Significance
In this paper, we describe the biophysical properties, stoichiometry, and activity of the Escherichia coli SecYEG–SecDF–YajC–YidC holo-translocon. This multiprotein complex consists of seven membrane protein subunits, including those components responsible for both protein secretion (SecYEG) and membrane protein insertion (YidC). We demonstrate the isolation of a stable complex containing YidC together with the core SecY translocon. The availability of this intact assembly allows us to reconstitute posttranslational protein export and cotranslational membrane protein insertion from purified components of known stoichiometry. The experiments demonstrate that protein secretion and insertion occur through a single complex. The reconstitution of membrane protein insertion from defined components is a novel development, breaking ground for the functional analysis of this largely unknown process.
Abstract
The SecY/61 complex forms the protein-channel component of the ubiquitous protein secretion and membrane protein insertion apparatus. The bacterial version SecYEG interacts with the highly conserved YidC and SecDF–YajC subcomplex, which facilitates translocation into and across the membrane. Together, they form the holo-translocon (HTL), which we have successfully overexpressed and purified. In contrast to the homo-dimeric SecYEG, the HTL is a hetero-dimer composed of single copies of SecYEG and SecDF–YajC–YidC. The activities of the HTL differ from the archetypal SecYEG complex. It is more effective in cotranslational insertion of membrane proteins and the posttranslational secretion of a β-barreled outer-membrane protein driven by SecA and ATP becomes much more dependent on the proton-motive force. The activity of the translocating copy of SecYEG may therefore be modulated by association with different accessory subcomplexes: SecYEG (forming SecYEG dimers) or SecDF–YajC–YidC (forming the HTL). This versatility may provide a means to refine the secretion and insertion capabilities according to the substrate. A similar modularity may also be exploited for the translocation or insertion of a wide range of substrates across and into the endoplasmic reticular and mitochondrial membranes of eukaryotes.
The essential SecY/61 complex selectively orchestrates the passage of newly synthesized proteins across and into the cytoplasmic and endoplasmic reticular (ER) membranes of prokaryotes and eukaryotes, respectively. Protein translocation is driven by associated cotranslating ribosomes or by specialized energy-transducing factors, such as the bacterial ATPase SecA. The protein-conducting channel is formed by a monomer of the SecYEG complex, encapsulated by two halves of SecY (1). The separation of transmembrane segments (TMSs) 1–5 from 6–10 along with the displacement of a central plug could help form a channel through the membrane to the outside, as well as laterally into the bilayer (1). SecYEG forms dimers in the membrane (2), required for association and activation of SecA (3–5). However, in vivo, the passive SecYEG complex is not rigidly fixed to the translocating copy, nor is it absolutely essential for transport (6).
Escherichia coli inner membranes harbor a “holo-translocon” (HTL) containing SecYEG and SecDF–YajC (7). YidC is a ubiquitous and essential membrane protein “insertase” (8, 9) that functions in concert with SecYEG during the biogenesis of many inner membrane (IM) proteins (10–12). In contrast, the insertion of small polypeptides such as the M13 procoat, Pf3 coat protein, and subunit c of the F1FO-ATP synthase are thought to occur through YidC alone (13–15). YidC and SecYEG may be bridged in the HTL by a subcomplex consisting of SecD, SecF, and YajC (16). SecDF may also act in the regulation of the interaction and activity of SecA with SecYEG (7, 17, 18). Like SecYEG, SecDF is thought to transduce the energy available in the transmembrane proton-motive force (PMF) to stimulate translocation (19–21), analogous to other members of the Resistance-Nodulation-Cell Division (RND) superfamily also conferring PMF-driven substrate efflux (22).
We have developed an expression system (23) that allows the simultaneous overexpression of all seven membrane proteins comprising the HTL. Its purification allows the exploration of unknown aspects of its organization, activity, and bioenergetics, providing insights into the general secretion and membrane protein insertion machinery.
Results
Production and Purification of the HTL: A Membrane Protein Complex of SecYEG–SecDF–YajC–YidC.
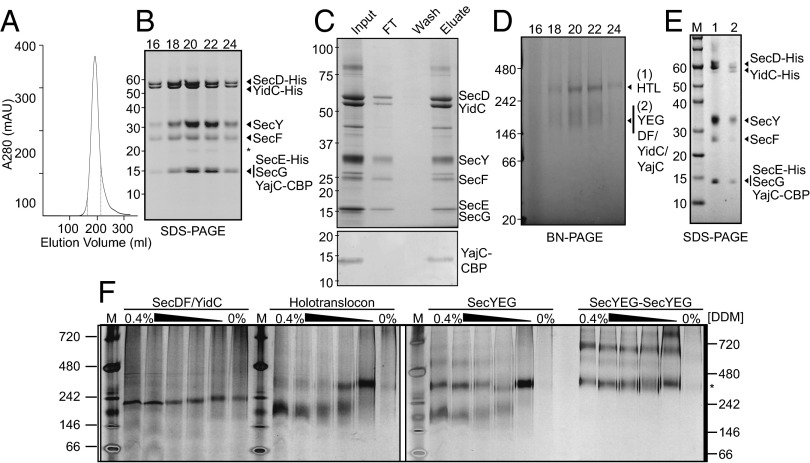
By using ACEMBL, a multigene expression system for complex production in E. coli (23), we constructed a single plasmid encoding and allowing for high-level expression of all seven subunits of the HTL (Fig. S1). The complex was purified by Ni2+ chromatography followed by size exclusion combined with an anion exchange step. The HTL eluted as a symmetrical peak in detergent (n-dodecyl-β-d-maltoside, DDM), characteristic of a single complex (Fig. 1A). All components of the complex but YajC were clearly visible by SDS/PAGE (Fig. 1B). The integrity of the complete complex was verified by applying the purified sample (input) onto a calmodulin column, which retained all seven subunits by virtue of the calmodulin binding peptide (CBP) attached to the C terminus of YajC (Fig. 1C and Fig. S1).
Fig. 1.
Purification of the E. coli HTL. (A) Representative gel filtration/ion exchange elution profile of the HTL (SI Materials and Methods) showing a single peak, demonstrating the copurification of all HTL components in a single complex. (B) SDS/PAGE representation of peak fractions 16–24 (corresponding to elution volumes 165–215 mL of the profile displayed in A, dotted lines). The asterisk represents a proteolytic fragment of SecY. (C) Coomassie-stained SDS/PAGE demonstrating that the purified complex (input) can be reisolated by running the HTL over a calmodulin column (Upper). Detection of the CBP peptide shows that YajC is indeed present in this complex (Lower). (D) BN-PAGE representation of peak fractions 16–24 from B. (E) Second-dimension SDS/PAGE of gel slices taken from the higher-MW (lane 1) and lower-MW (lane 2) complexes seen in D. Note that the slight shift in MW for all components of the HTL is the result of a gel slice being placed into the wells for the run. (F) BN-PAGE illustrating the susceptibility of various complexes [SecDF–YidC, HTL, SecYEG, or a covalently-linked dimer of SecYEG (38)] to changes in detergent concentration. Fifty nanograms of complexes purified in low concentrations of detergent (0.02–0.05%) were resuspended in TSG130 buffer containing decreasing concentrations of DDM from 0.4 to 0%. The asterisk represents migration of the SecYEG tandem dimer.
Stability of the HTL Complex.
Examination by blue-native (BN)-PAGE revealed one prominent and one diffuse band migrating respectively at ∼300 kDa and ∼150–200 kDa (Fig. 1D). Both bands were excised and subject to a second-dimension denaturing gel. The higher-molecular-weight (MW) form contained all seven subunits of the HTL (Fig. 1E, lane 1), with a combined mass of ∼250 kDa, most likely representing the intact complex. The appearance of the lower-MW bands (Fig. 1E, lane 2) presumably arises owing to the dissociation of the HTL into the SecYEG (∼75 kDa) and SecDF–YajC–YidC (∼175 kDa) subcomplexes. The tendency of the HTL heterodimer ([SecYEG]–[SecDF–YajC–YidC]) to separate is promoted by the dilute conditions used for BN-PAGE, and by increasing detergent concentration (Fig. 1F; right to left for each sample), reminiscent of the separation of SecYEG homo-dimers ([SecYEG]–[SecYEG]) into monomers (24). The SecDF–YidC complex had no tendency to oligomerize (Fig. 1F).
The intact HTL complex has the same apparent MW as SecYEG dimers (Fig. 1F, asterisk) and, therefore, can only contain single copies of SecYEG and SecDF–YajC–YidC. The extraction of tightly bound phospholipids by high detergent concentrations may account for the dissociation of the HTL complex, which is also the case for SecYEG dimers (25). The integrity of the complex could be directly demonstrated by negative-stain electron microscopy (Fig. S2, Left), which revealed the presence of uniformly sized particles of purified HTL complex, as shown in the selected 2D class averages (Fig. S2, Right).
Subunit Organization of the HTL Complex.
We performed in-membrane cross-linking experiments using the photo-inducible Tris-bipyridylruthenium(II) to investigate protein–protein interactions within the HTL. SecYEG was used as a control; as reported previously (4), we observed the formation of SecE–E and SecY–Y products upon cross-linking of inner membrane vesicles (IMVs) containing overexpressed SecYEG, indicative of the presence of SecYEG dimers (Fig. 2A). When IMVs containing overexpressed HTL complex were subjected to the same treatment, the SecE–E and SecY–Y products were no longer detectable (Fig. 2A). To confirm that this result is not due to the decreased signal from blotting with the SecE antibody, we used the homo-bifunctional amine-reactive reagent dithiobis[succinimidyl propionate] (DSP) as an alternative method for ex vivo cross-linking of the native membranes before HTL and SecYEG purification. In contrast to Tris-bipyridylruthenium(II), which couples neighboring residues without a linker, DSP extends the cross-linking range by virtue of its 12-Å spacer arm, allowing for identification of nearby interaction partners. In HTL- and SecYEG-overexpressing membranes, those cross-links occurring within the SecYEG subcomplex (SecY–E, SecY–G, and SecE–G) could be generated with similar efficiency (Fig. 2B, red boxes). Cross-links at the interface between SecYEG dimers (SecE–E, SecY–E–E, and SecY–Y; Fig. 2B, green boxes) were either lost or considerably diminished in the HTL complex.
Fig. 2.
Cross-linking of HTL subunits. (A) Western blots of photo-activatable Tris-bipyridylruthenium(II)–mediated cross-linking (with or without exposure to light radiation) of E. coli IMVs overexpressing either SecYEG or HTL. Y–Y represents a purified covalently-linked SecYEG dimer (38). 106x represents a purified SecE-E dimeric version of SecYEG (4). (B) IMVs were also subjected to DSP-mediated cross-linking, from where the respective complexes were isolated for further analysis by SDS/PAGE and Western blotting. Cross-linking adducts preserved between SecYEG and HTL are bounded in red boxes. Those that are eliminated in the HTL are bounded in green boxes. Newly formed adducts are bounded in blue boxes.
Notably, new higher-MW products were observed in the cross-linked HTL sample, indicative of contacts between SecYEG and SecDF–YajC–YidC subcomplexes (Fig. 2B, blue boxes). These HTL-specific bands cross-react with SecY, SecE, and SecG antibodies and are larger than the corresponding cross-links with SecY (Fig. 2B; Y–Y, Y–E, and Y–G, respectively) and therefore must have arisen from cross-links with the higher-MW subunits SecD or YidC. Mass spectrometry performed on corresponding bands excised from Coomassie-stained gels (Fig. S3, asterisk) confirmed the presence of both SecD and YidC. Taken together, the results show that the HTL complex contains only one copy of SecYEG, which contacts SecD and YidC in place of the second copy of SecYEG found in the dimeric form.
Interaction of the HTL Complex with SecA.
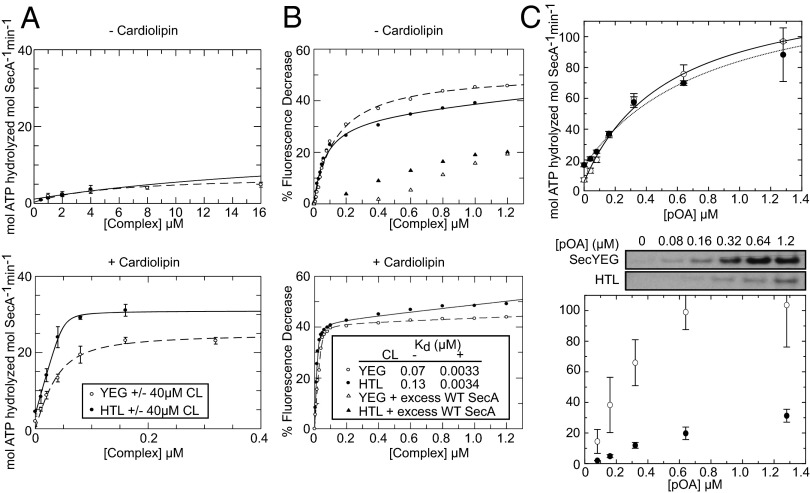
We analyzed the ability of the HTL complex to associate and activate the motor ATPase SecA for secretion. Both HTL and SecYEG had significantly increased rates of SecA-mediated ATP hydrolysis in the presence of cardiolipin (CL), a phospholipid previously demonstrated to stabilize and stimulate the activity of the SecYEG dimer (25) (Fig. 3A, compare Upper and Lower). The apparent affinity of the HTL for SecA was in fact too high to be measured accurately but is clearly higher than that for SecYEG, as is the stimulation of the ATPase activity (Fig. 3A, Lower).
Fig. 3.
SecYEG- and HTL-mediated stimulation of SecA ATPase activity is enhanced by CL. (A) Increasing amounts of SecYEG dimer (YEG2, open circles) or HTL complex (filled circles) were tested for their ability to activate the ATPase activity of SecA (0.3 µM) in the presence or absence of 40 µM CL. Data are representative of the mean of three independent experiments ± SD. Data were fitted as detailed in Materials and Methods. (B) Fluorescence quenching assays using fluorescein-tagged SecA (SecA795Fl) and SecYEG or HTL complex in the presence or absence of 40 µM CL. (Inset) The Kd values determined with and without CL. Note that the values of the Kd in the presence of CL are too low to be determined accurately. (C) Translocation ATPase activity of SecA as a function of substrate concentration (Upper) showing the average of three experiments fitted to the weak binding equation with y axis offset (SI Materials and Methods) and the corresponding translocation yields (Lower, with above representative blot) ± SEM.
The association of SecA and SecYEG in the ATP-bound state can be monitored by the quenching of fluorescein-labeled SecA in the presence of a nonhydrolyzable analog adenosine 5′-(β,γ-imido)triphosphate (AMPPNP) (4). This same effect was observed upon the addition of the HTL complex (Fig. 3B). The affinity of SecAATP for HTL is considerably increased (>10-fold) in the presence of CL. In fact, the Kd was again too low to be measured accurately. This effect is consistent with the binding of SecYEG to SecA (Fig. 3B). The results show that SecYEG dimers (25) and HTL act on SecA in a very similar CL-dependent fashion.
ATP- and PMF-Driven Protein Secretion Through the HTL Complex.
To assess the functionality of the isolated HTL complex, we examined its ability to transport the outer membrane precursor protein pro-OmpA across a lipid bilayer. The ATPase activity (Vmax) corresponding to translocation of pro-OmpA into vesicles containing either SecYEG core complex or HTL was about the same (Fig. 3C, Upper). However, the Km for proOmpA was lower for the HTL (0.55 μM compared with 0.80 μM), indicating a higher affinity of the translocating polypeptide. Much more striking was the conversion efficiency of ATP to transport, which is very much reduced for the HTL complex (Fig. 3C, Lower).
Next, translocation was assessed in the presence and absence of a transmembrane PMF. For PMF generation, the light-driven proton pump bacteriorhodopsin (BR) was incorporated into proteoliposomes harboring SecYEG or the HTL. The reconstitution efficiency was assessed by SDS/PAGE (Fig. S4). The levels of BR in both sets of proteoliposomes were similar and the quantities of SecY were consistent with their expected stoichiometries (i.e., twice as much in the SecYEG sample, owing to the presence of two copies of SecY, compared with one in the HTL). Thus, respective transport activities could be legitimately compared.
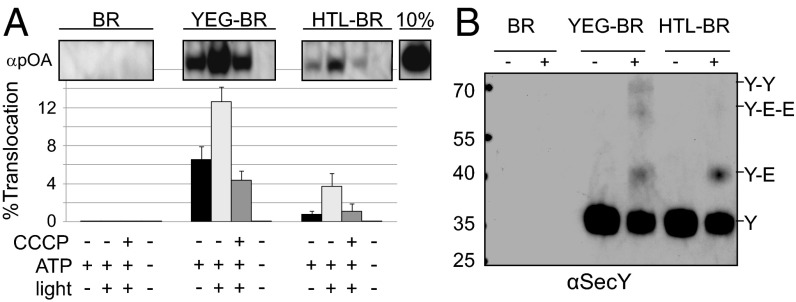
The translocation assay was monitored following addition of SecA, ATP, and proOmpA, in the presence (+PMF) or absence (−PMF) of a light source. The HTL complex is less effective in ATP-dependent SecA-driven protein secretion and more dependent on the PMF (Fig. 4A). To verify that the reduced secretion activity of the HTL complex was not the result of an asymmetric reconstitution favoring inwardly-facing cytosolic sites, the sidedness of the vesicles was investigated by exposure to trypsinolysis (Fig. S5). A 21-kDa band appeared, which corresponds to the N-terminal fragment of SecY, owing to cleavage in the cytosolic loop between TMSs 6 and 7 (26). In both proteoliposomes containing SecYEG and HTL complexes, SecY was sensitive to proteolysis (Fig. S5). Evidently, the reconstitution has favored the orientation with the cytosolic surface facing outward. Therefore, the observed reduction in secretion activity of the HTL complex is not the result of a reduction in available sites for translocation.
Fig. 4.
The E. coli holo-translocon is competent for protein secretion. (A) In vitro translocation assay showing the effect of a light-stimulated PMF on the efficiency of the reaction. SecYEG and HTL proteoliposomes were reconstituted together with BR for the purposes of generating a PMF. The graph shows the percentage of pro-OmpA (pOA) translocated into the liposome interior by SecYEG or the HTL. Values represent the mean of four independent experiments ± SEM. (B) SecY immunoblot of DSP cross-linking of the same proteoliposomes used in A, demonstrating that the organization of liposome-incorporated SecYEG and HTL is maintained.
To confirm that the secretion activity seen for the HTL was not due to dissociation of the [SecYEG]–[SecDF–YajC–YidC] heterodimer and reformation of SecYEG dimers, we performed DSP cross-linking on the proteoliposome samples used for translocation (Fig. 4B). Although the cross-linking efficiency was reduced, presumably owing to removal of the HTL from its native membrane environment, immunoblotting results for SecY in this context mirrored the trends observed in IMVs containing overexpressed SecYEG and HTL (Fig. 2B). Proteoliposomes containing SecYEG produced SecY–Y and SecY–E–E cross-links, owing to the presence of SecYEG dimers. These were not observed in cross-linked proteoliposomes containing the HTL complex (Fig. 4B). Therefore, in these assay conditions, the HTL complex contains single copies of SecYEG and SecDF–YajC–YidC and remains competent for secretion of proOmpA.
Ribosome Binding of HTL and Its Subcomplexes.
The HTL complex and its constituents were tested for their ability to associate with the ribosome by cosedimentation. HTL, SecYEG, YidC, and SecDF–YajC–YidC all displayed a preference for ribosomes displaying the nascent transmembrane helix of FtsQ (27) over nontranslating ribosomes (70S) (Fig. S6). In contrast, SecDF had an equal preference for translating and nontranslating ribosomes or the small ribosomal subunit (30S) (Fig. S6), suggestive of a nonspecific interaction. Therefore, the nascent membrane protein probably contacts both SecYEG and YidC.
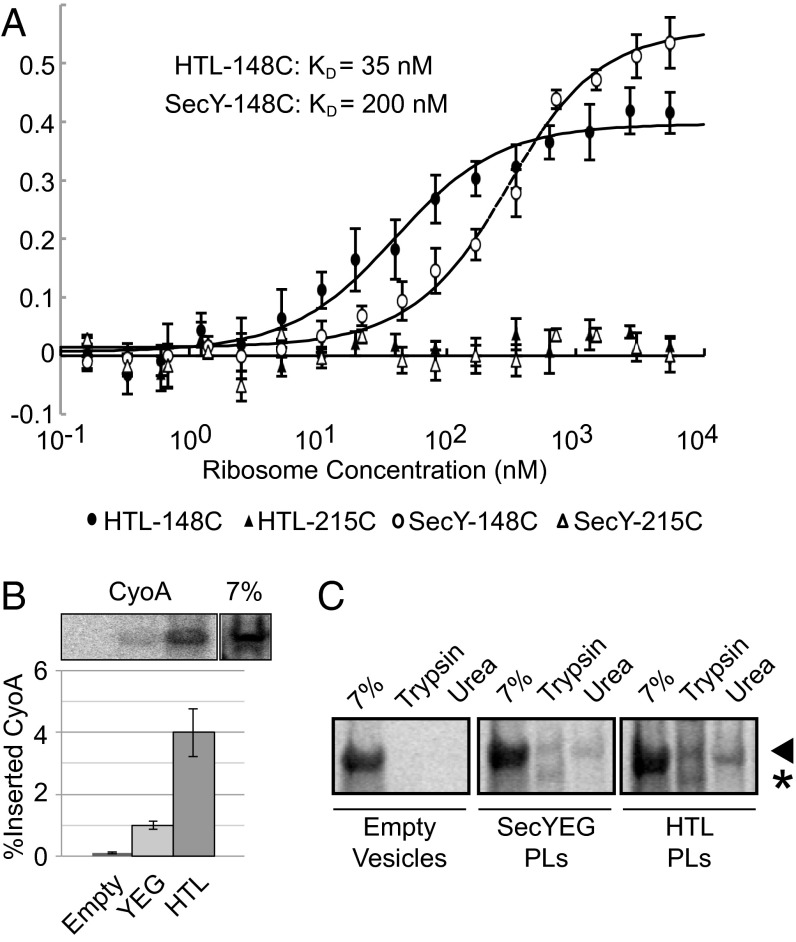
Using SecY labeled with Cy3 at position 148 and 215 as a control (which does not respond to ribosome binding), we determined the affinity of detergent-solubilized SecYEG and HTL, respectively, to 70S ribosomes by fluorescence analysis (Fig. 5A). The binding of ribosomes to SecYEG induces conformational changes, which can be monitored by environment-sensitive fluorophores at specific locations (28). The increase of Cy3 fluorescence at position 148, located at the periplasmic side of the lateral gate, was used to determine a Kd for HTL (∼35 nM) and SecYEG (∼200 nM). Therefore, SecDF–YajC and YidC consolidate the association of the translocon with the ribosome.
Fig. 5.
Interaction of the HTL with ribosomes supports membrane protein insertion. (A) Binding of detergent-solubilized HTL and SecYEG to 70S ribosomes followed by fluorescence intensity. Constant amounts of SecYEG and HTL containing SecY labeled with Cy3 at positions 148 and 215 were exposed to increasing concentrations of 70S ribosomes, leading to an increased fluorescence in the case of SecY labeled at position 148, but not at 215. (B) In vitro synthesized cyoA mRNA was incubated together with empty liposomes or proteoliposomes containing YEG or the HTL together with an E. coli S30 membrane-free cell extract including single-chain signal recognition particle-SRP receptor (scSRP) (39) to assess incorporation of 35S-labeled CyoA directly into an in vitro membrane bilayer. Values represent the mean of four independent experiments ± SEM. (C) Representative phosphorimaging of trypsin/urea-treated 35S-labeled CyoA. Arrowhead represents full-length CyoA; asterisk denotes protease-sensitive fragment.
Membrane Protein Insertion Through the HTL Complex.
The presence of SecYEG and YidC in the HTL suggests that the complex could be active in membrane protein insertion. Therefore, we compared the capability of SecYEG and the HTL for insertion of a nascent membrane protein, CyoA, into proteoliposomes. CyoA is a polytopic membrane protein subunit of the cytochrome bo3 oxidase with known dependencies on YidC and SecYEG for cotranslational insertion (29). Successfully incorporated protein was measured separately by resistance to urea extraction and proteolysis (Fig. 5 B and C and Fig. S7). By both criteria, CyoA was inserted more efficiently into proteoliposomes containing the HTL.
Discussion
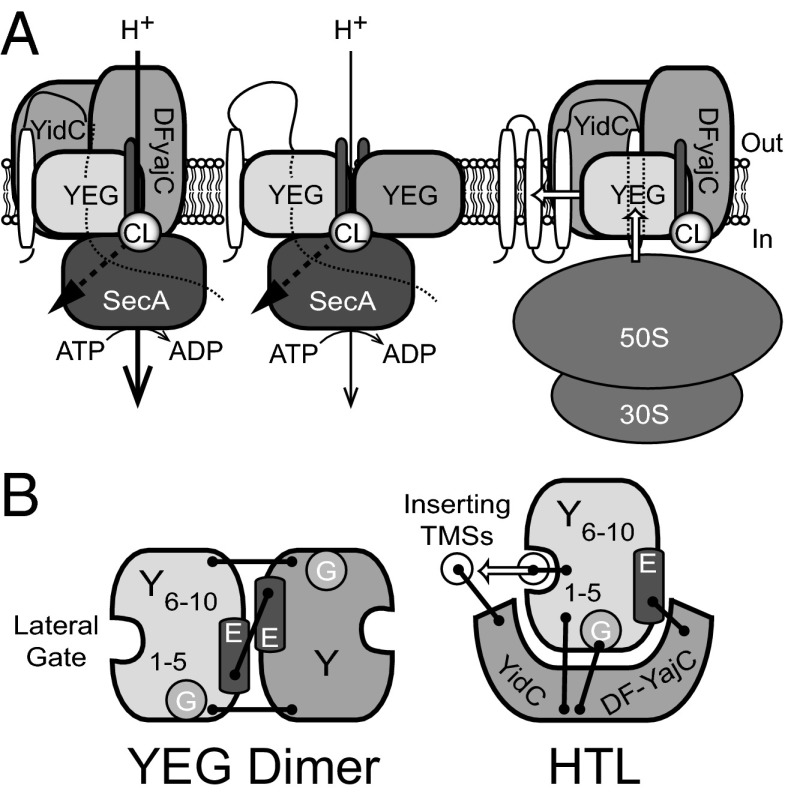
Our understanding of the integrated process of protein translocation has been restricted by the absence of a pure and stable complex capable of both secretion and membrane protein insertion. Protein secretion is driven through the center of SecYEG (1, 30) and membrane protein insertion through the lateral gate. The partitioning of translocating TMSs into the bilayer is thought to involve YidC (8), which in some cases may act alone (9). However, a physical interaction between SecYEG and YidC has yet to be demonstrated. The nature of this interaction and the mechanism of Sec-dependent membrane protein insertion have not been addressed. This work resolves this challenge in the production, purification, and functional reconstitution of a complex containing both SecYEG and YidC, as well as the accessory subcomplex SecDF–YajC—otherwise known as the HTL. The concomitant overexpression of all seven constituents of the HTL complex seems to have been a vital prerequisite for obtaining an intact and active complex. YidC was most likely incorporated as a component of the previously identified SecDF–YajC–YidC subcomplex (16). We show that the HTL is a hetero-dimeric assembly of single copies of this subcomplex and SecYEG (Fig. 6), the integrity of which depends on phospholipids.
Fig. 6.
Model for structural organization and activity of the HTL. (A) The SecYEG translocon can associate with SecDF–YajC–YidC (Left) to promote protein transport in a SecA-dependent fashion. This translocation reaction can be stimulated by the presence of a PMF and the anionic phospholipid CL. SecYEG dimers (Center), arranged in a back-to-back orientation via SecE–E interactions, are capable of mediating the same reaction, but the effect of the PMF is less. Membrane protein integration (Right) may preferentially use the SecYEG–SecDF–YajC–YidC setup (the holo-translocon) for insertion of newly synthesized transmembrane helices directly into the lipid bilayer. (B) Top view of proposed interactions for the YEG dimer and holo-translocon. Black bars represent subunit cross-links.
Cross-linking experiments have localized YidC to the lateral gate of SecY [TMSs 2b, 3, and 7 (12)], which also contacts translocating TMSs as they emerge from the translocon (10, 11, 31). We also show cross-links between SecD/YidC and SecY, E, and G. These findings, together with a reported functional interaction between SecG and SecDF–YajC (32), are suggestive of an interface involving the N-terminal half of SecY (TMSs 1–5), SecE, and SecG (Fig. 6).
A comparison of the activities of HTL and SecYEG shows that they are equally capable of inducing high levels of ATP turnover in SecA. The HTL complex associates more tightly with SecA and with the translocating substrate but is less capable of secretion. This difference in the energy transducing stoichiometry may be compensated by the higher dependence of the HTL on the PMF. The determinants and mechanistic basis governing the stimulation by the PMF are not immediately clear. The core SecYEG complex itself couples the PMF to translocation (20). The increased dependence of the HTL on the PMF may result from combined effects of SecYEG and SecDF, possibly also through a SecDF-dependent regulation of a membrane-associated state of the SecA ATPase (18).
The HTL complex is more proficient in cotranslational membrane protein insertion compared with SecYEG alone. The increased efficiency afforded by the presence of YidC in the HTL complex may be critical for rapid protein assembly, membrane biogenesis, and competitive survival. The different dependencies of SecYEG and HTL for secretion and insertion are presumably selected according to the specific requirements of the translocating substrate. Moreover, the dual capabilities of the HTL may be critical for the secretion of large extracellular domains of polytopic membrane proteins.
Different translocons may form either from single copies of SecYEG and SecDF–YajC–YidC (HTL), or two copies of SecYEG (Fig. 6), or of YidC alone. The estimated number of copies of SecYE (300–400 copies per cell), SecDF (30 copies per cell) (33), and YidC (2,700 copies per cell) (34) are very different. Thus, a typical cell might contain up to five copies of SecYEG dimers for every SecYEG–[SecDF–YajC–YidC] holo-translocon complex. The need for a large (100-fold) excess of YidC is unclear; it may in part be required for its Sec-independent activity.
The dynamic exchange of the accessory complexes bound to SecYEG may provide a means to modulate translocation activity (Fig. 6) and the composition of the membrane and envelope during different stages of growth or upon exposure to different environmental conditions. Similarly, the eukaryotic Sec61 complex and mitochondrial import machinery are likely to associate with a number of different accessory factors such as translocating chain-associating membrane protein or Oxa1, respectively, tailored to the specific needs of folding, assembly, and modification in the membranes of these organelles.
Materials and Methods
Strains, Plasmids, and Antisera.
E. coli C43(DE3) was used for overexpression of the HTL components and was a gift from John Walker, Medical Research Council Mitochondrial Biology Unit, Cambridge, UK. SecYEG and SecDF–YidC–YajC (DFYY) expression vectors were from our laboratory collection (35). The HTL expression plasmid (Fig. S1) was constructed using the ACEMBL expression system (23). SI Materials and Methods gives more details. The plasmids for SecDF–YidC and covalently linked SecYEG dimer expression were gifts from Franck Duong, University of British Columbia, Vancouver, BC, Canada. Mouse monoclonal antibodies to SecY, E, and G were from our laboratory collection.
Enzymes and Chemicals.
CL was obtained from Avanti Polar Lipids. DDM was from Glycon Biochemicals GmbH. Unless noted otherwise, all other reagents used in this study were from Sigma–Aldrich.
Purification of SecYEG, SecA, proOmpA, and SecDF–YidC.
SecYEG, SecA, and proOmpA proteins were purified according to well-established procedures (36). SecDF–YidC was purified in the same way as SecYEG.
Purification of the HTL.
E. coli C43(DE3) expressing pACEMBL:HTL was used for HTL purification by Ni2+ chromatography followed by size exclusion combined with an anion exchange step. SI Materials and Methods gives more details.
BN-PAGE Analysis.
Fifty nanograms of protein complexes were incubated in TSG130 buffer [20 mM Tris⋅Cl (pH 8.0), 130 mM NaCl, and 10% (vol/vol) glycerol] containing decreasing detergent concentrations on ice for 20 min. Coomassie G-250 was then added to a final concentration of 0.025% before loading onto 4–16% Bis-Tris NativePAGE gels (Invitrogen). Bands were visualized by silver stain (SilverQuest Staining Kit; Invitrogen).
In Vivo and in Vitro Cross-Linking.
Analysis of intersubunit organization within the HTL was probed using Tris-bipyridylruthenium(II) as described (4) or DSP. DSP cross-linking was performed in IMVs containing overexpressed SecYEG or HTL in HSG130 [50 mM Hepes (pH 7.5), 130 mM NaCl, and 10% (vol/vol) glycerol] buffer. IMVs were isolated as in ref. 4. DSP was then added to the IMVs at a final concentration of 150 µM before incubation at room temperature for 20 min. The reaction was quenched by addition of Tris⋅Cl, pH 8.0, to a final concentration of 50 mM. The cross-linked SecYEG or HTL was then examined by Western blot directly from IMVs or by further purification as above.
SecA ATPase Stimulation.
SecYEG- or HTL-mediated stimulation of the SecA ATPase was measured by titrating in increasing concentrations of each complex into solutions of SecA, as has been previously described (36), in the presence or absence of 40 µM CL. SI Materials and Methods gives the data analysis.
Affinity Measurements of SecA to Either SecYEG or HTL by Quenching of an Extrinsic Fluorescent Probe on SecA.
SecYEG or HTL were titrated into solutions of fluorescently labeled SecAA795C (SecA795Fl) as indicated. Fluorescence assays were performed in 20 mM Tris (pH 8.0), 130 mM NaCl, 10% (vol/vol) glycerol, 2 mM MgCl2, 0.02% DDM, 1 mM AMPPNP, and 10 nM SecA795Fl. SecA795Fl fluorescence quenching was monitored using a Jobin Yvon Fluorolog (Horiba Scientific) at an excitation wavelength of 495 nm and emission wavelength of 515 nm. CL (40 µM) was incorporated into the assay buffer where required, and SecYEG or HTL stocks were incubated with 40 µM CL for 1 h before titration. Specificity of the quenching of SecA795Fl was assessed by competition for SecYEG or HTL binding using 1 µM wild-type SecA. SI Materials and Methods gives the data analysis.
Coreconstitution of BR with Translocation Machinery.
For in vitro generation of a PMF in proteoliposomes, BR from Halobacterium halobium purple membranes was coreconstituted together with the HTL or SecYEG. Purple membranes were purified by standard methods (37) (SI Materials and Methods gives more details).
In Vitro Translocation Assay.
The protein transport activity of the HTL or SecYEG, respectively, was analyzed as described (36).
Affinity Measurements of Either SecYEG or HTL to 70S by Fluorescence Analysis.
One hundred nanomolar Cy3-labeled cysteine mutants of HTL or SecYEG in 50 mM Hepes/KOH (pH 7.5), 100 mM KOAc2, 20 mM MgOAc2, 10% (vol/vol) glycerol, and 0.03% DDM was mixed 1:1 with 0.3 nM to 11 µM 70S ribosomes in 20 mM Hepes/KOH (pH 7.5), 20 mM MgOAc2, 30 mM NH4Cl, and 1 mM DTT in a volume of 30 µL. Fluorescence was measured using a Monolith NT.115 and data were analyzed using the supplied software (Nanotemper). Each experiment was repeated fourfold and the fluorescence was normalized by division through the average of the first four data points.
In Vitro Transcription/Translation/Insertion Assay.
mRNA transcripts were generated using T7 RNA polymerase by in vitro transcription from PCR products containing cyoA downstream of a T7 promoter. These mRNAs were subsequently used in a coupled in vitro translation/insertion assay as detailed in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. John Bason for advice with the reconstitution of bacteriorhodopsin, Sir John Walker for the E. coli C43 expression strain, Prof. Franck Duong for SecDF–YidC and SecY–Y overexpression constructs, and Dr. Alice Robson for assistance with kinetic analysis. This work was supported by a Royal Society Leverhulme Research Fellowship (to I.C.) and Biotechnology and Biological Sciences Research Council Project Grants BB/F007248/1 and BB/I008675/1 (to I.C.). C.S. is supported by contract research “Methoden für die Lebenswissenschaften” of the Baden–Württemberg Stiftung, by the Agence Nationale de la Recherche (HOLOTRANS project, JC09_471873), and European Research Council Starting Grant 281331. I.B. is supported by the European Commission Framework Programme 7 project ComplexINC (279039).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 4739.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315901111/-/DCSupplemental.
References
- 1.Van den Berg B, et al. X-ray structure of a protein-conducting channel. Nature. 2004;427(6969):36–44. doi: 10.1038/nature02218. [DOI] [PubMed] [Google Scholar]
- 2.Breyton C, Haase W, Rapoport TA, Kühlbrandt W, Collinson I. Three-dimensional structure of the bacterial protein-translocation complex SecYEG. Nature. 2002;418(6898):662–665. doi: 10.1038/nature00827. [DOI] [PubMed] [Google Scholar]
- 3.Osborne AR, Rapoport TA. Protein translocation is mediated by oligomers of the SecY complex with one SecY copy forming the channel. Cell. 2007;129(1):97–110. doi: 10.1016/j.cell.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 4.Deville K, et al. The oligomeric state and arrangement of the active bacterial translocon. J Biol Chem. 2011;286(6):4659–4669. doi: 10.1074/jbc.M110.175638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalal K, Chan CS, Sligar SG, Duong F. Two copies of the SecY channel and acidic lipids are necessary to activate the SecA translocation ATPase. Proc Natl Acad Sci USA. 2012;109(11):4104–4109. doi: 10.1073/pnas.1117783109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park E, Rapoport TA. Bacterial protein translocation requires only one copy of the SecY complex in vivo. J Cell Biol. 2012;198(5):881–893. doi: 10.1083/jcb.201205140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duong F, Wickner W. Distinct catalytic roles of the SecYE, SecG and SecDFyajC subunits of preprotein translocase holoenzyme. EMBO J. 1997;16(10):2756–2768. doi: 10.1093/emboj/16.10.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scotti PA, et al. YidC, the Escherichia coli homologue of mitochondrial Oxa1p, is a component of the Sec translocase. EMBO J. 2000;19(4):542–549. doi: 10.1093/emboj/19.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samuelson JC, et al. YidC mediates membrane protein insertion in bacteria. Nature. 2000;406(6796):637–641. doi: 10.1038/35020586. [DOI] [PubMed] [Google Scholar]
- 10.Beck K, et al. YidC, an assembly site for polytopic Escherichia coli membrane proteins located in immediate proximity to the SecYE translocon and lipids. EMBO Rep. 2001;2(8):709–714. doi: 10.1093/embo-reports/kve154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urbanus ML, et al. Sec-dependent membrane protein insertion: sequential interaction of nascent FtsQ with SecY and YidC. EMBO Rep. 2001;2(6):524–529. doi: 10.1093/embo-reports/kve108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sachelaru I, et al. YidC occupies the lateral gate of the SecYEG translocon and is sequentially displaced by a nascent membrane protein. J Biol Chem. 2013;288(23):16295–16307. doi: 10.1074/jbc.M112.446583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samuelson JC, et al. Function of YidC for the insertion of M13 procoat protein in Escherichia coli: Translocation of mutants that show differences in their membrane potential dependence and Sec requirement. J Biol Chem. 2001;276(37):34847–34852. doi: 10.1074/jbc.M105793200. [DOI] [PubMed] [Google Scholar]
- 14.Chen M, et al. Direct interaction of YidC with the Sec-independent Pf3 coat protein during its membrane protein insertion. J Biol Chem. 2002;277(10):7670–7675. doi: 10.1074/jbc.M110644200. [DOI] [PubMed] [Google Scholar]
- 15.van der Laan M, Bechtluft P, Kol S, Nouwen N, Driessen AJ. F1F0 ATP synthase subunit c is a substrate of the novel YidC pathway for membrane protein biogenesis. J Cell Biol. 2004;165(2):213–222. doi: 10.1083/jcb.200402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nouwen N, Driessen AJ. SecDFyajC forms a heterotetrameric complex with YidC. Mol Microbiol. 2002;44(5):1397–1405. doi: 10.1046/j.1365-2958.2002.02972.x. [DOI] [PubMed] [Google Scholar]
- 17.Duong F, Wickner W. The SecDFyajC domain of preprotein translocase controls preprotein movement by regulating SecA membrane cycling. EMBO J. 1997;16(16):4871–4879. doi: 10.1093/emboj/16.16.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Economou A, Pogliano JA, Beckwith J, Oliver DB, Wickner W. SecA membrane cycling at SecYEG is driven by distinct ATP binding and hydrolysis events and is regulated by SecD and SecF. Cell. 1995;83(7):1171–1181. doi: 10.1016/0092-8674(95)90143-4. [DOI] [PubMed] [Google Scholar]
- 19.Tsukazaki T, et al. Structure and function of a membrane component SecDF that enhances protein export. Nature. 2011;474(7350):235–238. doi: 10.1038/nature09980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brundage L, Hendrick JP, Schiebel E, Driessen AJ, Wickner W. The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell. 1990;62(4):649–657. doi: 10.1016/0092-8674(90)90111-q. [DOI] [PubMed] [Google Scholar]
- 21.Arkowitz RA, Wickner W. SecD and SecF are required for the proton electrochemical gradient stimulation of preprotein translocation. EMBO J. 1994;13(4):954–963. doi: 10.1002/j.1460-2075.1994.tb06340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tseng TT, et al. The RND permease superfamily: An ancient, ubiquitous and diverse family that includes human disease and development proteins. J Mol Microbiol Biotechnol. 1999;1(1):107–125. [PubMed] [Google Scholar]
- 23.Bieniossek C, et al. Automated unrestricted multigene recombineering for multiprotein complex production. Nat Methods. 2009;6(6):447–450. doi: 10.1038/nmeth.1326. [DOI] [PubMed] [Google Scholar]
- 24.Bessonneau P, Besson V, Collinson I, Duong F. The SecYEG preprotein translocation channel is a conformationally dynamic and dimeric structure. EMBO J. 2002;21(5):995–1003. doi: 10.1093/emboj/21.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gold VA, et al. The action of cardiolipin on the bacterial translocon. Proc Natl Acad Sci USA. 2010;107(22):10044–10049. doi: 10.1073/pnas.0914680107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akiyama Y, Ito K. SecY protein, a membrane-embedded secretion factor of E. coli, is cleaved by the ompT protease in vitro. Biochem Biophys Res Commun. 1990;167(2):711–715. doi: 10.1016/0006-291x(90)92083-c. [DOI] [PubMed] [Google Scholar]
- 27.Schaffitzel C, Ban N. Generation of ribosome nascent chain complexes for structural and functional studies. J Struct Biol. 2007;158(3):463–471. doi: 10.1016/j.jsb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Lycklama a Nijeholt JA, Wu ZC, Driessen AJ. Conformational dynamics of the plug domain of the SecYEG protein-conducting channel. J Biol Chem. 2011;286:43881–43890. doi: 10.1074/jbc.M111.297507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.du Plessis DJ, Nouwen N, Driessen AJ. Subunit a of cytochrome o oxidase requires both YidC and SecYEG for membrane insertion. J Biol Chem. 2006;281(18):12248–12252. doi: 10.1074/jbc.M600048200. [DOI] [PubMed] [Google Scholar]
- 30.Cannon KS, Or E, Clemons WMJ, Jr, Shibata Y, Rapoport TA. Disulfide bridge formation between SecY and a translocating polypeptide localizes the translocation pore to the center of SecY. J Cell Biol. 2005;169(2):219–225. doi: 10.1083/jcb.200412019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klenner C, Kuhn A. Dynamic disulfide scanning of the membrane-inserting Pf3 coat protein reveals multiple YidC substrate contacts. J Biol Chem. 2012;287(6):3769–3776. doi: 10.1074/jbc.M111.307223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato Y, Nishiyama K, Tokuda H. Depletion of SecDF-YajC causes a decrease in the level of SecG: implication for their functional interaction. FEBS Lett. 2003;550(1–3):114–118. doi: 10.1016/s0014-5793(03)00847-0. [DOI] [PubMed] [Google Scholar]
- 33.Pogliano KJ, Beckwith J. Genetic and molecular characterization of the Escherichia coli secD operon and its products. J Bacteriol. 1994;176(3):804–814. doi: 10.1128/jb.176.3.804-814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urbanus ML, et al. Targeting, insertion, and localization of Escherichia coli YidC. J Biol Chem. 2002;277(15):12718–12723. doi: 10.1074/jbc.M200311200. [DOI] [PubMed] [Google Scholar]
- 35.Collinson I, et al. Projection structure and oligomeric properties of a bacterial core protein translocase. EMBO J. 2001;20(10):2462–2471. doi: 10.1093/emboj/20.10.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robson A, Gold VA, Hodson S, Clarke AR, Collinson I. Energy transduction in protein transport and the ATP hydrolytic cycle of SecA. Proc Natl Acad Sci USA. 2009;106(13):5111–5116. doi: 10.1073/pnas.0809592106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oesterhelt D, Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- 38.Duong F. Binding, activation and dissociation of the dimeric SecA ATPase at the dimeric SecYEG translocase. EMBO J. 2003;22(17):4375–4384. doi: 10.1093/emboj/cdg418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Estrozi LF, Boehringer D, Shan SO, Ban N, Schaffitzel C. Cryo-EM structure of the E. coli translating ribosome in complex with SRP and its receptor. Nat Struct Mol Biol. 2011;18(1):88–90. doi: 10.1038/nsmb.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.