1). NJST258_2 lacks one of these prophages (a phage analogous to 258.6, i.e., phage 6) and has three fewer IS, but the MGE content otherwise is similar to that of NJST258_1 (Fig. 1 and Table 1). ICE Kp258.1 and phages 258.1, 258.5, 258.7, and 258.8 also are present in ST11 K. pneumoniae strains (ST11 is a single-locus variant of ST258), HS11286 (GenBank accession no.: CP003200), and JM45 (accession no.: CP006656). Moreover, prophages 258.2 and 258.4 are present in JM45 and HS11286, respectively. However, these prophage elements are largely divergent in or absent from several other K. pneumoniae strains, including CG43, KCTC2242, Kp1084, Kp342, MGH78578, and NTUH-K2044 (Fig. 1). Whether the prophages or other mobile genetic elements (MGEs) present in the ST258 lineage have contributed to its recent success remains to be determined.
Table 1.
Key features of the ST258 genome
| NJST258_1 | NJST258_2 | |
| Size, bp | 5,266,518 | 5,293,301 |
| G+C content, % | 57.4 | 57.5 |
| No. of CDS | 5,497 | 5,454 |
| rRNA,n | ||
| 16S | 8 | 8 |
| 23S | 8 | 8 |
| 5S | 10 | 9 |
| tRNA, n | 77 | 86 |
| Plasmids, n | 5 | 3 |
| Prophages, n | 8 | 7 |
| Phage258.1 | + | + |
| Phage258.2 | + | + |
| Phage258.3 | + | + |
| Phage258.4 | + | + |
| Phage258.5 | + | + |
| Phage258.6 | + | — |
| Phage258.7 | + | + |
| Phage258.8 | + | + |
| ICEs, n | 2 | 2 |
| ICE258.1 | + | + |
| ICE258.2 | + | + |
| IS elements, n | 22 | 19 |
| IS1294 | 3 | 2 |
| ISKpn1 | 5 | 5 |
| IS5075 | 1 | 0 |
| ISKpn18 | 2 | 2 |
| ISSm1 | 1 | 0 |
| IS903 | 2 | 2 |
| IS5 | 7 | 7 |
| IS1400 | 1 | 1 |
CDS, coding sequences; ICE, integrated conjugative element; IS, insertion sequence. Data refer to the chromosome only, except where plasmids are indicated.
Plasmid and Genome-Encoded Antibiotic Resistance.
NJST258_1 and NJST258_2 are resistant to β-lactam antibiotics (including carbapenem antibiotics) and β-lactamase inhibitors (e.g., clavulanate and tazobactam), quinolones (ciprofloxacin and levofloxacin), and aminoglycosides (amikacin and tobramycin). NJST258_1 also is resistant to trimethoprim-sulfamethoxazole, gentamicin, minocycline, colistin, and polymyxin B but is susceptible to tigecycline (Dataset S1 and Table S1). We discovered that many of the antibiotic-resistance determinants are encoded on plasmids in these two strains.
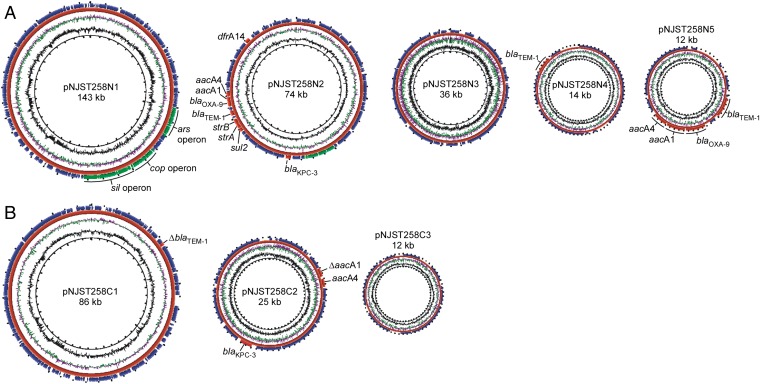
NJST258_1 and NJST258_2 contain five plasmids (pNJST258N1–pNJST258N5) and three plasmids (pNJST258C1–pNJST258C3), respectively, which vary in size from 10,925–142,768 bp and collectively encode resistance to multiple classes of antibiotics and heavy metals (Fig. 2 and Table S1). Five plasmids were identified in strain NJST258_1: pNJST258N1 (142,788 bp), pNJST258N2 (73,636 bp), pNJST258N3 (36,109 bp), pNJST258N4 (14,249 bp), and pNJST258N5 (10,925 bp) (Fig. 2A). pNJST258N2 has nine resistance determinants, including blaKPC-3, blaTEM-1, blaOXA-9 (β-lactam resistance), aacA4, aadA1, strA, strB (aminoglycoside modifying enzymes), sul2 (sulfonamide resistance), and dfrA14 (trimethoprim resistance), and a nickel-resistance operon (Table S1). pNJST258N4 has blaTEM-1, and pNJ258N5 has blaTEM-1, blaOXA-9, aacA4, and aadA1.
Fig. 2.
Plasmids and multidrug resistance. Plasmids present in NJST258_1 (A) and NJST258_2 (B). Antibiotic and heavy metal-resistance elements are indicated for each plasmid.
The three plasmids identified in NJST258_2 [pNJST258C1 (86,232 bp), pNJST258C2 (25,284 bp), and pNJST258C3 (12,399 bp)] collectively contained fewer antibiotic and/or heavy metal resistance determinants than did the plasmids in strain NJST258_1 (Fig. 2B). A single copy of blaSHV-11 was identified on the chromosome of NJST258_2.
The quinolone resistance-determining regions in both genomes, including gyrA, gyrB, parC, and parE genes, contained several amino acid replacements (Ser83Ile and Asp87Gly in GyrA, and Ser80Ile in ParC for NJST258_1; Ser83Ile and Asp87Asn in GyrA, and Ser80Ile in ParC for NJST258_2) that likely explain quinolone resistance in these strains. Taken together, our data indicate that NJST258_1 and NJST258_2 are carbapenem-resistant K. pneumoniae clinical isolates that encode extensive resistance to other classes of antibiotics, resulting in the critical multidrug resistance phenotype.
Genome Sequencing of Geographically Diverse ST258 Clinical Isolates.
To gain a better understanding of the emergence and evolution of the ST258 clone, we next performed whole-genome DNA sequencing of 83 additional K. pneumoniae clinical isolates (Dataset S1) and mapped the DNA sequence reads to reference strain NJST258_1. Isolates were selected based on the geographic and temporal distributions, KPC variants, and Tn4401 patterns. These clinical isolates were cultured from blood, urine, sputum, or skin of infected individuals or from a rectal swab over a time period encompassing 2003–2012 (Dataset S1). Isolates were obtained from healthcare facilities at diverse geographic locations in the United States (Florida, Illinois, Pennsylvania, New Jersey, New York, and Texas), Canada, Colombia, and Italy. Seventy-five (89%) of these strains were ST258; the others were ST379 (4), ST512 (4), or ST418 (1)—all single-locus variants of ST258.
There were 2,436 unique SNPs in the core genome (defined here as all nucleotide positions except those in plasmids and MGEs, which includes prophages, ICEs, and ISs) of all 84 isolates (including NJST258_2) compared with reference isolate NJST238_1 (Datasets S2 and S3). The 84 query isolates differed from NJST258_1 on average by 350 SNPs (range, 116–784 SNPs) in the core genome, indicating that the isolates are closely related (Dataset S3). In comparison, we previously reported less core genome genetic diversity—an average of ∼50 SNPs—among isolates of the epidemic community-associated methicillin-resistant Staphylococcus aureus clone, USA300 (16). Of the 2,436 unique SNPs among all isolates, 310 (12.7%) were intergenic, 881 (36.2%) were synonymous, and 1,245 (51.1%) were nonsynonymous. Details about the location of SNPs and ratio of nonsynonymous-to-synonymous SNPs are provided below.
Phylogenetic Analyses.
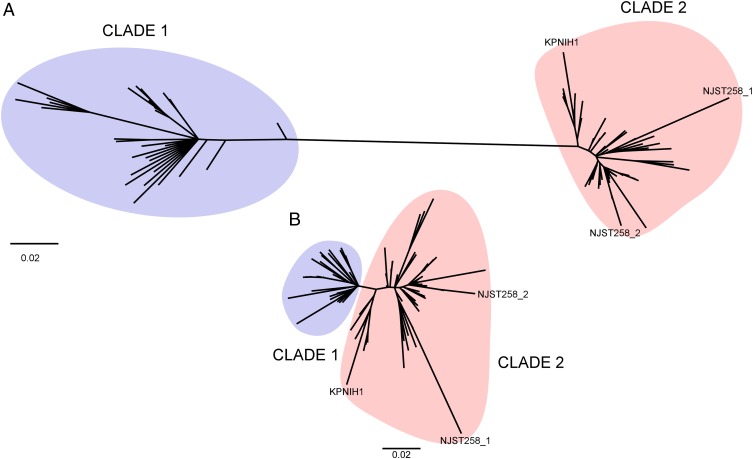
To estimate genetic relationships among the 85 K. pneumoniae clinical isolates (83 query isolates plus NJST258_2 and NJST258_1), we performed phylogenetic analyses using concatenated SNP nucleotides present in the core genome (Fig. 3). All isolates segregated into two distinct subclades, herein referred to as clade 1 and clade 2 (Fig. 3A and Dataset S3). Isolates within clade 1 differed on average by 136 SNPs (range, 29–291), and those within clade 2 differed by an average of 82 SNPs (range, 2–231). In comparison, the two clades differed from each other by an average of 529 SNPs (range, 310–784). Moreover, 96.3% of the KPC-producing isolates within clade 1 primarily contained plasmids encoding KPC2, whereas 96.2% of those in clade 2 encoded KPC3 (Dataset S1). Collectively, the data indicate that the two lineages have had distinct evolutionary histories and thus emerged independently. These findings were unexpected, because the ST258 lineage has been considered to be a single clone or strain (1, 8, 9).
Fig. 3.
SNP-based phylogenetic analysis of ST258 clinical isolates. (A) Phylogenetic analysis of 86 K. pneumoniae clinical isolates [85 study isolates and KPNIH1, an ST258 isolate originally recovered from a patient at the National Institutes of Health Clinical Center (4)] based on 2,436 unique, concatenated SNP nucleotides present in the core genome (excluding MGEs) of the 85 study isolates (Dataset S1). KPNIH1 was included because of its clinical significance. (B) Phylogenetic analysis of the clinical isolates shown in A but excluding the RD (∼215 kb). NJST258_1 is the fully closed mucoid ST258 reference strain to which all isolates are compared (see Fig. 1). NJST258_2 is the fully closed nonmucoid ST258 strain as presented in Fig. 1.
It also is noteworthy that genes encoding KPC2 and KPC3 are associated primarily with three blaKPC-harboring Tn4401 isoforms and plasmid integration sites (ISS) on blaKPC-harboring plasmids in the ST258 strains. Two of these blaKPC-harboring elements (Tn4401d-ISS-1 and Tn4401b-ISS-2) were associated exclusively with clade 2 (Dataset S1), supporting the idea that spread of the KPC gene occurs at least in part through clonal dissemination of ST258 strains. On the other hand, some plasmids [e.g., a pKpQIL-like plasmid (Dataset S1)] are present in both clades, suggesting there has been horizontal acquisition of the same or similar plasmids into each clade.
The ratio of nonsynonymous-to-synonymous SNPs was 1.4:1 among all isolates, but the ratios for the two clades (excluding MGEs) were quite different. The ratio of nonsynonymous-to-synonymous SNPs was 0.9:1 in the core genome of clade 1 but was 2.4:1 for clade 2, underscoring the notion that the two clades have had different evolutionary histories. Moreover, the relatively high ratio of nonsynonymous-to-synonymous SNPs in clade 2 suggests these isolates have undergone recent diversification. These findings are consistent with the recent prominence of KPC3-containing strains as a cause of human infections.
Identification of an ∼215-kbp Region of Divergence.
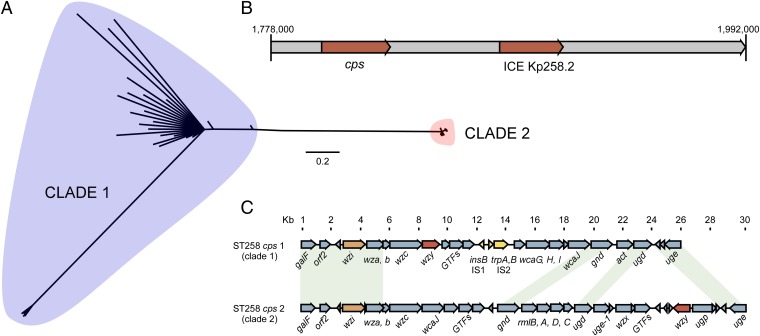
To understand better the molecular processes that underlie the distinct evolution of clades 1 and 2, we analyzed the distribution of SNPs in specific genes and/or regions of the core genome. Unexpectedly, we found an ∼215-kbp region of the ST258 genome (nucleotide position 1778210–1992999 in strain NJST258_1) that had a disproportionate number of SNPs (664 SNPs, approximately one SNP per 324 nucleotides), compared with those present in the remaining ∼5 Mbp of the genome (1,772 SNPs, approximately one SNP per 2,850 nucleotides) (Dataset S2). Phylogenetic analysis of the core genome minus this region of divergence (RD) and analysis of the RD alone revealed that the genetic differentiation in the core genomes between the two clades is largely attributed to SNPs in the RD (Figs. 3A and 4A). Notably, the ratio of nonsynonymous-to-synonymous SNPs within the RD was 0.2:1 for clade 1 and 3.9:1 for clade 2, whereas that for the reminder of the core genome these ratios were 2.2:1 and 2.4:1, respectively. These results suggest that the RD present in clade 1 isolates has undergone purifying selection and, more importantly, provide strong support for the idea that the RD of clade 1 likely originated from an unrelated K. pneumoniae donor strain. In comparison, the RD in clade 2 has undergone recent diversification (based on the relatively high ratio of nonsynonymous-to-synonymous SNPs), and this high ratio of nonsynonymous-to-synonymous SNPs is more similar to that of the core genomes of either reference strain than to the RD of clade 1. These findings indicate different evolutionary histories for clade 1 and clade 2, a finding that changes our fundamental understanding of the origins of the epidemic carbapenem-resistant ST258 organisms.
Fig. 4.
RD. (A) Phylogenetic analysis of 85 ST258 (or single-locus multilocus sequence type variants) K. pneumoniae clinical isolates based upon 664 unique, concatenated SNP nucleotides present in the RD alone. (B) Linear schematic of the RD indicating the location of the genes encoding the cps biosynthesis enzymes and ICE Kp258.2. (C) Comparison of the cps regions in clades 1 and 2.
Capsule polysaccharide (CPS) is a well-known contributor to the success of K. pneumoniae as a pathogen, in part because the variation in genes that encode enzymes involved in CPS synthesis results in serologic diversity. Notably, the CPS gene island, which encodes the capsule K-antigen in K. pneumoniae, is located within the RD (Fig. 1 and Fig. 4B). Currently, more than 78 different K-serotypes have been identified, some of which are significantly associated with serious human infections [e.g., K1 and K2 are associated with pyogenic liver abscess in Asian populations (17)]. Unexpectedly, we identified two distinct cps gene clusters in the genomes of the ST258 clinical isolates (ST258 cps 1 in clade 1 and cps 2 in clade 2) (Fig. 4C). The genetic differences between these two cps clusters are primarily responsible for the segregation of ST258 isolates into two distinct clades (Fig. 4 A and C).
To investigate further the genetic differences between the ST258 cps 1 and cps 2 regions, we aligned the DNA sequences of the wzy gene, which encodes the capsule polymerase, the core enzyme in capsule biosynthesis (16). The nucleotide similarity between cps 1 and cps 2 was only 44.5%, confirming the high degree of genetic variation in this region of the genome. We next designed cps 1- and cps 2-specific multiplex PCR primers to screen for the presence of wzy among our collection of ∼500 clinical K. pneumoniae isolates collected from 1995 to the present from more than 10 hospitals in New York and New Jersey. As expected, the wzy PCR assay differentiated ST258 strains into cps 1 and cps 2 types, but, surprisingly, the cps 1 wzy primers produced a positive PCR amplicon among seven of the ST42 K. pneumoniae isolates tested (collected in 2001–2002 from New York City hospitals). Further sequencing of the full-length wzy gene and the neighboring cps wzi gene in these seven ST42 isolates revealed that these genes had 100% identity to the ST258 cps 1 genes. One possible explanation for these observations is that the cps and RD present in the clade 1 ST258 isolates originated from the ST42 lineage, presumably as the result of a relatively large recombination event.
Concluding Remarks.
Carbapenem-resistant Enterobacteriaceae—most notably ST258 K. pneumoniae—now are classified as an “Urgent Threat” by the Centers for Disease Control and Prevention (CDC) (18). Although the absolute number of estimated deaths caused by carbapenem-resistant Enterobacteriaceae is relatively low (in the United States 610 deaths annually versus 11,000 caused by methicillin-resistant S. aureus and 14,000 caused by Clostridium difficile), the potential exists for transfer of multidrug resistance to Gram-negative community-associated pathogens such as Escherichia coli. As suggested by the CDC report on antibiotic resistance threats, a multitiered approach is needed to prevent or moderate the threat posed by carbapenem-resistant Enterobacteriaceae (18). Such an approach includes understanding the evolutionary genomic history of the microbe as a means to gain new insight into its success as a human pathogen, as we have done herein. Unexpectedly, we discovered that the ST258 “strain” is comprised of at least two distinct lineages or clades rather than being a single clone as previously suggested (1, 8, 9). The two clades are differentiated largely by an RD that encodes CPS biosynthesis machinery (cps 1 and cps 2), and this region of the genome was introduced into clade 1 by a relatively large chromosome recombination event (horizontal gene transfer) or possibly by multiple recombination events. Thus, independent acquisition of genetic material by an ancestral ST258 clone (rather than within-ST258 diversification) is the primary basis for segregation of these isolates into two clades.
Chromosomal recombination events are known to contribute to the genetic plasticity and ultimate success of many bacterial pathogens. For example, the origin and emergence of a highly successful serotype M1 group A Streptococcus clone was linked in part to the acquisition of a 36-kb region of DNA that contains NAD+-glycohydrolase and streptolysin O, which are proven streptococcal virulence factors (19). The basis for the success of ST258 (and related single-locus variants) as a prominent human pathogen worldwide has remained unclear, because carbapenem resistance is not unique to this multilocus sequence type and has been acquired by numerous other K. pneumoniae lineages. The large (∼215 kb) RD of K. pneumoniae reported herein contains the genes encoding CPS biosynthesis, which is one of the primary determinants of antigenicity and antigenic diversity among K. pneumoniae. Notably, capsule polysaccharides of K. pneumoniae contribute to the evasion of innate host defense and thus are known to be important for survival in the host (20–22). We speculate that the cps-containing RD is a factor that contributes to the global success of ST258 above and beyond antibiotic resistance. Preliminary studies with human neutrophils in vitro revealed little or no killing of isolates from clades 1 and 2 under our assay conditions, although there was some isolate-to-isolate variance (Fig. S1). Further investigation is needed to elucidate the basis for the inability of neutrophils to kill K. pneumoniae in vitro and to determine whether isolates from these clades have enhanced capacity to circumvent killing by neutrophils as compared with isolates representative of other K. pneumoniae lineages.
Our molecular deconvolution of the evolutionary history of ST258 strains was made possible by sequencing to closure two representative carbapenem-resistant ST258 clinical isolates, coupled with our comparative genomics investigative strategy. This information will assist our efforts to develop diagnostics, therapeutics, and vaccines designed to diagnose, treat, and/or prevent infections caused by multidrug resistant K. pneumoniae.
Materials and Methods
For full details, see SI Materials and Methods.
K. pneumoniae Isolates.
We selected two representative ST258 carbapenem-resistant K. pneumoniae isolates for genome sequencing based upon the presence of KPC3 and mucoid and nonmucoid phenotypes. The selection of the isolates used for comparative genome sequencing was based on their geographical and temporal distributions, KPC variants, and Tn4401 isoforms (Dataset S1). K. pneumoniae isolates were obtained from healthcare facilities at diverse geographic locations in the United States, Canada, Colombia, and Italy.
Characterization of K. pneumoniae Strains.
Minimum inhibitory concentrations of K. pneumoniae isolates were determined by broth microdilution in cation-adjusted Mueller–Hinton broth using Sensititre GNX2F panels (Thermo Fisher Scientific) according to Clinical and Laboratory Standards Institute methods and interpretations (23, 24).
K. pneumoniae de Novo DNA Sequencing.
The genomes of K. pneumoniae strains NJST258_1 and NJST258_2 were sequenced using Roche 454/Life Sciences GS FLX (454 Life Sciences) and Illumina HisEq 2000 (Illumina) sequencers. The genome and plasmid DNA sequences of NJST258_1 and NJST258_2 have been deposited in GenBank under the National Center for Biotechnology Information (NCBI) accession nos. CP006923 (NJST258_1 chromosome), CP006927 (pNJST258N1), CP006926 (pNJST258N2), CP006925 (pNJST258N3), CP006928 (pNJST258N4), and CP006924 (pNJST258N5) and NCBI accession nos. CP006918 (NJST258_2 chromosome), CP006922 (pNJ258C1), CP006919 (pNJ258C2), and CP006921 (pNJ258C3).
K. pneumoniae Comparative Genome Sequencing.
Genome sequencing for 83 query ST258 K. pneumoniae isolates was performed using an Illumina HisEq 2000 sequencer as described in SI Materials and Methods. Metadata were deposited in the NCBI sequence read archive (SRA) (www.ncbi.nlm.nih.gov/sra) under the accession no. SRP036874.
Supplementary Material
Acknowledgments
We thank Dan Bruno, Stacy Ricklefs, Jennifer Hashimoto, and Kent Barbian of the National Institute of Allergy and Infectious Diseases (NIAID) for technical assistance with genome sequencing and Anita Mora (NIAID) and Heather Murphy (NIAID) for assistance with graphic illustration. This work was supported in part by National Institutes of Health (NIH) Grant 1R01AI090155 (to B.N.K.), and by the Intramural Research Program of the NIAID, NIH.
Footnotes
Conflict of interest statement: B.N.K. holds two patents that focus on using DNA sequencing to identify bacterial pathogens.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession numbers CP006918, CP006919, CP006921, CP006922, CP006923, and CP006925–CP006928) and in the National Center for Biotechnology Information Sequence Read Archive (accession no. SRP036874).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321364111/-/DCSupplemental.
References
- 1.Munoz-Price LS, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13(9):785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Endimiani A, et al. Emergence of blaKPC-containing Klebsiella pneumoniae in a long-term acute care hospital: A new challenge to our healthcare system. J Antimicrob Chemother. 2009;64(5):1102–1110. doi: 10.1093/jac/dkp327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee GC, Burgess DS. Treatment of Klebsiella pneumoniae carbapenemase (KPC) infections: A review of published case series and case reports. Ann Clin Microbiol Antimicrob. 2012;11:32. doi: 10.1186/1476-0711-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-David D, et al. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect. 2012;18(1):54–60. doi: 10.1111/j.1469-0691.2011.03478.x. [DOI] [PubMed] [Google Scholar]
- 5.Snitkin ES, et al. NISC Comparative Sequencing Program Group Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med. 2012;4(148):ra116. doi: 10.1126/scitranslmed.3004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borer A, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol. 2009;30(10):972–976. doi: 10.1086/605922. [DOI] [PubMed] [Google Scholar]
- 7.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29(12):1099–1106. doi: 10.1086/592412. [DOI] [PubMed] [Google Scholar]
- 8.Kitchel B, et al. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: Clonal expansion of multilocus sequence type 258. Antimicrob Agents Chemother. 2009;53(8):3365–3370. doi: 10.1128/AAC.00126-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuzon G, et al. Worldwide diversity of Klebsiella pneumoniae that produce beta-lactamase blaKPC-2 gene. Emerg Infect Dis. 2010;16(9):1349–1356. doi: 10.3201/eid1609.091389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuzon G, Naas T, Nordmann P. Functional characterization of Tn4401, a Tn3-based transposon involved in blaKPC gene mobilization. Antimicrob Agents Chemother. 2011;55(11):5370–5373. doi: 10.1128/AAC.05202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naas T, et al. Genetic structures at the origin of acquisition of the beta-lactamase bla KPC gene. Antimicrob Agents Chemother. 2008;52(4):1257–1263. doi: 10.1128/AAC.01451-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: An evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25(4):682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fouts DE, et al. Complete genome sequence of the N2-fixing broad host range endophyte Klebsiella pneumoniae 342 and virulence predictions verified in mice. PLoS Genet. 2008;4(7):e1000141. doi: 10.1371/journal.pgen.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu KM, et al. Genome sequencing and comparative analysis of Klebsiella pneumoniae NTUH-K2044, a strain causing liver abscess and meningitis. J Bacteriol. 2009;191(14):4492–4501. doi: 10.1128/JB.00315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu P, et al. Complete genome sequence of Klebsiella pneumoniae subsp. pneumoniae HS11286, a multidrug-resistant strain isolated from human sputum. J Bacteriol. 2012;194(7):1841–1842. doi: 10.1128/JB.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy AD, et al. Epidemic community-associated methicillin-resistant Staphylococcus aureus: Recent clonal expansion and diversification. Proc Natl Acad Sci USA. 2008;105(4):1327–1332. doi: 10.1073/pnas.0710217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: A new invasive syndrome. Lancet Infect Dis. 2012;12(11):881–887. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention (2013) Antibiotic Resistance Threats in the United States, 2013. Available at: www.cdc.gov/drugresistance/threat-report-2013/ Accessed February 27, 2014.
- 19.Sumby P, et al. Evolutionary origin and emergence of a highly successful clone of serotype M1 group a Streptococcus involved multiple horizontal gene transfer events. J Infect Dis. 2005;192(5):771–782. doi: 10.1086/432514. [DOI] [PubMed] [Google Scholar]
- 20.Domenico P, Salo RJ, Cross AS, Cunha BA. Polysaccharide capsule-mediated resistance to opsonophagocytosis in Klebsiella pneumoniae. Infect Immun. 1994;62(10):4495–4499. doi: 10.1128/iai.62.10.4495-4499.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollack M. Significance of circulating capsular antigen in Klebsiella infections. Infect Immun. 1976;13(6):1543–1548. doi: 10.1128/iai.13.6.1543-1548.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simoons-Smit AM, Verweij-van Vught AM, MacLaren DM. The role of K antigens as virulence factors in Klebsiella. J Med Microbiol. 1986;21(2):133–137. doi: 10.1099/00222615-21-2-133. [DOI] [PubMed] [Google Scholar]
- 23.Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard. 9th Ed. Wayne, PA: CLSI; 2012. pp. M07–A9. [Google Scholar]
- 24. Clinical and Laboratory Standards Institute (2013) Performance standards for antimicrobial susceptibility testing. Twenty-third informational supplement, M100-S23. (CLSI, Wayne, PA)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.